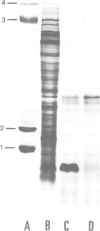
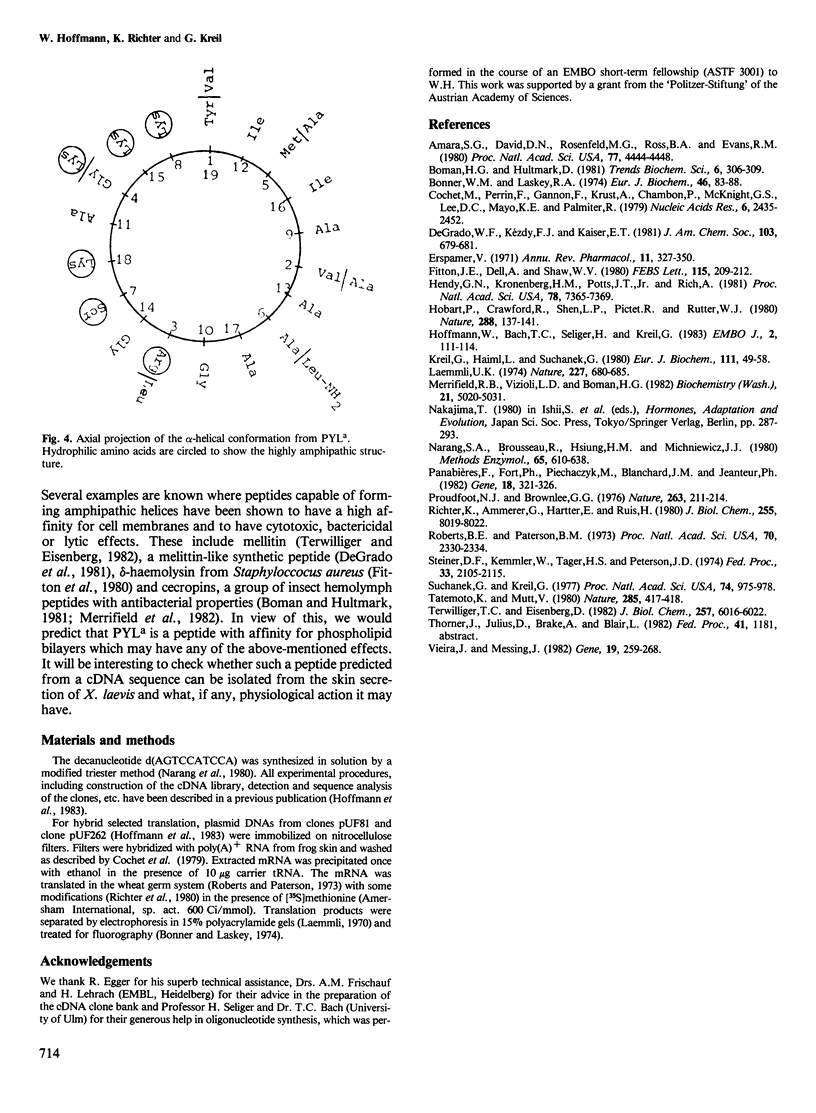
Abstract
A variety of peptides closely related to mammalian hormones and neurotransmitters are secreted from amphibian skin. Using cDNA clones of mRNA isolated from skin of Xenopus laevis, we have been searching for precursors of some of these constituents. Here we present the sequences of parts of cloned mRNAs which code for precursors of a novel peptide. In the predicted polypeptides, pairs of basic residues flank a sequence of 25 amino acids terminating with glycine, the signal for the formation of a terminal amide. The predicted final product liberated from these precursors would be a peptide comprised of 24 amino acids starting with tyrosine and ending with leucine amide, which has therefore been designated PYLa. This peptide can form an amphipathic helix similar to that found in peptides with cytotoxic, bacteriostatic and/or lytic properties.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., David D. N., Rosenfeld M. G., Roos B. A., Evans R. M. Characterization of rat calcitonin mRNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4444–4448. doi: 10.1073/pnas.77.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cochet M., Perrin F., Gannon F., Krust A., Chambon P., McKnight G. S., Lee D. C., Mayo K. E., Palmiter R. Cloning of an almost full-length chicken conalbumin double-stranded cDNA. Nucleic Acids Res. 1979 Jun 11;6(7):2435–2452. doi: 10.1093/nar/6.7.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V. Biogenic amines and active polypeptides of the amphibian skin. Annu Rev Pharmacol. 1971;11:327–350. doi: 10.1146/annurev.pa.11.040171.001551. [DOI] [PubMed] [Google Scholar]
- Fitton J. E., Dell A., Shaw W. V. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 1980 Jun 30;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Hendy G. N., Kronenberg H. M., Potts J. T., Jr, Rich A. Nucleotide sequence of cloned cDNAs encoding human preproparathyroid hormone. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7365–7369. doi: 10.1073/pnas.78.12.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann W., Bach T. C., Seliger H., Kreil G. Biosynthesis of caerulein in the skin of Xenopus laevis: partial sequences of precursors as deduced from cDNA clones. EMBO J. 1983;2(1):111–114. doi: 10.1002/j.1460-2075.1983.tb01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Haiml L., Suchanek G. Stepwise cleavage of the pro part of promelittin by dipeptidylpeptidase IV. Evidence for a new type of precursor--product conversion. Eur J Biochem. 1980 Oct;111(1):49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Narang S. A., Brousseau R., Hsiung H. M., Michniewicz J. J. Chemical synthesis of deoxyoligonucleotides by the modified triester method. Methods Enzymol. 1980;65(1):610–620. doi: 10.1016/s0076-6879(80)65063-0. [DOI] [PubMed] [Google Scholar]
- Panabières F., Fort P., Piechaczyk M., Blanchard J. M., Jeanteur P. A warning on the use of synthetic DNA primers for initiation of reverse transcription on RNA templates: unexpected initiation at a mismatched nucleotide. Gene. 1982 Oct;19(3):321–326. doi: 10.1016/0378-1119(82)90022-1. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Richter K., Ammerer G., Hartter E., Ruis H. The effect of delta-aminolevulinate on catalase T-messenger RNA levels in delta-aminolevulinate synthase-defective mutants of Saccharomyces cerevisiae. J Biol Chem. 1980 Sep 10;255(17):8019–8022. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Suchanek G., Kreil G. Translation of melittin messenger RNA in vitro yields a product terminating with glutaminylglycine rather than with glutaminamide. Proc Natl Acad Sci U S A. 1977 Mar;74(3):975–978. doi: 10.1073/pnas.74.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980 Jun 5;285(5764):417–418. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982 Jun 10;257(11):6016–6022. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]