Kononenko highlights Goo et al.’s discovery that lysosomes can be positioned in neuronal dendritic spines in response to synapse activity.
Abstract
In neurons, lysosomes regulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor levels at the plasma membrane, although their presence at distal dendrites is controversial. In this issue, Goo et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201704068) show for the first time that neuronal activity positions lysosomes at the dendritic spines to facilitate synaptic remodeling through local protein degradation.
Our brain stores information by strengthening or weakening existing synapses. Such changes in synaptic connections are essential for learning and memory formation, although the exact mechanisms underlying continuous synaptic remodeling remain enigmatic. During the last few decades, close attention has been drawn to dendritic spines, which are small protrusions along neuronal dendrites, known for their remarkable plasticity in response to input from the presynaptic terminal. The ability of dendritic spines to generate a long-lasting increase or decrease in synaptic strength, known as long-term potentiation and long-term depression, respectively, critically relies on the presence of the glutamate-gated ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) on their postsynaptic membrane. Various forms of membrane trafficking regulate the synaptic abundance of AMPARs. Depending on the type of stimulation, internalized AMPARs undergo complex endosomal sorting processes that direct them either to recycle back to the plasma membrane or to be degraded by the lysosomal pathway. Such changes in synaptic abundance of AMPARs are a prerequisite for the expression of Hebbian-type synaptic plasticity, the process by which neurons are thought to adapt during learning. However, given the fact that a neuron receives multiple presynaptic inputs, precisely how the synaptic abundance of AMPARs at individual spines is regulated remains unclear. In this issue, Goo et al. discovered that lysosome positioning is a key determinant of postsynaptic remodeling at individual spines. They found that the neuronal activity at single dendritic spines recruits the digestive compartments of the cell (i.e., the lysosomes) to the spine head. Blocking lysosomal function decreases the spine number and increases the inter-event interval of miniature excitatory postsynaptic currents (mEPSC). These data provide the first evidence that synaptic remodeling upon neuronal activity might be at least partially mediated by local lysosome-dependent degradation of synaptic proteins.
Protein degradation plays crucial roles in neuronal physiology and pathology. The first evidence linking synaptic plasticity to protein degradation came from the sea slug Aplysia californica, where application of a modulatory neurotransmitter serotonin increased proteolysis via the ubiquitin–proteasome system, which contributed to the initiation and consolidation of memory (Hegde et al., 1997). Neurons also use the lysosome system, which degrades a wide variety of membrane-bound receptors that affect synaptic plasticity, including AMPARs. Two main degradative pathways converge at the lysosome: the ESCRT (Endosomal Sorting Complex Required for Transport) and autophagy pathways. In the ESCRT pathway, endocytosed membrane proteins are routed to the lysosome via their sorting to multivesicular bodies, whereas during autophagy the cargo is first engulfed by a bowl-shaped membrane, the so-called phagophore. The resulting autophagosome is subsequently delivered to the lysosome for the breakup and recycling of the enclosed cellular components. Trafficking of AMPARs to the lysosome was initially described by Michael Ehlers, who showed that application of AMPA targets AMPARs to late endosome/lysosome compartments (Ehlers, 2000). Subsequently, it was discovered that AMPARs can be degraded both via the endosomal sorting (Fernández-Monreal et al., 2012) and the autophagy pathways (Shehata et al., 2012), and that such activity-dependent lysosomal degradation of AMPARs is important for synaptic depression and amyloid-β–induced loss of AMPARs (Hou et al., 2011; Rodrigues et al., 2016).
Although the lysosomal degradation of AMPARs is well established, one particular feature of neurons complicates the accomplishment of this type of protein turnover. Up to now, lysosomes were found mostly within the cell body, whereas the synapses are located up to hundreds of micrometers away. This raises the question of how lysosomal degradation takes place in distal dendrites. A simple scenario suggests that endocytosed proteins are transported from the dendritic spine to the cell body for lysosomal degradation. A second possibility is that lysosomes are restricted locally to the site where degradation takes place. Goo et al. (2017) now provide evidence in favor of the second hypothesis. By using immunocytochemistry in combination with overexpression techniques, the authors detected LAMP1-positive structures in the soma as well as throughout primary and secondary dendrites. LAMP1 is a classical marker of lysosomes but is also known to be associated with late endosomes, which, in contrast to lysosomes (pH 4.5–5.0), possess only a slightly acidic milieu (pH 5.5–6.0). To prove that the LAMP1-positive structures in dendrites are functional lysosomes, Goo et al. (2017) undertook a series of elegant approaches. First, by using LysoTracker, a fluorescent acidotropic probe for lysosome labeling, they showed that the LAMP1-GFP–positive structures in dendrites are indeed acidic organelles. Second, they treated LysoTracker-stained dendrites with glycyl-L-phenylalanine-β-naphthylamide (GPN), which is a substrate of lysosomal protease cathepsin C that triggers osmotic lysis of lysosomes after cleavage. Cleavage of GPN by cathepsin C diminished LysoTracker fluorescence of LAMP1-GFP–positive organelles, confirming that they are lysosomes. Are the structures found in dendrites hundreds of micrometers away from the soma indeed functional lysosomes? To provide more evidence of the lysosomal nature of the LAMP1-positive organelles, Goo et al. (2017) took advantage of the fact that lysosomes are important sources of intracellular calcium. They reasoned that treatment with GPN should not only cause osmosis but also lead to the release of Ca2+ into the intracellular space. Goo et al. (2017) treated neurons with GPN and measured the Ca2+ efflux from the lysosomes with the GCaMP3 probe fused to Mucolipin transient receptor potential channel 1 (TRPML1), a major receptor mediating the release of Ca2+ from the lysosomes. Strikingly, the GPN treatment dramatically increased the TRPML1-GCaMP3 fluorescence in distal dendrites. Finally, to obtain higher resolution images of lysosomes in dendrites, the authors engineered a construct with the cytoplasmic tail of LAMP1 fused to a genetically encoded electron microscopy tag called APEX (enhanced ascorbate peroxidase). After treatment with the diaminobenzidine, dendrites expressing LAMP1-APEX2 revealed a dark stain around the membranes of lysosomes, detected by electron microscopy. Collectively, these results support the initial unexpected observation made by Goo et al. (2017) that lysosomes are indeed present in distal neurites.
Equally unexpected is the finding that functional lysosomes can be found not only in dendritic shafts but also in the head of the dendritic spine, both in vitro and in vivo. This observation raises two important questions: How are lysosomes recruited to dendritic spines, and does such a mechanism have a functional role in basal postsynaptic transmission? The trafficking of membrane-bound organelles is known to be regulated by synaptic activity (Kennedy and Ehlers, 2006). Hence, to understand the mechanism behind lysosomal positioning at dendritic spines, Goo et al. (2017) performed a series of experiments to test whether neuronal activity regulates lysosomal trafficking in dendrites. They found that application of AMPA profoundly increases the number of spine heads containing lysosomes. Furthermore, application of a high concentration of glycine, a treatment paradigm known to potentiate neurons through the activation of synaptic N-methyl-d-aspartate receptors (NMDARs), caused a significant increase in the number of lysosomes found at dendritic spines, which was abolished by application of the NMDAR antagonist AP5. Strikingly, application of glutamate to single spines by two-photon uncaging of MNI-glutamate was enough to induce the repositioning of lysosomes from the dendrites to the base of the spine. Collectively, the aforementioned findings indicate that the positioning of lysosomes at individual dendritic spines can be regulated by neuronal activity.
Whether and how AMPARs are degraded after activity-dependent endocytosis have been controversial questions for the last decade. Both Rab7-dependent endosomal sorting (Fernández-Monreal et al., 2012) and autophagy have been implicated in the degradation of AMPARs in dendrites (Shehata et al., 2012). To determine whether lysosomes convene at the synapse to degrade synaptic proteins, Goo et al. (2017) performed a bulk surface membrane internalization assay in neurons transfected with LAMP1-GFP and treated with leupeptin to prevent lysosomal degradation. Endocytosed biotinylated cell surface proteins were found with and juxtaposed to LAMP1-GFP–positive organelles in dendrites. To evaluate whether AMPARs were specifically degraded by lysosomes, they cotransfected GFP-GluA1 together with LAMP1-RFP and followed the internalization of these AMPARs in neurons pretreated with leupeptin and stimulated with AMPA. They found that in a subset of spines, surface-labeled GFP-GluA1 colocalized and cotrafficked with LAMP1-RFP, suggesting that lysosomes are positioned in place to facilitate the degradation of this membrane protein cargo.
If lysosomes degrade synaptic proteins, altered lysosomal function should have severe consequences for the maintenance of excitatory synapses. To test this hypothesis Goo et al. (2017) analyzed mEPSCs in leupeptin-treated neurons. They found that the amplitudes of evoked responses were unaltered, suggesting that synaptic strength is unchanged when lysosomes are inhibited. Although unexpected, these results are in agreement with previous findings where lysosomes were found to be dispensable for long-term depression induction in hippocampal slices (Fernández-Monreal et al., 2012). What then is the functional role of lysosomal positioning at the synapse? To answer this question, Goo et al. (2017) further investigated their surprising observation that perturbations of lysosomal function caused a significant decrease in the frequency of mEPSCs. Although decreased mEPSC frequency usually indicates changes in presynaptic function, it can also be a result of decreased synapse number. Indeed, the number of spines was significantly decreased in neurons under conditions in which lysosomal function was inhibited. Thus, these data provide the first evidence that the structural dynamics of dendritic spines can at least be partially controlled by lysosomal degradation pathways. As both formation and elimination of spines are critical determinants of long-term memory and cognition, the cell biological results presented by Goo et al. (2017) are of great importance with respect to systems neuroscience and neurodegeneration because cognitive decline is one of the earliest signs of the lysosomal storage disease in patients.
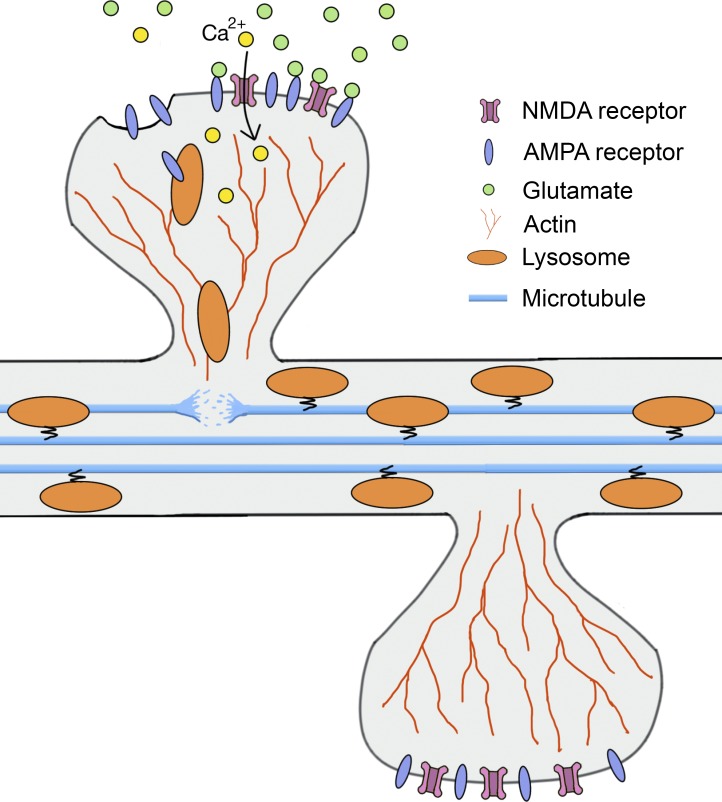
Finally, what is the molecular mechanism governing the recruitment of lysosomes to dendritic spines? Both plus end– and minus end–directed microtubule motors, including dynein and kinesins, are known to control trafficking of late endosomes and lysosomes in neurons (Franker and Hoogenraad, 2013), whereas in nonneuronal cells actin filaments cooperate with microtubules for the movement of lysosomes (Cordonnier et al., 2001). Hence, to unravel the mechanism behind lysosomal positioning in dendrites Goo et al. (2017) destabilized microtubules in cultured neurons and analyzed the motility of lysosomes. As expected, this treatment significantly increased the number of stationary lysosomes compared with the control condition. A surprising twist came when Goo et al. (2017) measured lysosomal motility under conditions that inhibit actin polymerization: Latrunculin treatment significantly increased lysosomal trafficking in dendrites. Furthermore, lysosomes were found to be coembedded with F-actin and the number of spine-containing lysosomes was increased under microtubule-destabilizing conditions. Because actin is the major cytoskeletal component of dendritic spines, these results suggest that microtubules and actin might cooperate in lysosomal positioning at the synapse (Fig. 1). Under steady-state conditions, lysosomes will be trafficked via microtubules along the dendrites, whereas neuronal activity would lead to increased interaction of lysosomes with F-actin, likely via the destabilization of microtubules as a result of a local increase in Ca2+. Further experiments are required to test this hypothesis in more detail.
Figure 1.
Hypothetical model for activity-dependent regulation of lysosome trafficking in dendrites. Under steady-state condition, lysosomes are trafficked along microtubules in dendrites. Neuronal activity induces the release of glutamate from presynaptic terminal that binds to postsynaptic AMPARs and NMDARs. Synaptic activation of NMDARs leads to influx of Ca2+ ions into the head of the spine. Local increase in Ca2+ destabilizes the microtubules, thereby releasing the lysosomes from their tracks and, in turn, facilitating their association with actin filaments in the head of the spine.
Dendritic spine pathology and dysfunctional synaptic plasticity are hallmarks of many neuropsychiatric and neurodegenerative disorders. Despite intense study, it is only in the past decade that a combination of advanced cell biology with neuroscience approaches have enabled the analysis of synaptic plasticity at the single-spine level. This pioneering work by Goo et al. (2017) provides us with the first evidence that lysosomes have a novel, previously undiscovered, function in the regulation of spine dynamics during neuronal activity. Although this work has improved our understanding of the mechanisms regulating local synapse remodeling, it also raises several important questions. For example, what is the exact molecular mechanism capturing lysosomes at the dendritic spines? Is this targeting mechanism lysosome specific or would other endosomal and autophagosomal compartments also be recruited by neuronal activity to dendritic spines? Which kind of synaptic proteins are degraded at the synapse and what is the functional implication of such local protein degradation for synaptic plasticity? Although there are many unresolved questions, with the identification of neuronal activity–dependent lysosome trafficking in dendrites, Goo et al. (2017) set the stage for future studies aimed at addressing the detailed mechanisms governing activity-dependent protein turnover at the synapse.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (KO 5091/2-1) and the Cologne Cluster of Excellence in Cellular Stress Responses in Aging-Associated Diseases (Exc. 229).
The author declares no competing financial interests.
References
- Cordonnier M.-N., Dauzonne D., Louvard D., and Coudrier E.. 2001. Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes. Mol. Biol. Cell. 12:4013–4029. 10.1091/mbc.12.12.4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M.D. 2000. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 28:511–525. 10.1016/S0896-6273(00)00129-X [DOI] [PubMed] [Google Scholar]
- Fernández-Monreal M., Brown T.C., Royo M., and Esteban J.A.. 2012. The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J. Neurosci. 32:13200–13205. 10.1523/JNEUROSCI.0061-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franker M.A.M., and Hoogenraad C.C.. 2013. Microtubule-based transport—basic mechanisms, traffic rules and role in neurological pathogenesis. J. Cell Sci. 126:2319–2329. 10.1242/jcs.115030 [DOI] [PubMed] [Google Scholar]
- Goo M.S., Sancho L., Slepak N., Boassa D., Deerinck T.J., Ellisman M.H., Bloodgood B.L., and Patrick G.N.. 2017. Activity-dependent trafficking of lysosomes in dendrites and dendritic spines. J. Cell Biol.:jcb.201704068 10.1083/jcb.201704068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde A.N., Inokuchi K., Pei W., Casadio A., Ghirardi M., Chain D.G., Martin K.C., Kandel E.R., and Schwartz J.H.. 1997. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 89:115–126. 10.1016/S0092-8674(00)80188-9 [DOI] [PubMed] [Google Scholar]
- Hou Q., Gilbert J., and Man H.-Y.. 2011. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 72:806–818. 10.1016/j.neuron.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.J., and Ehlers M.D.. 2006. Organelles and trafficking machinery for postsynaptic plasticity. Annu. Rev. Neurosci. 29:325–362. 10.1146/annurev.neuro.29.051605.112808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues E.M., Scudder S.L., Goo M.S., and Patrick G.N.. 2016. Aβ-Induced synaptic alterations require the E3 ubiquitin ligase Nedd4-1. J. Neurosci. 36:1590–1595. 10.1523/JNEUROSCI.2964-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M., Matsumura H., Okubo-Suzuki R., Ohkawa N., and Inokuchi K.. 2012. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J. Neurosci. 32:10413–10422. 10.1523/JNEUROSCI.4533-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]