Summary
Adipose tissue–derived “stem cells” have been increasingly used by “stem-cell clinics” in the United States and elsewhere to treat a variety of disorders. We evaluated three patients in whom severe bilateral visual loss developed after they received intravitreal injections of autologous adipose tissue–derived “stem cells” at one such clinic in the United States. In these three patients, the last documented visual acuity on the Snellen eye chart before the injection ranged from 20/30 to 20/200. The patients’ severe visual loss after the injection was associated with ocular hypertension, hemorrhagic retinopathy, vitreous hemorrhage, combined traction and rhegmatogenous retinal detachment, or lens dislocation. After 1 year, the patients’ visual acuity ranged from 20/200 to no light perception.
Age-related macular degeneration (AMD) is the leading cause of vision loss in persons older than 75 years of age in the United States.1 Progressive dysfunction and loss of retinal pigment epithelium cells and photoreceptors lead to poor visual acuity in patients with non-neovascular AMD.2 The potential role of delivering subretinal human retinal pigment epithelium, photoreceptor cells, or both, differentiated from pluripotent stem cells, to replace the damaged cells in patients with non-neovascular AMD is being investigated in several clinical trials registered by the Food and Drug Administration (FDA) and approved by institutional review boards.3 As of November 2, 2016, at least 13 trials of intravitreal injections of various stem-cell populations were registered on ClinicalTrials.gov. Of these, 4 were based in the United States and 3 were recruiting. One trial (ClinicalTrials.gov number, NCT01736059)4 focused on intravitreal injection of CD34+ bone marrow–derived stem cells isolated with the use of Good Manufacturing Practices. A second trial (NCT02320812) involves retinal progenitor cells tested in patients with retinitis pigmentosa. A third trial (NCT01920867)5,6 involves bone marrow–derived stem cells delivered through up to six different routes of administration for a broad swath of ocular diseases, including non-neovascular AMD. A fourth trial (NCT02024269), which was withdrawn on September 15, 2015, before enrollment had begun, focused on the use of intravitreal autologous adipose tissue–derived stem cells in patients with non-neovascular AMD.
Adipose tissue–derived “stem cells” have been increasingly used by “stem-cell clinics” because of the ease of obtaining and preparing these cells. Many of the clinics that provide these stem-cell therapies have done so under the auspices of patient-funded, institutional review board–approved research, and the research is listed on ClinicalTrials.gov without an investigational new drug application with the FDA.7–10 We report three cases of vision loss after patients with AMD received bilateral intravitreal injections of autologous adipose tissue–derived stem cells at a stem-cell clinic, which was the study site for the fourth trial described above (NCT02024269). After treatment, in June 2015, the patients were referred to two university-based ophthalmology practices. The funding organizations had no role in the design or conduct of the current study.
Stem-Cell Procedure
According to the documentation that we obtained, the procedure was carried out in the patients as follows. An adipose-rich periumbilical area was injected subcutaneously with an anesthetic solution to prepare for tumescent liposuction. Fifteen to 20 minutes later, additional local anesthetic was injected, followed by a 60-ml liposuction aspiration, and the sample of adipose tissue was processed to isolate the putative stem cells.
In parallel, standard phlebotomy was performed to collect 35 ml of whole blood, which was centrifuged to separate a platelet-rich plasma fraction. The liposuction aspirate was washed in 60 ml of cell-wash solution with gentle agitation for 2 to 4 minutes, after which the aqueous fraction was removed and the cells underwent enzyme treatment for 12 minutes at 37°C, with 30 seconds of vigorous shaking every 6 minutes. After dissociation, the cells were centrifuged at 1800 rpm for 5 minutes, creating a pellet of stromal vascular cells. These cells were resuspended, washed in cell-wash solution, and centrifuged again.
The pelleted cells were resuspended in 1 to 6 ml of platelet-rich plasma and used immediately for intravitreal injection into both eyes as described below. Patients were instructed to use one drop of topical moxifloxacin three times per day for 3 days in both eyes, to limit activity (swimming, heavy lifting, exercise, and application of eye makeup) for 3 days, and to keep their eyes dry for 3 days.
Case Reports
Patient 1
A 72-year-old woman with a history of non-neovascular AMD and a best-corrected Snellen visual acuity of 20/60 in the right eye and 20/30 in the left eye underwent bilateral intravitreal injection of autologous adipose tissue–derived stem cells for the treatment of non-neovascular AMD. The patient underwent the procedure at a stem-cell clinic that had an institutional review board–approved research trial (NCT02024269) listed on ClinicalTrials.gov at the time; however, the written information provided to the patient did not mention participation in a clinical trial, review or approval by an institutional review board, or an association with a trial listed on ClinicalTrials.gov.
The patient reported that she had found the stem-cell clinic through its listing on Clinical-Trials.gov. She also reported that she was under the impression that she was participating in a clinical trial and that she had met the criteria of the trial. She paid $5,000 for the bilateral procedure. The consent form indicated the risk of blindness.
The patient presented to the Bascom Palmer Eye Institute in Miami 3 days after the intravitreal injection. On presentation, her visual acuity was perception of hand motion in both eyes, and the intraocular pressure was 66 mm Hg in the right eye and 59 mm Hg in the left eye. Examination of the anterior segment showed conjunctival injection, corneal microcystic edema, fixed, middilated pupils, and peripheral iridocorneal touch in both eyes. The nuclear sclerotic cataract was anteriorly displaced in both eyes, as shown by high-resolution ultrasonography (Fig. 1A). The patient had a dense vitreous hemorrhage in both eyes, which obscured the view of the posterior pole. Posterior segment ultrasonography showed mildly to moderately dense, mobile, vitreous opacities with macular thickening in both eyes and possible retinal detachment and vitreoretinal adhesions in the left eye (Fig. 1B).
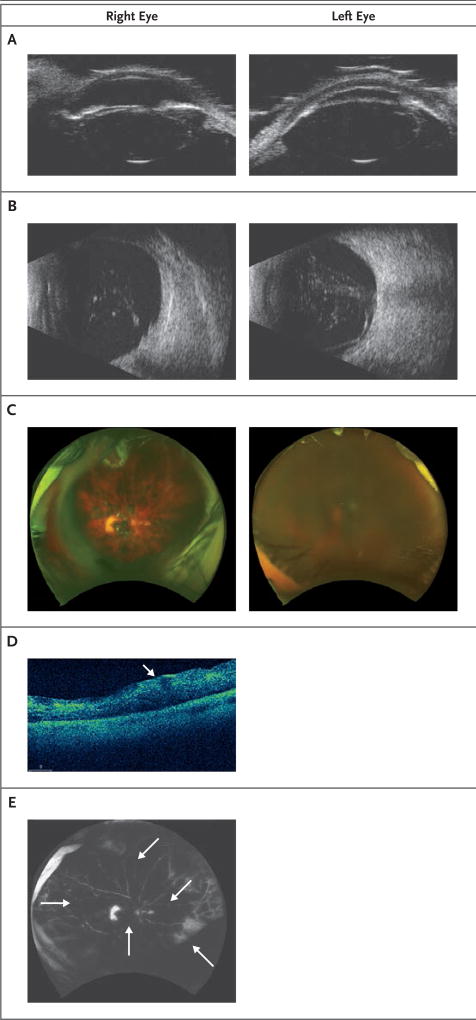
Figure 1. Findings on Ophthalmologic Examination in Patient 1.
In Panel A, a high-resolution ultrasonographic image shows anterior subluxation of the lens of both eyes, more prominently in the left eye. In Panel B, a posterior segment ultrasonographic image shows moderately dense, mobile vitreous opacities with macular thickening in both eyes and possible retinal detachment and vitreoretinal adhesions in the left eye. In Panel C, fundus photographs of both eyes show diffuse intraretinal hemorrhage. The view is hazy in the left eye because of corneal edema. In Panel D, an optical coherence tomographic image of the right eye shows macular thickening without cystoid macular edema. The thickening is more prominent in the inner retina (arrow). In Panel E, a fluorescein angiogram of the right eye shows blockage from the hemorrhages (arrows), a window defect in the temporal macula, and an area of staining in the temporal periphery. No vasculitis was seen. Fluorescein angiography of the left eye could not be performed because of severe corneal edema.
Because of the anterior displacement of the crystalline lens and elevated intraocular pressure in both eyes, bilateral pars plana vitrectomies and lensectomies were performed. Histologic examination showed histiocytes and no malignant cells. After removal of the dense vitreous hemorrhage, diffuse intraretinal hemorrhage was identified in both eyes (Fig. 1C). A localized rhegmatogenous retinal detachment that was identified in the right eye was managed with endolaser barricade of the tear and silicone oil tamponade. Silicone oil tamponade was chosen for tamponade in all patients during repair of retinal detachment because it facilitates visual rehabilitation and travel better than does air or gas tamponade, which greatly limits vision and precludes flight in commercial aircraft. Postoperative optical coherence tomography (OCT) revealed retinal thickening without cystoid macular edema in the right eye (Fig. 1D). Fluorescein angiography of the right eye showed blockage from the hemorrhages and a temporal macula window defect, but it did not show changes consistent with vasculitis (Fig. 1E). OCT and fluorescein angiography could not be performed in the left eye because of corneal edema.
Over time, both of the patient’s retinas became markedly atrophic. One year after the vitrectomies and lensectomies, her right retina was detached, with severe proliferative vitreoretinopathy, and she had no light perception in both eyes because of atrophy. Her intraocular pressure was controlled with topical medications for glaucoma.
Patient 2
Quiescent neovascular AMD was diagnosed in a 78-year-old woman with a best-corrected Snellen visual acuity of 20/50 in the right eye and 20/100 in the left eye after she received bilateral injections of anti–vascular endothelial growth factor (VEGF) drugs in both eyes over the course of 2 years and before she received bilateral intravitreal injections of autologous adipose tissue– derived stem cells at the same stem-cell clinic mentioned previously. Like Patient 1, she was aware of the clinical trial posted on ClinicalTrials.gov by the stem-cell clinic. She also paid $5,000 for the same procedure that Patient 1 had undergone.
Approximately 2 days after Patient 2 received bilateral intravitreal injections, she presented to both the Bascom Palmer Eye Institute and to the Center for Sight in Sarasota, Florida. On presentation, her visual acuity was such that she could count fingers with both eyes, and the intraocular pressure was 13 mm Hg in both eyes. Examination of the anterior segment of each eye showed conjunctival injection and grade 1+ anterior chamber cells in both eyes. The patient had bilateral vitreous hemorrhages and diffuse intraretinal and preretinal hemorrhages (Fig. 2A). OCT showed an epiretinal membrane with fundus thickening without cystoid macular edema in the right eye and geographic atrophy and fundus thickening without cystoid macular edema in the left eye (Fig. 2B). Fluorescein angiography showed blockage from the hemorrhages, but it did not show vasculitis (Fig. 2C). She underwent serial observation, but 16 days after the injection, a combined tractional and rhegmatogenous retinal detachment with proliferative vitreoretinopathy developed in the right eye and was treated with scleral buckle, pars plana vitrectomy, membrane peel, and silicone oil tamponade. Consistent with zonular weakness, there was anterior dislocation of the patient’s intraocular lens and lens capsule during the fluid–air exchange intraoperatively.
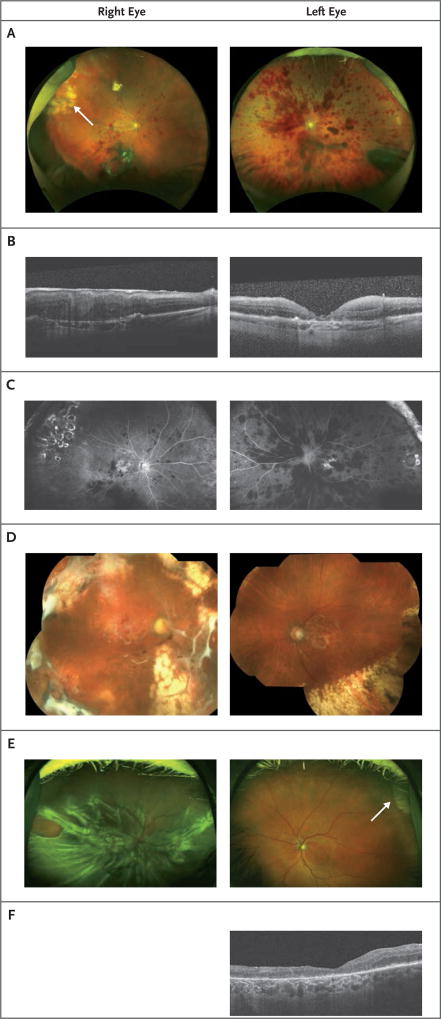
Figure 2. Findings on Ophthalmologic Examination in Patients 2 and 3.
In Panel A, fundus photographs of both eyes in Patient 2 show intraretinal hemorrhage and vitreous hemorrhages. In Panel B, an optical coherence tomographic (OCT) image shows macular thickening without cystoid macular edema and an epiretinal membrane in the right eye and retinal thickening without cystoid macular edema and geographic atrophy in the left eye in Patient 2. An old scar caused by laser retinopexy is visible in the superotemporal quadrant of the right eye (arrow). In Panel C, a fluorescein angiogram shows blockage from the hemorrhages, areas of staining temporally, and a window defect in the central macula in both eyes in Patient 2. No vasculitis was seen. In Panel D, montage fundus photographs of both eyes in Patient 2 show bilateral attached retinas with peripheral laser chorioretinal scars. In Panel E, fundus photographs of both eyes in Patient 3 show severe combined tractional and rhegmatogenous retinal detachment with proliferative vitreoretinopathy in the right eye and geographic atrophy in the left. An old scar caused by cryopexy is visible in the superotemporal quadrant of the left eye (arrow). In Panel F, an OCT image of the left eye in Patient 3 shows geographic atrophy.
Thirty-eight days after the patient received the bilateral intravitreal injections, a combined tractional and rhegmatogenous retinal detachment with proliferative vitreoretinopathy developed in the left eye, which was treated with pars plana vitrectomy and silicone oil. Postoperatively, the patient’s retinas were attached after 1 year (Fig. 2D), and her vision was perception of hand motion in the right eye and 20/200 in the left eye.
Patient 3
An 88-year-old woman with a visual acuity of 20/40 in the right eye and 20/200 in the left eye had a history of non-neovascular AMD with bilateral geographic atrophy and a retinal tear treated with cryopexy in the left eye 30 years before she received bilateral intravitreal stem-cell injections, as described in Patients 1 and 2. Patient 3 received injections at the same stem-cell clinic for $5,000.
Patient 3 presented 1 week after the procedure to the Dean McGee Eye Institute, Oklahoma City. On presentation, the patient’s visual acuity was light perception in the right eye and 20/200 in the left eye, and her intraocular pressure was 12 mm Hg in the right eye and 16 mm Hg in the left eye. She had an afferent pupillary defect in the right eye. Examination of the anterior segment of each eye was clinically significant for pseudophakia in both eyes. The patient had a total retinal detachment with proliferative vitreoretinopathy in the right eye and geographic atrophy with a superotemporal cryopexy scar in the left eye (Fig. 2E). OCT imaging of the left eye showed geographic atrophy (Fig. 2F). OCT imaging of the right eye was not performed.
The patient’s right eye was managed with a scleral buckle, pars plana vitrectomy, membrane peel, peripheral localized retinectomy, and silicone oil tamponade. Four weeks after the injection, a total retinal detachment with proliferative vitreoretinopathy developed in the patient’s left eye. Of note, during the intraoperative fluid–air exchange, there was anterior dislocation of the patient’s intraocular lens and lens capsule in both eyes consistent with zonular weakness. One year after surgery, the patient’s retinas were attached, and her visual acuity was perception of hand motion in the right eye and light perception in the left.
Discussion
Here we describe a consecutive case series of serious adverse events associated with intravitreal stem-cell injections performed in the United States. Blinding visual outcomes occurred in three patients after they received bilateral intravitreal injection of autologous adipose tissue–derived stem cells at the same stem-cell clinic (Table 1). These 1-year outcomes are dramatically worse than the typical 1-year visual-loss outcomes that have been reported with the use of vitamin supplementation for non-neovascular AMD, which has been associated with a moderate visual loss in 3.3% of patients,11 and the 1-year outcomes that have been reported with the use of anti-VEGF therapy for neovascular AMD, which has been associated with a moderate visual loss in less than 5% of patients.12
Table 1.
Clinical Course of Three Patients Who Received Bilateral Intravitreal Injection of Autologous Adipose Tissue–Derived Stem Cells.
| Patient Number |
Preinjection Visual Acuity |
Presenting Visual Acuity |
Lens Subluxation | Intraretinal Hemorrhage | Macular Thickening | Retinal Detachment | Last Measured Visual Acuity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | ||
|
| |||||||||||||||
| 1 | 20/60 | 20/30 | Hand motion | Hand motion | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No light perception | No light perception | |
|
| |||||||||||||||
| 2 | 20/50 | 20/100 | Count fingers | Count fingers | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Hand motion | 20/200 | |
|
| |||||||||||||||
| 3 | 20/40 | 20/200 | Light perception | 20/200 | Yes | Yes | No | No | No | No | Yes | Yes | Hand motion | Light perception | |
Although numerous stem-cell therapies for medical disorders are being investigated at research institutions with appropriate regulatory oversight, many stem-cell clinics are treating patients with little oversight and with no proof of efficacy.10 A distinction has been made between clinical studies of stem-cell therapies that are founded on solid preclinical research with strong scientific design and programs that lack preclinical research justification. These programs are often funded by patients at nonacademic centers,8 and they may not receive FDA oversight if these procedures are performed without the filing of an investigational new drug application with the FDA, which requires extensive safety data. At least one of the patients thought the procedure was performed within the context of a clinical trial (NCT02024269). However, the consent forms signed by all three patients do not mention a clinical trial. The patients paid for a procedure that had never been studied in a clinical trial, lacked sufficient safety data, and was performed in both eyes on the same day. Experimental bilateral intravitreal injections are both atypical and unsafe.
In patients with non-neovascular AMD, stem cells have been investigated as a way to replace the diseased retinal pigment epithelium, photoreceptors in the macula, or both. Previous studies have shown that suspensions of autologous retinal pigment epithelium cells were limited in their ability to recreate a monolayer on the diseased Bruch’s membrane in patients with nonneovascular AMD.13 Moreover, although suspensions of human embryonic stem cell–derived retinal pigment epithelium that have been injected under the retina in patients with non-neovascular AMD have been shown to have a good safety profile, they have limited benefit with respect to visual acuity in patients with geographic atrophy.14 In addition, there is sparse evidence that cultures of mesenchymal or adipose-derived stem cells can differentiate into retinal pigment epithelium or photoreceptors.15,16
Since ophthalmologists have administered millions of intravitreal injections of other medications for various retinal diseases without similar complications, the complications seen in the three patients described in this series are probably due to the stem-cell preparations rather than to the injection procedure. If the eyes had been injected with excessive volume, then we would expect elevation of the intraocular pressure, pain, and transient loss of vision due to ischemia of the retina or optic nerve. However, retinal detachments with severe proliferative vitreoretinopathy developed in all six eyes in this series while the patients were undergoing observation after the injection.
Since pluripotent stem cells injected into the vitreous may undergo transformation to myofibroblast-like cells, it is possible that the injected cells transformed into myofibroblasts. If this is the case, like retinal pigment epithelium cells that may be present on the surface of the retina after rhegmatogenous retinal detachments, they may have caused proliferative vitreoretinopathy and retinal detachment.17 Retinal detachment rates of 29% in a phase 1 trial and 15% in a phase 1–2a trial of subretinal human umbilical tissue–derived cells have been reported.18,19 In contrast, the rate of retinal detachment after intravitreal injection of anti-VEGF is 0 to 0.67%.20
All three patients in this series also had profound zonular weakness, which can occur when enzymes such as trypsin are used in the preparation of the stem cells and contaminate the injections.21 In addition to the vision loss related to intraocular pressure and retinal detachment, vision loss may have developed because of a combination of factors, including toxic effects on the retina or optic nerve caused by the injected material, which may have included the enzymes used in the preparation. The loss of vision in Patient 1 is most likely due to optic-nerve atrophy caused by prolonged elevated intraocular pressure, since rapid progression to no light perception developed when her retina was attached in both eyes.
The outcome of poor visual acuity in these three patients arouses concern about the performance of procedures at stem-cell clinics that charge patients for their services and that lack clinical or preclinical data to support their practices. Many stem-cell clinics have claimed that autologous stem-cell injections do not fall under the auspices of FDA regulations.7–10 Our case reports establish the possibility of devastating outcomes from such procedures. Other case reports have shown a lack of efficacy in cases of medical tourism in which nothing was injected into the patient’s eye.8 The need for oversight of such clinics and for the education of patients by physicians and regulatory bodies is paramount to protecting patients while advancing proper research and innovation.22–24
Acknowledgments
Supported by grants from the National Institutes of Health (Center Core grant, P30EY014801), Research to Prevent Blindness, the Department of Defense (W81XWH-09-1-0675), and the Klorfine Foundation (to Dr. Albini). The funders named were not associated with the clinical trial described.
We thank Jeffrey S. Heier, M.D., chair of the Research and Safety in Therapeutics Committee of the American Society of Retina Specialists, for assistance in collecting and reviewing these case reports.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–93. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 3.Nazari H, Zhang L, Zhu D, et al. Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res. 2015;48:1–39. doi: 10.1016/j.preteyeres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Park SS, Bauer G, Abedi M, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2014;56:81–9. doi: 10.1167/iovs.14-15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing autoimmune optic neuropathy. Neural Regen Res. 2015;10:1507–15. doi: 10.4103/1673-5374.165525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss JN, Levy S, Malkin A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a preliminary report. Neural Regen Res. 2015;10:982–8. doi: 10.4103/1673-5374.158365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner LG. Federal regulatory oversight of US clinics marketing adipose-derived autologous stem cell interventions: insights from 3 new FDA draft guidance documents. Mayo Clin Proc. 2015;90:567–71. doi: 10.1016/j.mayocp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Turner L. US stem cell clinics, patient safety, and the FDA. Trends Mol Med. 2015;21:271–3. doi: 10.1016/j.molmed.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Turner LG. US clinics marketing unproven and unlicensed adipose-derived autologous stem cell interventions. Regen Med. 2015;10:397–402. doi: 10.2217/rme.15.10. [DOI] [PubMed] [Google Scholar]
- 10.Taylor-Weiner H, Graff Zivin J. Medicine’s Wild West — unlicensed stem-cell clinics in the United States. N Engl J Med. 2015;373:985–7. doi: 10.1056/NEJMp1504560. [DOI] [PubMed] [Google Scholar]
- 11.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Lee E, MacLaren RE. Sources of retinal pigment epithelium (RPE) for replacement therapy. Br J Ophthalmol. 2011;95:445–9. doi: 10.1136/bjo.2009.171918. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 15.Moviglia GA, Blasetti N, Zarate JO, Pelayes DE. In vitro differentiation of adult adipose mesenchymal stem cells into retinal progenitor cells. Ophthalmic Res. 2012;48(Suppl 1):1–5. doi: 10.1159/000339839. [DOI] [PubMed] [Google Scholar]
- 16.Rezanejad H, Soheili ZS, Haddad F, et al. In vitro differentiation of adiposetissue-derived mesenchymal stem cells into neural retinal cells through expression of human PAX6 (5a) gene. Cell Tissue Res. 2014;356:65–75. doi: 10.1007/s00441-014-1795-y. [DOI] [PubMed] [Google Scholar]
- 17.Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res. 2014;40:16–34. doi: 10.1016/j.preteyeres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Francis PJ, Birch DG, Davis JL, et al. A Phase 1 open-label, non-comparative study evaluating the safety of a single, unilateral subretinal administration of CNTO2476 (human umbilical tissue-derived cells [hUTC]) in advanced retinitis pigmentosa (RP) Invest Ophthalmol Vis Sci. 2010;51(13):4789. abstract. [Google Scholar]
- 19.Ho AC. Microcatheter delivery of cell therapy CNTO 2476 for atrophic age related macular degeneration. Presented at the Angiogenesis, Exudation, and Degeneration 2015 Conference; Miami. February 7, 2015; abstract. [Google Scholar]
- 20.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27:787–94. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takei Y, Smelser GK. Electron microscopic studies on zonular fibers. II. Changes of the zonular fibers after the treatment with collagenase, alpha-chymotrypsin and hyaluronidase. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;194:153–73. doi: 10.1007/BF00496873. [DOI] [PubMed] [Google Scholar]
- 22.Bowman M, Racke M, Kissel J, Imitola J. Responsibilities of health care professionals in counseling and educating patients with incurable neurological diseases regarding “stem cell tourism”: caveat emptor. JAMA Neurol. 2015;72:1342–5. doi: 10.1001/jamaneurol.2015.1891. [DOI] [PubMed] [Google Scholar]
- 23.Knoepfler PS. From bench to FDA to bedside: US regulatory trends for new stem cell therapies. Adv Drug Deliv Rev. 2015;82–83:192–6. doi: 10.1016/j.addr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarzeczny A, Clark M. Unproven stem cell-based interventions & physicians’ professional obligations; a qualitative study with medical regulatory authorities in Canada. BMC Med Ethics. 2014;15:75. doi: 10.1186/1472-6939-15-75. [DOI] [PMC free article] [PubMed] [Google Scholar]