Abstract
Objective
To determine whether hyperuricosuria was a predisposing factor for urate urolithiasis in Bulldogs and Black Russian Terriers (BRTs) and to estimate the allele frequency of the Cys181Phe genetic mutation in urate transporter SLC2A9 in these breeds.
Animals
192 Bulldogs, 101 BRTs, 10 Dalmatians, and 9 dogs of other breeds.
Procedures
Uric acid (UA) and creatinine (Cr) concentrations were quantified in urine samples collected from all dogs via midstream catch during natural voiding. Buccal swab or blood samples were also obtained, and DNA was extracted and used to genotype SLC2A9 sequence variants by use of pyrosequencing assays. A urine test for hyperuricosuria was validated in adult dogs by comparing urinary UA:Cr ratios between known hyperuricosuric and nonhyperuricosuric dogs.
Results
Significantly higher UA:Cr ratios were found in some Bulldogs and BRTs, compared with ratios in other dogs from these breeds. These dogs were also homozygous for the SLC2A9 Cys181Phe mutation. The allele frequency of the Cys181Phe mutation was 0.16 in Bulldogs and 0.51 in BRTs. On the basis of these allele frequencies, 3% of the Bulldog population and 27% of the BRT population were estimated to be hyperuricosuric.
Conclusions and Clinical Relevance
Results suggested the genetic mutation associated with hyperuricosuria, first identified in Dalmatians, also appears to cause hyperuricosuria in Bulldogs and BRTs, indicating that similar management strategies for urate urolithiasis can be used in these breeds. The allele frequency of the mutation was high in both breeds, and DNA testing can be used to select against the mutation.
Purine catabolism in humans, great apes, and all dogs of 1 particular breed, Dalmatian, results in hyperuricosuria, which is the excess excretion of uric acid in the urine.1,2 In other mammals and most dogs, uric acid is oxidized in the liver to allantoin, a soluble product that is excreted in the urine. The capability to produce the enzyme that oxidizes uric acid to allantoin, urate oxidase, was lost during the evolution of great apes (including humans).3,4 In Dalmatians, urate oxidase is functional5 and hyperuricosuria is a result of alteration of the urate transporter SLC2A9,6,7 which likely affects the transport of uric acid into the liver and kidney.5,8–10 It has been proposed that SLC2A9 transports uric acid into the liver so the molecule can be oxidized by urate oxidase. In the kidney, SLC2A9 likely facilitates the reabsorption of uric acid into the circulatory system.6,11 A defect in SLC2A9 accounts for the more pronounced hyperuricosuria and less marked hyperuricemia that exist in Dalmatians6 because more uric acid is excreted in the urine than is reabsorbed by the kidneys.12
Hyperuricosuria has been studied extensively in Dalmatians and has been associated with the high incidence of urate urolithiasis in that breed,13–16 which may cause urinary obstruction.17 Dalmatians are reportedly 229 times as likely as other breeds to have a urolith composed of urate. In addition to Dalmatians, Bulldogs are approximately 16 times as likely to have a urate urolith, compared with other breeds.18 The risk for urate urolithiasis in Hungarian BRTs is as high as that in Hungarian Dalmatians.19 In Dalmatians, the mean age at the time urate uroliths are submitted for analysis is 4.2 years.18 Similarly, in Bulldogs, the mean age is 3.7 years18,20; in BRTs, it is reportedly 4.7 years.19 The risk of urate urolithiasis and the reported age of BRTs at the time of urolith submission are based on a single publication,19 which is likely attributable to the rarity of the breed. Although urate urolithiasis is commonly detected in dogs with portosystemic shunts,14 Bulldogs and BRTs are not reported to have a high prevalence of this congenital defect21 and the etiology of urate urolithiasis in these 2 breeds has not been determined.
Because Dalmatians have been studied with regard to hyperuricosuria and urate urolithiasis for many years,1,10,14–16,22 treatment protocols for the conditions are well established. Dalmatians with urate urolithiasis are often medically managed with allopurinol to reduce the amount of uric acid excreted and prevent recurrence of urate urolith formation. Collection of urine over a 24-hour period is recommended to quantify uric acid concentrations so allopurinol dose can be adjusted and increased production of xanthine, which is insoluble and can form uroliths, can be avoided.16,23 However, 24-hour urine collection is an invasive and expensive procedure because it requires a metabolic cage and catheterization of the urinary tract23; as a result, it is not commonly used.
In Dalmatians, hyperuricosuria is a single-gene trait with an autosomal recessive mode of inheritance.13,24 Identification of the mutation was performed by use of a multigenerational Dalmatian × Pointer backcross.25 Segregation of the typical allele for hyperuricosuria was obtained from 1 Pointer used in this pedigree. Subsequent backcrossing to purebred Dalmatians yielded dogs that excrete low amounts of uric acid in their urine (LUA Dalmatians) as well as dogs that excrete high amounts of uric acid (HUA Dalmatians). To differentiate between LUA and HUA Dalmatians, urinary UA:Cr ratios were measured in 3- to 7-week-old puppies (UA:Cr ratios decrease with age15,26). The UA:Cr ratios ranged between 0.3 and 0.6 in LUA Dalmatian puppies and 1.3 and 4.6 in HUA Dalmatian puppies.15
When the Dalmatian × Pointer backcross progeny were used, a missense mutation in exon 5 of the SLC2A9 gene (SLC2A9 542G>T; in reference to variant O, CFA03: 72,222,637–72,416,753) was identified.6 This mutation causes a cysteine to phenylalanine substitution at amino acid 181 of the protein (Cys181Phe). As reported, 4 urate stone–forming dogs (2 Bulldogs and 2 BRTs) were homozygous for the same SLC2A9 mutation that was identified in Dalmatians.6
In humans, SLC2A9 is a urate transporter that is expressed in the liver and kidney.7 Two isoforms of SLC2A9 exist in humans, mice, and dogs. In dogs, these variants, N and O, differ in the first 28 amino acids of the N terminus because of a change in the start codon of the first exon of SLC2A9.6 A difference in the degree of mRNA expression was detected in variant O between Dalmatian and non-Dalmatian dogs in kidney and liver tissues.6 Two SNPs were identified in the promoter region (the region responsible for mRNA transcription) of canine SLC2A9.6 These 2 promoter SNPs were homozygous and identical in all purebred Dalmatians tested but were also detected in LUA non-Dalmatian dogs, indicating that they were not sufficient to cause hyperuricosuria.6 The purpose of the study reported here was to determine whether hyperuricosuria was a predisposing factor for urate urolithiasis in Bulldogs and BRTs and to estimate the allele frequency of the Cys181Phe mutation in urate transporter SLC2A9 in these breeds.
Materials and Methods
Dogs
To establish a test for hyperuricosuria in adult dogs, urine samples were collected from 3 groups of dogs: an affected control group of purebred adult Dalmatians, a carrier group of adult LUA Dalmatians from a Dalmatian × Pointer cross that were phenotyped as puppies15 and were expected to be heterozygous for the hyperuricosuria locus, and a healthy control group of LUA non-Dalmatian dogs from breeds that are not known to develop urate stones. Urine samples were also randomly collected from Bulldogs and BRTs at dog shows or randomly selected from owner submissions. All dogs from which urine samples were obtained had their genotypes confirmed upon DNA testing. The study protocol was reviewed and approved by the University of California-Davis Institutional Animal Care and Use Committee.
Selection of animals for genotyping
Owners of Bulldogs and BRTs were notified of the study via postings by breed club members of a request for buccal swab samples. Interested owners contacted researchers and subsequently collected and submitted the samples. Additional samples from these breeds in the form of blood were obtained from patients of the William R. Pritchard Veterinary Medical Teaching Hospital of the University of California-Davis.
Urine uric acid and creatinine assays
Urine samples were collected via midstream catch during natural voiding into clean containers and transferred into 15-mL tubes. Urine uric acid and creatinine concentrations were measured by use of colorimetric assays. Uric acid concentration was assayed by means of a commercial kit,a and creatinine concentration was assayed by use of another kitb via the Jaffe method. Uric acid concentrations were evaluated in relation to creatinine concentrations as a UA:Cr ratio to allow for differences in urine concentration.15,26
Genotyping of samples
Extraction of DNA from samples was performed by use of a commercial assay.c Genotyping of samples was performed with 2 methods. Initially, a restriction fragment length polymorphism assay was used to evaluate the Cys181Phe mutation, as described elsewhere.6 A pyrosequencing assay was later designed for higher throughput and better accuracy for both the Cys181Phe mutation and the variant O promoter SNPs (99 and 101 bp 5′ to the start codon). The PCR assay products were generated in 40-µL reactions containing approximately 30 ng of DNA, 1.5mM MgCl2, 200µM deoxyribonucleotide triphosphate, 6 pmol each of the respective forward and reverse primers (Appendix), and 0.75 U of DNA polymerase.d Amplification was performed by use of a PCR systeme at 95°C for 15 minutes, 45 cycles of 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. Pyrosequencing27 was performed in a reaction containing 3 µL of polysaccharide beads,f 37 µL of binding buffer,g 15 pmol of sequencing primer, 35 µL of annealing buffer,g and 40 µL of PCR assay product. Pyrosequencing data were read and analyzed with pyrosequencing software.h
Determination of allele and genotype class frequencies
Because the Cys181Phe mutant allele is prevalent in Bulldogs and BRTs, selection for hyperuricosuria was evaluated. Allele frequencies were calculated on the basis of genotype results from the Bulldog and BRT sample populations. Estimation of genotypic class distributions in the population was performed on the basis of the calculated allele frequencies by use of the Hardy-Weinberg equilibrium equation.
Calculation of incidence of stone formation in hyperuricosuric dogs
Male dogs older than the mean age of stone formation in the breed and homozygous for the mutant allele of the Cys181Phe mutation were included in the assessment. In Bulldogs, no dogs fit the criteria; in BRTs, 6 dogs fit the criteria. Formation of urate stones was determined from owner disclosures in health questionnaires that had been completed at the time of DNA sample collection.
Statistical analysis
Statistical analyses were performed by use of statistical software.i To determine whether UA:Cr ratios would reliably define hyperuricosuria in adult dogs, urine uric acid concentrations were compared among 3 groups of adult dogs of known hyperuricosuria phenotype: HUA Dalmatians and LUA Dalmatians that had been phenotyped as puppies15 and LUA non-Dalmatian dogs from breeds that are not reported to be predisposed to urate urolithiasis. Dogs were grouped on the basis of genotype (unaffected, carrier, and affected), and urinary UA:Cr ratios were compared among the 3 genotype classes by means of ANOVA. Comparison between 2 groups was achieved by use of the Student t test, assuming unequal variance; the reported P value is for 1-tail analysis. A χ2 test was performed to compare the observed and expected (based on Hardy-Weinberg equilibrium) genotypic class frequencies, to determine whether selection for the mutation occurred.
Although the promoter SNPs are not sufficient to cause hyperuricosuria, they may contribute to the phenotype by altering the expression of SLC2A9. To evaluate whether the promoter SNPs affected the phenotype, urine UA:Cr ratios were compared between adult dogs with identical Cys181Phe genotypes and differing promoter SNP genotypes by use of a Student t test. A value of P < 0.05 was considered significant for all analyses.
Results
Dogs
One hundred ninety-two Bulldogs, 101 BRTs, 10 Dalmatians, and 9 dogs of other breeds were included in the study. Adult dogs of known hyperuricosuria phenotype consisted of the following: HUA Dalmatians (n = 5), LUA Dalmatians (5), and LUA non-Dalmatians (from breeds not reported to be predisposed to urate urolithiasis; 9).
UA:Cr ratios
Values for UA:Cr ratio differed significantly (P < 0.001) among the 3 groups of dogs with known hyperuricosuria phenotype. The mean ratio values were 0.73 (range, 0.58 to 0.95) for HUA Dalmatians, 0.11 (range, 0.03 to 0.15) for LUA Dalmatians, and 0.5 (range, 0.03 to 0.09) for LUA non-Dalmatians. Because there was no overlap of UA:Cr ratio values between HUA and LUA dogs, it was concluded that this method could be used to distinguish these groups, but because there was overlap of values between LUA Dalmatians and LUA non-Dalmatians, the method could not be used to differentiate LUA dogs by breed.
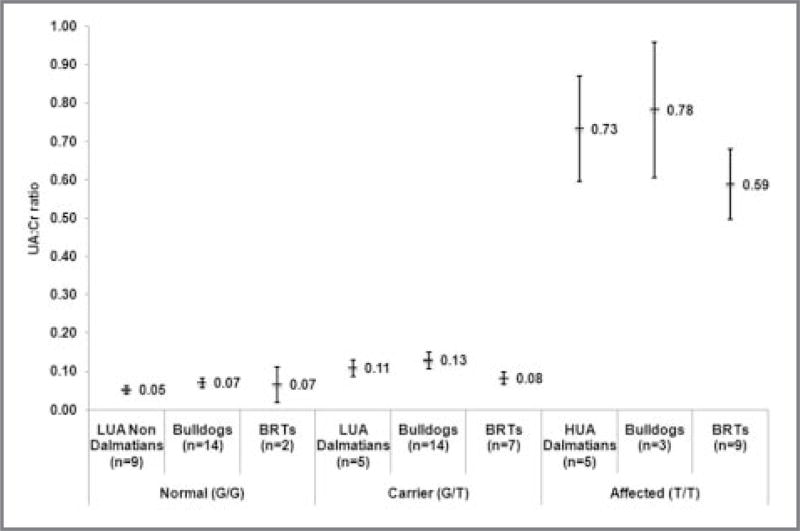
Urinary UA:Cr ratios were determined for adult Bulldogs and BRTs to determine whether hyperuricosuria was associated with the various Cys181Phe genotypes (Figure 1). In both Bulldogs and BRTs, a significant (Student t test; P = 0.009 and P < 0.001, respectively) difference was detected between the hyperuricosuric (affected) and the LUA (unaffected and carrier) groups, and the UA:Cr ratio ranges did not overlap. In Bulldogs, the UA:Cr ratio ranges of homozygous, affected dogs were 0.65 to 0.96, and those of unaffected and carrier dogs were 0.03 to 0.2. In BRTs, the UA:Cr ratios were 0.34 to 0.8 in affected dogs and 0.04 to 0.11 in unaffected and carrier dogs. Similar to the results in the LUA control groups, overlap between unaffected and carrier UA:Cr ratio ranges in Bulldogs and BRTs was detected, despite the fact that a t test revealed mean values were significantly different. No significant difference was found when HUA Bulldogs, BRTs, and Dalmatians were compared, indicating a similarity in the phenotype across these breeds.
Figure 1.
Mean and 95% CI urine UA:Cr ratios in control dogs (LUA non-Dalmatian, LUA Dalmatian, and HUA Dalmatian), Bulldogs, and BRTs. Dogs are grouped according to their genotypes: normal (both alleles contain guanine [G] at nucleotide No. 542), carrier (1 allele contains G, and the other contains thymine [T]), and affected (both alleles contain T) for the SLC2A9 Cys181Phe mutation. Urine UA:Cr values differed significantly among the 3 genotype groups in the control group (P < 0.001), Bulldogs (P < 0.001), and BRTs (P < 0.001).
Genotype and allele frequency
All samples were genotyped via the pyrosequencing method, and half of these samples were also genotyped with the restriction fragment length polymorphism assay. Both methods yielded identical results except for 3 samples, for which results conflicted. Attempts to obtain DNA sequences of the SLC2A9 gene in these 3 samples failed, likely because of poor-quality DNA; therefore, data from these samples were not included in the analyses.
Other than the Cys181Phe mutation, 2 additional sequence variations were identified in the promoter region of SLC2A9, 99 (promoter SNP 1) and 101 (promoter SNP 2) bp 5′ to the start codon of SLC2A9 variant O.6 These 2 promoter SNPs were previously found to be homozygous in all purebred Dalmatians tested (adenine/adenine for promoter SNP 1 and cytosine/cytosine for promoter SNP 2) but were also identified in LUA dogs.6 The pyrosequencing assay designed to determine the genotypes at promoters SNP 1 and SNP 2 revealed that HUA Bulldogs and BRTs that were homozygous and affected for the Cys181Phe mutation had the same homozygous genotypes for both promoter SNPs that are found in Dalmatians. Additionally, LUA (unaffected and carrier) dogs from these breeds also had the same promoter genotypes as affected dogs (Table 1). When urine UA:Cr ratios were compared between adult dogs with identical Cys181Phe genotypes and differing promoter SNP genotypes, no significant differences were found.
Table 1.
Urine UA:Cr ratios in adult dogs with and without hyperuricosuria, grouped by SNP combinations for the SLC2A9 Cys181Phe genetic mutation.
| Genotype | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Promoter SNP1 | Promoter SNP2 | ys181Phe | No. of dogs | UA:Cr ratio (range) | Mean | P value |
| A/A | C/C | T/T | 15 | 0.4–0.95 | 0.67 | |
| T/A | C/C | T/G | 5 | 0.08–0.11 | 0.09 | 0.35 |
| A/A | C/C | T/G | 2 | 0.06–0.11 | 0.09 | |
| T/A | C/A | T/G | 5 | 0.09–0.15 | 0.11 | |
| A/A | C/C | G/G | 4 | 0.04–0.09 | 0.06 | 0.64 |
| T/A | C/C | G/G | 1 | 0.04 | 0.04 | |
| T/A | C/A | G/G | 4 | 0.03–0.06 | 0.05 | |
A = Adenine. C = Cytosine. G = Guanine. T = Thymine.
On the basis of the allele frequency of the mutant allele of the Cys181Phe mutation, disease frequency could be established in the Bulldogs and BRTs in the study (Table 2). In Bulldogs (n = 192), the mutant allele frequency ± SE was 0.16 ± 0.02, whereas in BRTs (101), it was 0.51 ± 0.04. The estimated (expected) percentage of affected dogs in the Bulldog breed was 3%, and 27% of the population was estimated to be carriers. For the BRT breed, it was estimated that 27% were affected and 50% were carriers. Genetic selection for the Cys181Phe mutation was assessed, and results of a χ2 test indicated there was no significant difference between the observed and expected values in either breed (ie, it is unlikely that the high mutant allele frequency in these breeds was attributable to selection).
Table 2.
Distribution (number [%]) and allele frequencies of Bulldogs (n = 192) and BRTs (101) with normal (both alleles contain guanine [G] at nucleotide No. 542), carrier (1 allele contains G, and the other contains (thymine [T]), and affected (both alleles contain T) genotypes of the SLC2A9 Cys181Phe mutation, associated with hyperuricosuria in these breeds.
| Genotype | Allele frequency | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Breed | Normal | Carrier | Affected | G | T | SE |
| Bulldog | 137 (71.4) | 49 (25.5) | 6 (3.1) | 0.84 | 0.16 | 0.02 |
| BRT | 27 (26.7) | 44 (43.6) | 30 (29.7) | 0.49 | 0.51 | 0.04 |
Stone formation in hyperuricosuric dogs
The percentage of HUA dogs that had clinical signs of the disease was estimated on the basis of owner disclosure of urate stone occurrence. Of 73 Bulldogs that were selected randomly and for which health questionnaires had been completed by owners, 3 were homozygous for the Cys181Phe mutation. These dogs were males < 3.5 years of age and had no history of urolith formation at the time of sample collection (as reported by their owners). In BRTs, the estimation was made by use of data from affected males that were at least 4 years of age.19 Of the 101 BRTs that were randomly selected, 6 dogs matched the criteria and 3 of them had urate stones prior to DNA collection. Six younger males and 15 females that were homozygous and affected for the Cys181Phe mutation were not reported to have had urate stones at the time of DNA collection.
Discussion
The hyperuricosuria mutation present in all Dalmatians was also commonly found in Bulldogs and BRTs. Although the allele frequency of the SLC2A9 Cys181Phe genetic mutation was unexpectedly high in Bulldogs and BRTs, selection for hyperuricosuria was not evident. The differences in allele frequency of the mutation between these 2 breeds may be attributed to their different population structure. The Bulldog is an old breed (first classified in the 1630s28) and has a large population size, whereas the BRT is a relatively new breed of small population size.28
In the present study, the frequency of the Cys181Phe mutant allele was estimated as 0.16 in Bulldogs and 0.51 in BRTs. Given the high allele frequency, the number of dogs genotyped was sufficient because such a high frequency could not have been a result of coincidental sampling. It is still possible that there was ascertainment bias in DNA sample collection caused by the increased interest of owners whose dogs had a history of urate urolithiasis. However, given the responses on owner-completed questionnaires, most DNA samples submitted were from dogs with no history of urate urolithiasis at the time of sample collection. The mutant allele frequency detected in Bulldogs and BRTs was calculated without taking into account the relatedness of the dogs from which samples were submitted. Data for Bulldogs were obtained from multiple DNA databases, as well as from samples volunteered by owners from the United States and Canada. Although most samples were collected from households in the west coast of the United States, Bulldogs are a popular breed (eighth most popular breed in the United States according to American Kennel Club registration statistics29) and artificial insemination is a common practice (that involves shipping of semen), indicating that the estimated frequency was not likely to be limited to the geographic area in which the samples were collected. Because of the more recent development of the BRT breed and rarity of such dogs, most BRTs are related. The samples from this breed were sent in from all over the world; therefore, the allele frequencies in the sample population were likely to have been representative of the breeding population.
In the present study, findings suggested the Cys181Phe mutation segregated with hyperuricosuria in Bulldogs and BRTs, but a possible contribution of the promoter SNPs had to be ruled out because expression of SLC2A9 could be affected by these SNPs. The promoter SNPs did not appear to segregate with hyperuricosuria, although all hyperuricosuric Bulldogs and BRTs were homozygous for both SNPs (identical to the genotype of Dalmatians). We could not completely rule out that the promoter SNPs had some contribution to the phenotype of affected (homozygous for the Cys181Phe mutation) dogs because a single sample of urine is not an established quantitative method of determining long-term uric acid excretion in dogs23 and because if the difference in UA:Cr ratios is subtle, then a larger number of urine samples in each genotypic group may be required to detect a difference. An analysis of the promoter SNP effect on affected dogs could not be performed because all affected dogs in our study had the same genotypes for the promoter SNPs.
Because affected dogs had a significantly higher urine UA:Cr ratio than unaffected dogs, a urine test of the UA:Cr ratio could be used to qualitatively identify hyperuricosuria in adult dogs. The broad range of UA:Cr ratios detected in HUA dogs (Dalmatian and affected Bulldogs and BRTs) was consistent with the wide range of uric acid quantified via 24-hour urine collection.14 A study23 involving unaffected dogs, in which results for 24-hour urine collection were compared with those for single urine samples obtained at various time points, revealed that uric acid excretion is not consistent throughout the day. Additional studies are necessary to determine whether a single urine sample can be used to quantify uric acid for medical management of dogs with hyperuricosuria. Despite the significant difference detected between LUA Dalmatians (carriers for Cys181Phe) and LUA non-Dalmatians (unaffected for Cys181Phe) and despite the fact that the UA:Cr ratios were within a narrow range in both groups, there was overlap in the UA:Cr ratio values between these 2 groups. This indicated that the urine test cannot be used to distinguish between carrier and unaffected individuals and that the genetic test for hyperuricosuria is required. It may be possible to use the urine test to identify dogs that have hyperuricosuria of a different etiology than the SLC2A9 Cys181Phe mutation, such as hyperuricosuria resulting from portosystemic shunts,30,31 but additional studies are required to validate the use of the test for this purpose.
The phenotype test for hyperuricosuria was used to distinguish hyperuricosuric dogs from Bulldog and BRT breeds, and these dogs were also homozygous for the mutant allele of the Cys181Phe mutation. The phenotypic appearance of hyperuricosuria in Dalmatians, Bulldogs, and BRTs is similar on the basis of a comparison of the UA:Cr ratio values of HUA dogs. The similarity of genotypes and phenotypes between breeds suggested that the same treatment is appropriate in these breeds. Because treatment for Dalmatian urate urolithiasis is well established,14,16,23 applying the same protocols to Bulldogs and BRTs should be fairly straightforward. In addition, by determining the genotypes of young Bulldogs and BRTs, particularly males and offspring of known affected or carrier dogs, preventive measures can be taken against urate urolithiasis.
The commonness of urate urolithiasis in Bulldogs and BRTs is accounted for by the high prevalence of the SLC2A9 Cys181Phe mutation identified in these breeds. Although not all hyperuricosuric dogs form uroliths, as is true for Dalmatians,13–15,32 it is probable that hyperuricosuria is a prerequisite for urate urolithiasis. When responses to the health questionnaires and genotypes of dogs were considered, half of the homozygous, affected male BRTs > 4 years of age had formed urate uroliths prior to DNA collection. Such an estimate in Bulldogs was not possible because none of those dogs fit our inclusion criteria. An estimate of the proportion of hyperuricosuric Bulldogs that have clinical signs will require a long-term follow-up because dogs sampled at an earlier age may form stones after DNA samples are collected and completed health questionnaires are submitted.
The Cys181Phe mutation may lead to urate urolithiasis in other dog breeds because the 3 breeds in which it has been identified have no known close ancestry. This may indicate that the mutation predates the initial classification of dog breeds.33 To test this hypothesis, screening of other breeds for the Cys181Phe variant is required.
Selection against hyperuricosuria in the Bulldog and BRT breeds is possible by DNA-based testing and has the potential to decrease the production of affected dogs and development of urate urolithiasis in Bulldogs and BRTs. In Bulldogs, 71% of the population is estimated to be unaffected, and the popularity of the breed allows for a more rapid selection against hyperuricosuria. Because of the high mutant allele frequency (only 24% of the population is unaffected) and the modest population size of the BRT breed, selection against the mutant allele will need to be done gradually.
In agreement with findings in a previous report,6 findings of our study confirmed that the promoter SNPs are not sufficient to cause hyperuricosuria and that the Bulldog and BRT breeds carry the SLC2A9 Cys181Phe mutation. A DNA-based test for hyperuricosuria is available to assist veterinarians in the diagnosis of the underlying cause of urate urolithiasis. This DNA test may also be used to genotype breeding dogs and aid breeders in reducing the incidence of hyperuricosuria and urate urolithiasis in susceptible breeds.
Acknowledgments
Supported by the Center for Companion Animal Health, the Veterinary Science Training Program, YEAR-Long Exposure to Advanced Research Program (T32 RR021312), and Students Training in Advanced Research (STAR) Program, School of Veterinary Medicine, University of California-Davis.
The authors thank Dr. Jodi Westropp and Annette Ruby from the Stone Analysis Laboratory at the University of California-Davis.
Abbreviations
- BRT
Black Russian Terrier
- CI
Confidence interval
- HUA
High urine uric acid concentration
- LUA
Low urine uric acid concentration
- SNP
Single nucleotide polymorphism
- UA:Cr
Uric acid concentration-to-creatinine concentration.
Appendix
Polymerase chain reaction assay and pyrosequencing primers for detection of a genetic mutation in SLC2A9.
| Assay | Primer type | Primer sequence |
|---|---|---|
| Exon 5 SNP | Forward* | CTCACCCAAGGAGATCCG |
| Reverse | GTCAGTAGCCACGCTTACCTT | |
| Sequence | CCCGGTGAACACACCGA | |
| Promoter SNPs | Forward* | CCAGGCAACCCCTCCATTT |
| Reverse | ATGTGCTGAGTCCTTTTCACTGA | |
| Sequence | TCACCAATACGTAATTAGG |
Labeled with 5′-biotin.
Footnotes
UA Plus kit, Roche Diagnostics, Indianapolis, Ind.
CREA kit, Roche Diagnostics, Indianapolis, Ind.
QIAmp DNA Blood Mini Kit, Qiagen, Valencia, Calif.
AmpliTaq Gold, Applied Biosystems, Foster City, Calif.
GeneAmp 9700, Applied Biosystems, Foster City, Calif.
Sepharose beads, Amersham Biosciences, Piscataway, NJ.
Biotage, Charlottesville, Va.
PSQ 96MA 2.1, Pyrosequencing AB, Uppsala, Sweden.
Microsoft Office Excel 2007 and Microsoft Office Enterprise 2007, Microsoft Corp, Redmond, Wash.
References
- 1.Benedict SR. Uric acid in its relations to metabolism. Harvey Lect. 1916;11:346–365. [Google Scholar]
- 2.Wells HG. The purine metabolism of the Dalmatian coach hound. J Biol Chem. 1918;35:221–225. [Google Scholar]
- 3.Friedman TB, Polanco GE, Appold JC, et al. On the loss of uricolytic activity during primate evolution—I. Silencing of urate oxidase in a hominoid ancestor. Comp Biochem Physiol B. 1985;81:653–659. doi: 10.1016/0305-0491(85)90381-5. [DOI] [PubMed] [Google Scholar]
- 4.Oda M, Satta Y, Takenaka O, et al. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 5.Giesecke D, Tiemeyer W. Defect of uric acid uptake in Dalmatian dog liver. Experientia. 1984;40:1415–1416. doi: 10.1007/BF01951919. [DOI] [PubMed] [Google Scholar]
- 6.Bannasch D, Safra N, Young A, et al. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet. 2008;4:e1000246. doi: 10.1371/journal.pgen.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 8.Appleman RM, Hallenbeck GA, Shorter RG. Effect of reciprocal allogeneic renal transplantation between Dalmatian and non-Dalmatian dogs on urinary excretion of uric acid. Proc Soc Exp Biol Med. 1966;121:1094–1097. doi: 10.3181/00379727-121-30975. [DOI] [PubMed] [Google Scholar]
- 9.Kocken JM, Borel Rinkes IH, Bijma AM, et al. Correction of an inborn error of metabolism by intraportal hepatocyte transplantation in a dog model. Transplantation. 1996;62:358–364. doi: 10.1097/00007890-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Simkin PA. The Dalmatian defect: a hepatic endocrinopathy of urate transport. Arthritis Rheum. 2005;52:2257–2262. doi: 10.1002/art.21241. [DOI] [PubMed] [Google Scholar]
- 11.Caulfield MJ, Munroe PB, O’Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5:e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roch-Ramel F, Wong NL, Dirks JH. Renal excretion of urate in mongrel and Dalmatian dogs: a micropuncture study. Am J Physiol. 1976;231:326–331. doi: 10.1152/ajplegacy.1976.231.2.326. [DOI] [PubMed] [Google Scholar]
- 13.Bannasch DL, Ling GV, Bea J, et al. Inheritance of urinary calculi in the Dalmatian. J Vet Intern Med. 2004;18:483–487. doi: 10.1892/0891-6640(2004)18<483:ioucit>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Bartges JW, Osborne CA, Lulich JP, et al. Canine urate urolithiasis. Etiopathogenesis, diagnosis, and management. Vet Clin North Am Small Anim Pract. 1999;29:161–191. doi: 10.1016/s0195-5616(99)50010-7. [DOI] [PubMed] [Google Scholar]
- 15.Schaible RH. Genetic predisposition to purine uroliths in Dalmatian dogs. Vet Clin North Am Small Anim Pract. 1986;16:127–131. doi: 10.1016/s0195-5616(86)50007-3. [DOI] [PubMed] [Google Scholar]
- 16.Sorenson JL, Ling GV. Diagnosis, prevention, and treatment of urate urolithiasis in Dalmatians. J Am Vet Med Assoc. 1993;203:863–869. [PubMed] [Google Scholar]
- 17.Albasan H, Lulich JP, Osborne CA, et al. Evaluation of the association between sex and risk of forming urate uroliths in Dalmatians. J Am Vet Med Assoc. 2005;227:565–569. doi: 10.2460/javma.2005.227.565. [DOI] [PubMed] [Google Scholar]
- 18.Bartges JW, Osborne CA, Lulich JP, et al. Prevalence of cystine and urate uroliths in Bulldogs and urate uroliths in Dalmatians. J Am Vet Med Assoc. 1994;204:1914–1918. [PubMed] [Google Scholar]
- 19.Bende B, Nemeth T. High prevalence of urate urolithiosis in the Russian Black Terrier. Vet Rec. 2004;155:239–240. doi: 10.1136/vr.155.8.239. [DOI] [PubMed] [Google Scholar]
- 20.Ling GV, Franti CE, Ruby AL, et al. Urolithiasis in dogs. II: breed prevalence, and interrelations of breed, sex, age, and mineral composition. Am J Vet Res. 1998;59:630–642. [PubMed] [Google Scholar]
- 21.Tobias KM, Rohrbach BW. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2,400 cases (1980–2002) J Am Vet Med Assoc. 2003;223:1636–1639. doi: 10.2460/javma.2003.223.1636. [DOI] [PubMed] [Google Scholar]
- 22.Sorenson JL, Ling GV. Metabolic and genetic aspects of urate urolithiasis in Dalmatians. J Am Vet Med Assoc. 1993;203:857–862. [PubMed] [Google Scholar]
- 23.Bartges JW, Osborne CA, Felice LJ, et al. Reliability of single urine and serum samples for estimation of 24-hour urinary uric acid excretion in six healthy Beagles. Am J Vet Res. 1994;55:472–476. [PubMed] [Google Scholar]
- 24.Trimble HC, Keeler CE. The inheritance of “high uric acide excretion” in dogs. J Hered. 1938;29:281–289. [Google Scholar]
- 25.Safra N, Schaible RH, Bannasch DL. Linkage analysis with an interbreed backcross maps Dalmatian hyperuricosuria to CFA03. Mamm Genome. 2006;17:340–345. doi: 10.1007/s00335-005-0137-5. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman JM, Greene ML, Seegmiller JE. Urine uric acid to creatinine ratio—a screening test for inherited disorders of purine metabolism. Phosphoribosyltransferase (PRT) deficiency in X-linked cerebral palsy and in a variant of gout. J Pediatr. 1968;73:583–592. doi: 10.1016/s0022-3476(68)80274-4. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadian A, Ehn M, Hober S. Pyrosequencing: history, biochemistry and future. Clin Chim Acta. 2006;363:83–94. doi: 10.1016/j.cccn.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox B, Walkowicz C. Atlas of dog breeds of the world. 5. Neptune City, NJ: TFH Publications; 1995. [Google Scholar]
- 29.American Kennel Club website. Dog registration statistics. [Accessed Apr 17, 2009]; Available at: www.akc.org/reg/dogreg_stats.cfm.
- 30.da Silva Curiel JM, Pope ER, O’Brien DP, et al. Ammonium urate urolith resulting in hydronephrosis and hydroureter in a dog with a congenital portosystemic shunt. Can Vet J. 1990;31:116–117. [PMC free article] [PubMed] [Google Scholar]
- 31.Gutman AB, Yu TF. Uric acid nephrolithiasis. Am J Med. 1968;45:756–779. doi: 10.1016/0002-9343(68)90209-x. [DOI] [PubMed] [Google Scholar]
- 32.Keeler CE. The inheritance of predisposition to renal calculi in the Dalmatian. J Am Vet Med Assoc. 1940;96:507–510. [Google Scholar]
- 33.Neff MW, Robertson KR, Wong AK, et al. Breed distribution and history of canine mdr1–1Delta, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proc Natl Acad Sci U S A. 2004;101:11725–11730. doi: 10.1073/pnas.0402374101. [DOI] [PMC free article] [PubMed] [Google Scholar]