Abstract
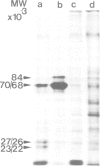
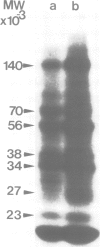
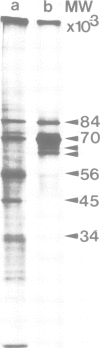
Ribonucleoprotein complexes of Drosophila melanogaster Kc tissue culture cells grown at 24°C or heat-shocked at 37°C were cross-linked in vivo by u.v. irradiation. Cross-linked heterogeneous nuclear ribonucleoprotein (hnRNP) complexes were fractionated by oligo(dT)-cellulose chromatography and CsCI density centrifugation. The hnRNP complexes of both 24°C and 37°C culture cells possess buoyant densities in CsCI between ϱ = 1.38 g/cm-3 and 1.43 g/cm-3. The 35S-labelled proteins bound to the hnRNA of 37°C culture cells correspond in mol. wt. to the so-called heat-shock proteins of 70 K, 68 K, 27 K, 26 K, 23 K and 22 K. The 70 K and 68 K proteins are also present in hnRNP complexes of 24°C culture cells. In addition, several other Drosophila hnRNPs of 140 K, 56 K, 45 K, 43 K, 38 K, 37 K and 34 K, whose synthesis is strongly repressed under heat-shock conditions, could be identified. The results demonstrate that the so-called heat-shock proteins possess a function as RNPs.
Keywords: heat shock, u.v. cross-linking, hnRNP, protein analysis
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. Isolement, en cultures in vitro, de lignées cellulaires diploïdes de Drosophila melanogaster. C R Acad Sci Hebd Seances Acad Sci D. 1969 Mar 31;268(13):1771–1773. [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Proteins crosslinked to messenger RNA by irradiating polyribosomes with ultraviolet light. Nucleic Acids Res. 1980 Dec 11;8(23):5685–5701. doi: 10.1093/nar/8.23.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Ultraviolet light-induced crosslinking of mRNA to proteins. Nucleic Acids Res. 1979 Feb;6(2):715–732. doi: 10.1093/nar/6.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P. M., Johnson R. M., Sommerville J. Interaction of the hnRNA of amphibian oocytes with fibril-forming proteins. Eur J Biochem. 1982 Oct;127(2):301–308. doi: 10.1111/j.1432-1033.1982.tb06870.x. [DOI] [PubMed] [Google Scholar]
- Kloetzel P. M., Knust E., Schwochau M. Analysis of nuclear proteins in primary spermatocytes of Drosophila hydei: The correlation of nuclear proteins with the function of the Y chromosomal loops. Chromosoma. 1981;84(1):67–86. doi: 10.1007/BF00293364. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lengyel J., Penman S. hnRNA size and processing as related to different DNA content in two dipterans: Drosophila and Aedes. Cell. 1975 Jul;5(3):281–290. doi: 10.1016/0092-8674(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller K., Brimacombe R. Specific cross-linking of proteins S7 and L4 to ribosomal RNA, by UV irradiation of Escherichia coli ribosomal subunits. Mol Gen Genet. 1975 Dec 9;141(4):343–355. doi: 10.1007/BF00331455. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Welch W. J., Mathews M. B., Feramisco J. R. Molecular and cellular effects of heat-shock and related treatments of mammalian tissue-culture cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):985–996. doi: 10.1101/sqb.1982.046.01.092. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., Sonoda S., Bugaisky G., Lindquist S. Is the major Drosophila heat shock protein present in cells that have not been heat shocked? J Cell Biol. 1983 Jan;96(1):286–290. doi: 10.1083/jcb.96.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M., Tanguay R. M. Different intracellular distributions of heat-shock and arsenite-induced proteins in Drosophila Kc cells. Possible relation with the phosphorylation and translocation of a major cytoskeletal protein. J Mol Biol. 1982 Dec 5;162(2):365–378. doi: 10.1016/0022-2836(82)90532-0. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]