Abstract
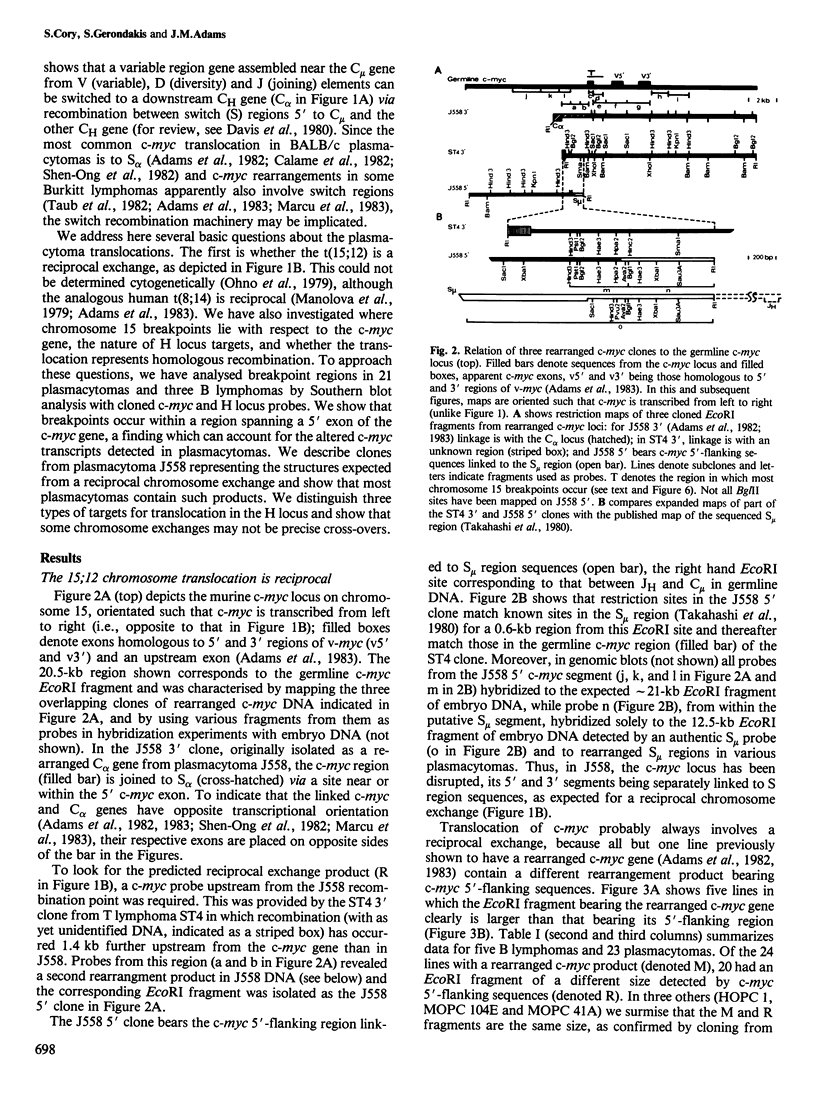
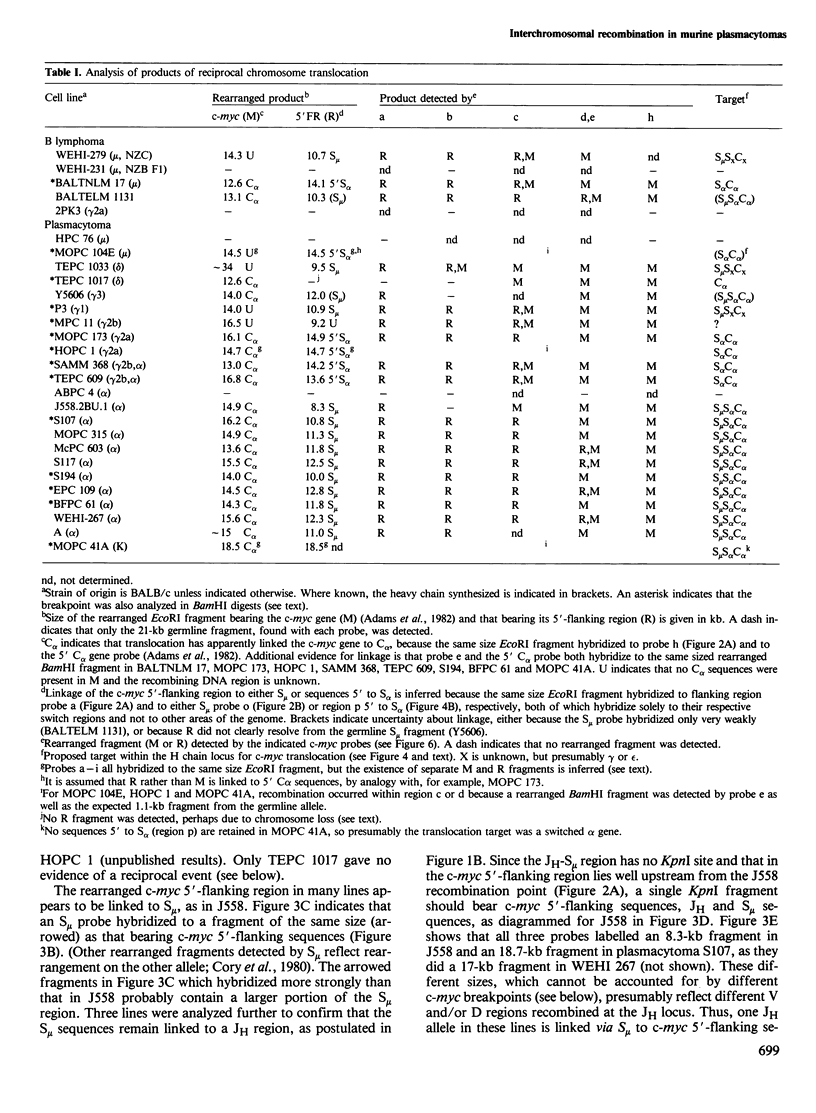
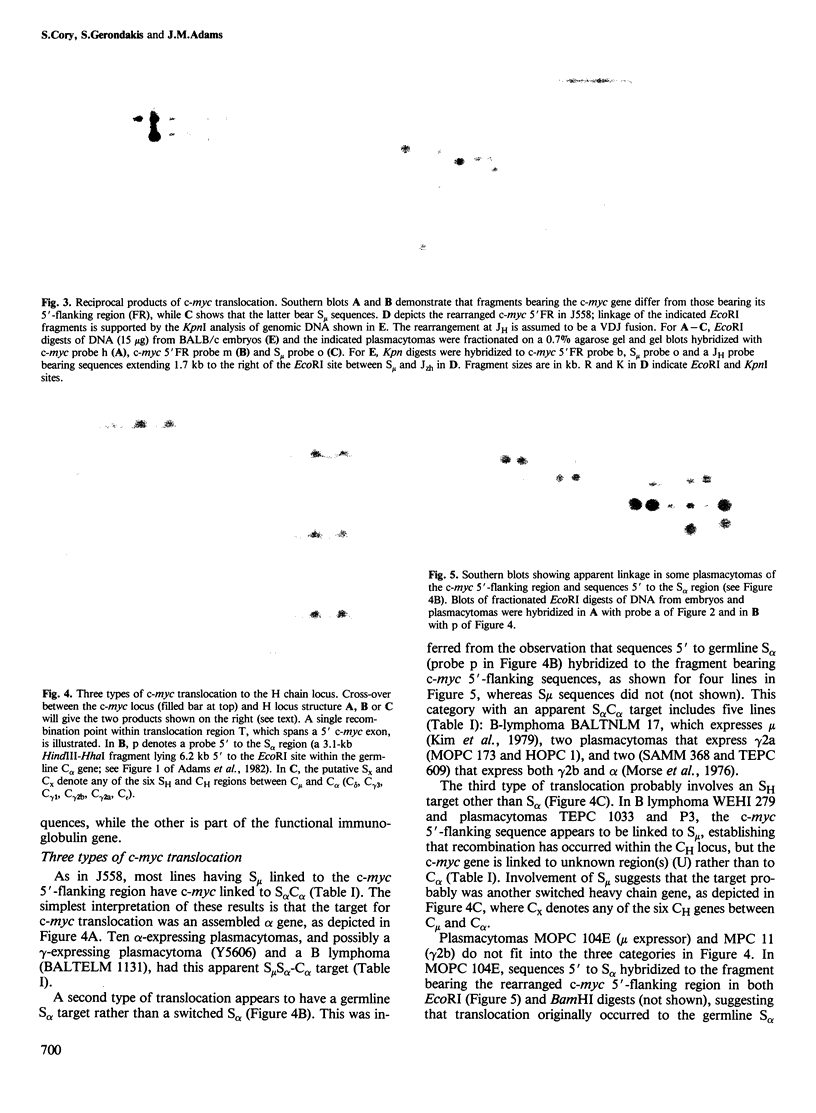
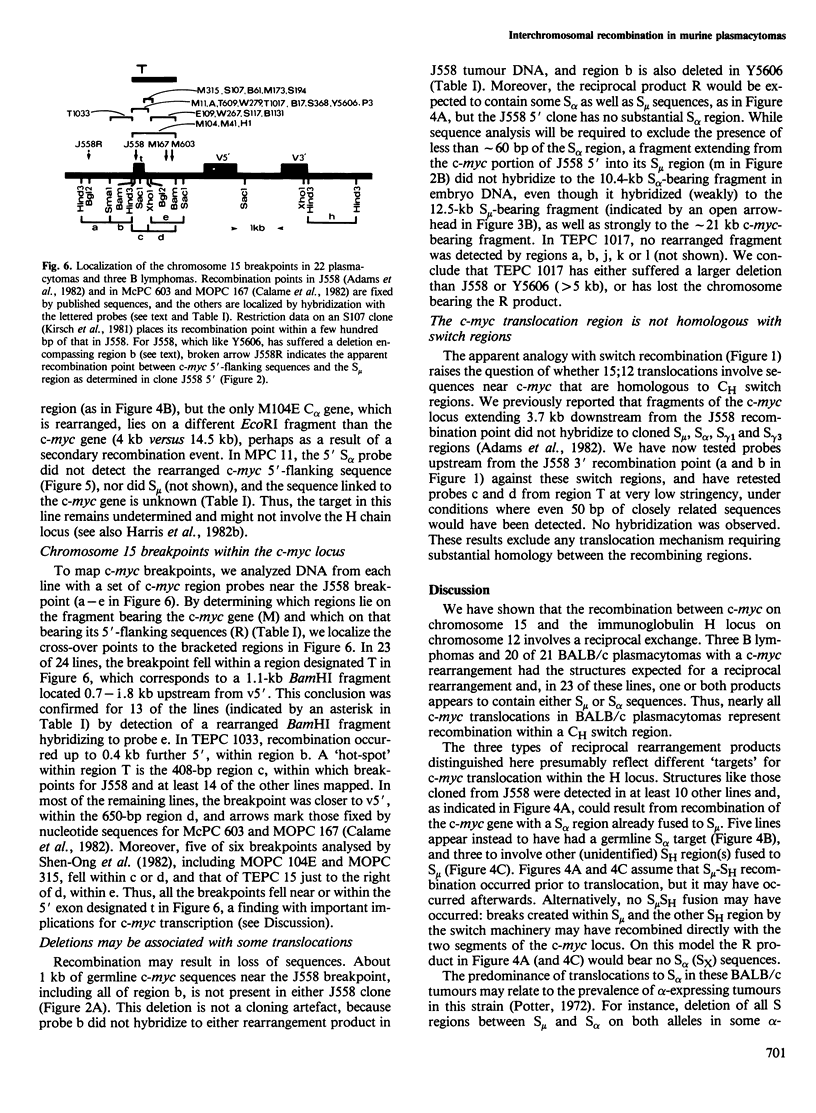
The 15:12 chromosome translocations found in most murine plasmacytomas involve the cellular gene (c-myc) homologous to the oncogene (v-myc) of avian retrovirus MC29, Translocation links the c-myc gene of chromosome 15 to the immunoglobulin heavy (H) chain locus of chromosome 12, often within the switch recombination (S) region 5' to the alpha constant region (C alpha) gene. We have investigated c-myc rearrangements in 21 BALB/c plasmacytomas and three B lymphomas by Southern blot analysis. We show that the t(15;12) is a reciprocal chromosome exchange since most tumours contain not only a c-myc gene linked to the S alpha C alpha region but also a separate structure with S mu or S alpha linked to the c-myc 5'-flanking region. Analysis of the two rearrangement products cloned from plasmacytoma J558 suggests that one type of H locus target for translocation is an S alpha region recombined with S mu; two other targets appear to be other switched heavy chain genes and an unrearranged C alpha gene. Nearly all the chromosome 15 breakpoints fall within a 1.1-kb region spanning a 5' c-myc exon; hence scission of the transcriptional unit by translocation can account for the altered c-myc transcription in plasmacytomas. The c-myc breakpoint region lacks substantial homology with S mu or S alpha, arguing against homologous recombination as the translocation mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Mitchell J., Bernard O., Cory S. Transcriptionally active DNA region that rearranges frequently in murine lymphoid tumors. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6966–6970. doi: 10.1073/pnas.79.22.6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Calame K., Kim S., Lalley P., Hill R., Davis M., Hood L. Molecular cloning of translocations involving chromosome 15 and the immunoglobulin C alpha gene from chromosome 12 in two murine plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6994–6998. doi: 10.1073/pnas.79.22.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Gerondakis S. D., Miller J. F., Gamble J., Wiener F., Spira J., Francke U. Fusion of DNA region to murine immunoglobulin heavy chain locus corresponds to plasmacytoma-associated chromosome translocation. EMBO J. 1983;2(2):213–216. doi: 10.1002/j.1460-2075.1983.tb01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Jackson J., Adams J. M. Deletions in the constant region locus can account for switches in immunoglobulin heavy chain expression. Nature. 1980 Jun 12;285(5765):450–456. doi: 10.1038/285450a0. [DOI] [PubMed] [Google Scholar]
- Crews S., Barth R., Hood L., Prehn J., Calame K. Mouse c-myc oncogene is located on chromosome 15 and translocated to chromosome 12 in plasmacytomas. Science. 1982 Dec 24;218(4579):1319–1321. doi: 10.1126/science.7146913. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. Immunoglobulin class switching: developmentally regulated DNA rearrangements during differentiation. Cell. 1980 Nov;22(1 Pt 1):1–2. doi: 10.1016/0092-8674(80)90145-2. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gutman G. A., Warner N. L., Harris A. W., Bowles A. Use of [75Se]selenomethionine in immunoglobulin biosynthetic studies. J Immunol Methods. 1978;21(1-2):101–109. doi: 10.1016/0022-1759(78)90227-2. [DOI] [PubMed] [Google Scholar]
- Gutman G. A., Warner N. L., Harris A. W. Immunoglobulin production by murine B-lymphoma cells. Clin Immunol Immunopathol. 1981 Feb;18(2):230–244. doi: 10.1016/0090-1229(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Harris L. J., D'Eustachio P., Ruddle F. H., Marcu K. B. DNA sequence associated with chromosome translocations in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6622–6626. doi: 10.1073/pnas.79.21.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Lang R. B., Marcu K. B. Non-immunoglobulin-associated DNA rearrangements in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4175–4179. doi: 10.1073/pnas.79.13.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Kirsch I. R., Morton C. C., Nakahara K., Leder P. Human immunoglobulin heavy chain genes map to a region of translocations in malignant B lymphocytes. Science. 1982 Apr 16;216(4543):301–303. doi: 10.1126/science.6801764. [DOI] [PubMed] [Google Scholar]
- Kirsch I. R., Ravetch J. V., Kwan S. P., Max E. E., Ney R. L., Leder P. Multiple immunoglobulin switch region homologies outside the heavy chain constant region locus. Nature. 1981 Oct 15;293(5833):585–587. doi: 10.1038/293585a0. [DOI] [PubMed] [Google Scholar]
- Klein G., Ohno S., Rosenberg N., Wiener F., Spira J., Baltimore D. Cytogenetic studies on abelson-virus-induced mouse leukemias. Int J Cancer. 1980 Jun 15;25(6):805–811. doi: 10.1002/ijc.2910250617. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Lane M. A., Sainten A., Cooper G. M. Stage-specific transforming genes of human and mouse B- and T-lymphocyte neoplasms. Cell. 1982 Apr;28(4):873–880. doi: 10.1016/0092-8674(82)90066-6. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R., Potter M., Humphrey W., Jr, Mushinski E. B., Vrana M. Multiple individual and cross-specific indiotypes on 13 levan-binding myeloma proteins of BALB/c mice. J Exp Med. 1975 Jul 1;142(1):106–119. doi: 10.1084/jem.142.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie M. R., Gutman G. A., Warner N. L. The binding of murine IgM to Staphylococcal A protein. Scand J Immunol. 1978;7(5):367–370. doi: 10.1111/j.1365-3083.1978.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Malcolm S., Barton P., Murphy C., Ferguson-Smith M. A., Bentley D. L., Rabbitts T. H. Localization of human immunoglobulin kappa light chain variable region genes to the short arm of chromosome 2 by in situ hybridization. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4957–4961. doi: 10.1073/pnas.79.16.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolova Y., Manolov G., Kieler J., Levan A., Klein G. Genesis of the 14q+ marker in Burkitt's lymphoma. Hereditas. 1979;90(1):5–10. doi: 10.1111/j.1601-5223.1979.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Mathieson B. J., Campbell P. S., Potter M., Asofsky R. Expression of Ly 1, Ly 2, Thy 1, and TL differentiation antigens on mouse T-cell tumors. J Exp Med. 1978 Apr 1;147(4):1267–1279. doi: 10.1084/jem.147.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Pumphrey J. G., Potter M., Asofsky R. Murine plasma cells secreting more than one class of immunoglobulin heavy chain. I. Frequency of two or more M-components in ascitic fluids from 788 primary plasmacytomas. J Immunol. 1976 Aug;117(2):541–547. [PubMed] [Google Scholar]
- Neel B. G., Gasic G. P., Rogler C. E., Skalka A. M., Ju G., Hishinuma F., Papas T., Astrin S. M., Hayward W. S. Molecular analysis of the c-myc locus in normal tissue and in avian leukosis virus-induced lymphomas. J Virol. 1982 Oct;44(1):158–166. doi: 10.1128/jvi.44.1.158-166.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Jhanwar S. C., Chaganti R. S., Hayward W. S. Two human c-onc genes are located on the long arm of chromosome 8. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7842–7846. doi: 10.1073/pnas.79.24.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Babonits M., Wiener F., Spira J., Klein G., Potter M. Nonrandom chromosome changes involving the Ig gene-carrying chromosomes 12 and 6 in pristane-induced mouse plasmacytomas. Cell. 1979 Dec;18(4):1001–1007. doi: 10.1016/0092-8674(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Owen F. L., Riblet R., Taylor B. A. The T suppressor cell alloantigen Tsud maps near immunoglobulin allotype genes and may be an heavy chain constant-region marker on a T cell receptor. J Exp Med. 1981 Apr 1;153(4):801–810. doi: 10.1084/jem.153.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Ralph P. Retention of lymphocyte characteristics by myelomas and theta + -lymphomas: sensitivity to cortisol and phytohemagglutinin. J Immunol. 1973 Jun;110(6):1470–1475. [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kataoka T., Honjo T. Nucleotide sequences of class-switch recombination region of the mouse immunoglobulin gamma 2b-chain gene. Gene. 1980 Oct;11(1-2):117–127. doi: 10.1016/0378-1119(80)90092-x. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener F., Babonits M., Spira J., Bregula U., Klein G., Merwin R. M., Asofsky R., Lynes M., Haughton G. Chromosome 15 trisomy in spontaneous and carcinogen-induced murine lymphomas of B-cell origin. Int J Cancer. 1981 Jan 15;27(1):51–58. doi: 10.1002/ijc.2910270109. [DOI] [PubMed] [Google Scholar]