Abstract
Objective
Research demonstrates heterogeneous neuropsychological profiles among individuals with mild cognitive impairment (MCI). However, few studies have included visuoconstructional ability or used latent mixture modeling to statistically identify MCI subtypes. We therefore examined whether unique neuropsychological MCI profiles could be ascertained using latent profile analysis (LPA), and subsequently investigated cerebrospinal fluid (CSF) biomarkers, genotype, and longitudinal clinical outcomes between the empirically-derived classes.
Methods
806 participants diagnosed via the Alzheimer’s Disease Neuroimaging Initiative (ADNI) MCI criteria received a comprehensive neuropsychological battery assessing visuoconstructional ability, language, attention/executive function, and episodic memory. Test scores were adjusted for demographic characteristics using standardized regression coefficients based on “robust” normal control performance (n=260). Calculated z-scores were subsequently used in the LPA, and CSF-derived biomarkers, genotype, and longitudinal clinical outcome were evaluated between the LPA-derived MCI classes.
Results
Statistical fit indices suggested a 3-class model was the optimal LPA solution. The 3-class LPA consisted of a mixed impairment MCI class (n=106), an amnestic MCI class (n=455), and an LPA-derived normal class (n=245). Additionally, the amnestic and mixed classes were more likely to be APOE e4+ and have worse AD CSF biomarkers than LPA-derived normal subjects.
Conclusions
Our study supports significant heterogeneity in MCI neuropsychological profiles using LPA and extends prior work (Edmonds et al., 2015) by demonstrating a lower rate of progression in the approximately one-third of ADNI MCI individuals who may represent “false-positive” diagnoses. Our results underscore the importance of using sensitive, actuarial methods for diagnosing MCI, as current diagnostic methods may be over-inclusive.
Keywords: Mild cognitive impairment (MCI), Assessment of cognitive disorders/dementia, Alzheimer’s disease, Neuropsychological Profiles, Multivariate Mixture Modeling, Latent profile analysis, Biomarkers
Introduction
Mild cognitive impairment (MCI), a prodromal state between normal aging and dementia, has been conventionally classified as “amnestic” or “non-amnestic” with single-versus multi-domain impairment (Petersen, 2004; Winblad et al., 2004). The criteria for MCI diagnosis used in many large-scale studies rely on subjective complaints, rating scales, and evidence of impaired performance on a single cognitive test. This approach to diagnosing MCI is epitomized in several clinical trials targeting MCI (Petersen & Morris, 2005) and in many large-scale studies like the Alzheimer’s Disease Neuroimaging Initiative (ADNI; Weiner et al., 2013). However, recent research has challenged the empirical validity of this conventional diagnostic approach, as statistical clustering techniques used to characterize MCI subtypes have identified considerable neuropsychological heterogeneity (Clark et al., 2013; Delano-Wood et al., 2009; Edmonds et al., 2015; Libon et al., 2010). For example, Edmonds et al. (2015) examined 825 MCI subjects from ADNI via cluster analysis. Results produced four unique cognitive phenotypes: an amnestic MCI group (34.9%), a dysnomic MCI group (18.5%), a dysexecutive MCI group (12.5%) and a large fourth cluster (34.2%) characterized by intact neuropsychological performance despite their MCI diagnosis. The “cluster-derived normal” group performed within normal limits on all neuropsychological cluster measures despite subjective complaints and impaired scores on the Wechsler Memory Scale-Revised (WMS-R) Logical Memory-II Story A and the Clinical Dementia Rating (CDR) scale that led to their ADNI MCI diagnosis. The notion that individuals in this group were assigned a diagnosis of MCI in error was further supported by normal cerebrospinal fluid (CSF) Alzheimer’s disease (AD) biomarker profiles and low rates of progression to AD and high rates of reversion to “cognitively normal” diagnoses (Bondi et al., 2014; Edmonds et al., 2015).
Despite the significant findings from Edmonds et al. (2015), two limitations of their study warrant further analysis of the ADNI MCI cohort. First, the authors only examined the neuropsychological domains of attention/executive functioning, language, and episodic memory, omitting any form of visuospatial skills. In clinical practice, visuoconstructional ability – which integrates visuospatial, organizational, and motor skills – is routinely assessed in the neuropsychological evaluation of older adults (Grossi & Trojano, 2001; Lezak, 2012). Significant visuospatial/constructional deficits are quite common among neurodegenerative disorders and dementia syndromes (Freedman & Dexter, 1991; Geldmacher, 2003), consequently representing an important component in any neuropsychological protocol. For example, Nielson, Cummings & Cotman (1996) demonstrated in autopsy-confirmed AD subjects a significant correlation between impaired visuoconstructional ability and hyperphosphorylated tau in occipital cortex. Moreover, visuoconstructional ability was not correlated with hyperphosphorylated tau in other brain regions, and language and memory functions were unrelated to hyperphosphorylated tau in occipital cortex. Prominent, differential visuospatial impairment is also a core diagnostic criterion of posterior cortical atrophy, a syndrome often attributable to AD pathology (Crutch et al., 2012; Crutch et al., 2013), and represents a key neuropsychological feature of Lewy body dementia (Ferman et al., 2006; Hamilton et al., 2008; Johnson, Morris & Galvin, 2005; Kao, et al., 2009; McKeith et al., 1996). Furthermore, individuals with non-amnestic MCI who progress to pathologically-confirmed Lewy body dementia have been shown to initially present with visuospatial/constructional as well as attentional impairments (Ferman et al., 2013; Molano et al., 2010). Visuospatial dysfunction has also been reported in multi-domain amnestic MCI (Mapstone, Steffenella & Duffy, 2003). Importantly, a cluster analysis of amnestic and non-amnestic MCI subjects by Clark et al. (2013) revealed four unique subtypes, with three demonstrating visuoconstructional impairment: a single-domain visuoconstructional MCI subgroup (23.8%); an MCI subgroup with predominant executive and visuoconstructional dysfunction (16.3%); and a multi-domain MCI subgroup with mixed episodic memory, executive function, language and visuoconstructional impairment (17.5%). The fourth MCI subgroup was characterized by single-domain amnestic impairment only (42.5%), a consistent finding among all previous MCI neuropsychological classification studies (Delano-Wood et al., 2009; Edmonds et al., 2015; Libon et al., 2010). However, results in the visuospatial domain lack replication due to the exclusion of any representative assessment, such as visuoconstruction, in the MCI classification literature. Thus, the contribution of visuoconstructional testing available in ADNI has potentially been overlooked by past studies identifying neuropsychological MCI subtypes (Bondi et al., 2014; Edmonds et al., 2015).
Another limitation of Edmonds et al. (2015) involves the use of traditional cluster analysis to identify subgroups. Newer latent mixture models, such as latent profile analysis (LPA), offer several statistical advantages over traditional cluster analysis given its model-driven classification approach. For example, while cluster analysis assigns each individual to subgroups in binary fashion, LPA utilizes maximum likelihood estimation to generate posterior probabilities and model the classification uncertainty of each individual in each latent class (Berlin, Williams, & Parra, 2014; Magidson & Vermunt, 2002; Muthén, 2004). These posterior probabilities are used to account for measurement error, consequently decreasing estimation bias and improving the accuracy of standard errors in analyses (Asparouhov & Muthén, 2015; Bray, Lanza, & Tan, 2015; Clark & Muthén, 2009; Magidson & Vermunt, 2002). LPA also produces information criterion and likelihood fit indices to guide determination of the number of optimal classes (Berlin, Williams & Parra, 2014; Muthén, 2004). This statistical comparison of nested models inherently increases objectivity and minimizes the arbitrary nature of subgroup selection in cluster analysis (Magidson & Vermunt, 2002). Other benefits of LPA include the ability to handle missing data points in analyses (Roesch, Villodas, & Villodas, 2010), accommodation of multiple data types such as categorical and continuous variables (Magidson & Vermunt, 2002), incorporation of predictor variables and distal outcomes in the model (Magidson & Vermunt, 2002; Muthén, 2004), and model verification with independent samples (Shao, Liang, Yuan, & Bian, 2014).
Therefore, we employed LPA to investigate unique MCI subtypes within ADNI across four neurocognitive domains (visuoconstructional ability, language, attention/executive function, and episodic memory), and subsequently evaluate class differences on exploratory outcomes of CSF and genetic AD biomarkers, longitudinal outcome, and other ADNI measures. We hypothesized that the optimal LPA solution would generate five classes: four subgroups similar in size and neuropsychological profile to the Edmonds et al. (2015) study – including a class with normal neuropsychological performance– as well as the emergence of a small, fifth subtype predominantly characterized by visuoconstructional impairment. Additionally, we predicted that visuoconstructional deficits would be present in a class analogous to the dysexecutive MCI subgroup from Edmonds et al. (2015), thus representing a subtype with “mixed” neuropsychological impairment. Among exploratory outcomes, we hypothesized that the classes would differ on AD biomarkers and longitudinal outcomes; classes with impairment across multiple domains would display increased levels of AD-positive markers and higher conversion rates than classes with mild, circumscribed deficits. Furthermore, the normal neuropsychological class was predicted to demonstrate lower rates of AD-positive biomarkers, lower longitudinal conversion to AD, and higher reversion to normal than all other classes.
Methods
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org. Research was conducted in accordance with the Declaration of Helsinki and the current study approved by the University of California, San Diego IRB.
Participants
Participants included 825 individuals diagnosed with MCI and 260 healthy elderly participants. MCI was diagnosed at a screening evaluation using conventional diagnostic criteria, as operationalized by ADNI (Petersen et al., 2010): 1) Subjective memory complaint; 2) Mini-Mental State Examination (MMSE) score greater than or equal to 24; 3) Global Clinical Dementia Rating Scale (CDR) score of 0.5; 4) Impairment on WMS-R Logical Memory-II Story A Recall (WMS-R LM II) after education adjustment; and 5) Intact global cognition and preserved activities of daily living/ instrumental activities of daily living. For the current study, we required MCI participants to fall within the demographic boundaries of the elderly normative control group. 19 MCI subjects were subsequently excluded due to age (i.e., >90 or <60), resulting in a final sample of 806 MCI participants. We required that all healthy elderly control subjects (n=260) have complete data on the neuropsychological variables examined and that they remained cognitively intact upon longitudinal re-evaluation (follow-up range: 1–7 years). Table 1 provides demographic information on these “robust” normal control participants and the entire MCI sample for descriptive purposes.
Table 1.
Demographic Characteristics of Total MCI Sample and Robust Normal Controls*
| Age (years) |
Education (years) |
Gender | GDS | MMSE at Screening |
|
|---|---|---|---|---|---|
| MCI(n=806) | 73.90 (6.94) | 15.95 (2.81) | 40.0% F | 1.65 (1.42) | 27.58 (1.81) |
| Robust Control (n=260) | 75.25 (5.62) | 16.20 (2.68) | 48.8% F | 0.62 (0.06) | 29.05 (1.17) |
Data summarized as Mean (Standard Deviation), unless otherwise noted
Abbreviations: GDS = Geriatric Depression Scale; MMSE = Mini-Mental State Examination; MCI = Mild Cognitive Impairment; F = Female
Neuropsychological Measures
Eight neuropsychological variables were selected from seven cognitive tests in ADNI’s neuropsychological battery. These variables were balanced across the domains of visuoconstructional ability (Mini-Mental State Examination [MMSE] Pentagons & Clock Drawing Test [CDT]); language (Animal Fluency & Boston Naming Test [BNT]); attention/executive function (Trail Making Test [TMT], Part A & TMT, Part B); and episodic memory (Rey Auditory Verbal Learning Test [AVLT] Delay Free Recall & AVLT Recognition). These specific neuropsychological test variables were selected from available ADNI measures as they were administered across all three ADNI phases and represent well-researched assessments in older adults that are commonly employed and easily interpreted in clinical practice (Lezak, 2012). We did not use WMS-R Logical Memory in our test corpus due its primary use in MCI diagnosis, thereby circumventing criterion contamination. All neuropsychological variables were significantly correlated with every other neuropsychological measure (all p’s<0.003), as presented in Supplemental Table e-1. Moreover, variables within a cognitive domain derived from the same neuropsychological test (AVLT Recall & Recognition; TMT, Part A & B) produced the largest correlations than variables from separate tests (CDT & MMSE Pentagons; Animal Fluency & BNT).
MMSE Pentagons
Raw MMSE baseline data were obtained via the ADNI website and participant copies of the interlocking pentagons were re-coded using an 8-point error scoring system previously published by Jefferson et al. (2002). This scoring system was chosen to increase the possible range (i.e., 0 to 8 points vs. the standard 0 or 1 scoring system) and minimize potential ceiling effects. Additionally, past research by Jefferson et al. (2002) has shown differential performance in patients with cortical vs. subcortical neurodegenerative disorders using this 8-point scoring system. Errors include 1) size distortion, 2) number of figures, 3) improper pentagon intersection, 4) tremor/segmentation, 5) absence of five angles, 6) significant rotation, 7) interminable motor perseveration, and 8) pull-to-stimulus. For further information and operational definitions of the scoring system, please refer to Jefferson et al. (2002).
Two raters were trained on the 8-point scoring system and established reliability on a randomly selected subset (n=54) of MMSE pentagons from the ADNI sample. After establishing satisfactory reliability (single measure intra-class correlation: 0.906, 95% CI: 0.838 – 0.945; range of kappa values for individual error types: 0.673 – 1.000) each rater was randomly assigned half of the remaining MMSE pentagons for recoding with the 8-point error scoring system.
The MMSE pentagons could not be retrospectively obtained via archives for 17.7% of our MCI sample. According to ADNI representatives, data were missing due to technical problems with raw file upload rather than lack of administration or inability to complete the test. Missing values analysis indicated that MMSE pentagons were not missing completely at random (Little’s MCAR test: χ2(7)=22.156, p=0.002) when evaluated with the other 7 neuropsychological variables. However, original MMSE pentagon scores (0 or 1) were available in ADNI for all MCI participants. These original scores and the 8-point error scoring system were significantly correlated with a medium-large effect (r= –0 .387, p<0.001), supporting their use as a reasonable proxy to examine the missing data. The proportion of individuals with correct versus incorrect original scores did not differ (χ2(1)=2.519, p=0.112) by presence (n=663; Correct: 87.6%, Incorrect: 12.4%) or absence (n=143; Correct: 92.3%, Incorrect: 7.7%) of raw files. Therefore, raw files were not absent because of poor performance secondary to underlying disease etiology and were assumed missing at random (MAR).
Clock Drawing Test
Clock drawing to command and copy was administered and scored according to ADNI procedures (Alzheimer’s Disease Neuroimaging Initiative, 2008; Goodglass & Kaplan, 1983). Briefly, participants were instructed on command to “draw the face of a clock showing the numbers and two hands set to ten after eleven” on blank paper. The participant was then presented a response form with the model clock at the top and requested to “copy this clock (point to the model) in the space provided below”.
Clock drawings to command and copy were each scored using the same 0 – 5 point scale. Clock scoring criteria as outlined in the ADNI-2 Procedures Manual (ADNI, 2008) include 1) approximately circular, 2) symmetry of number placement, 3) correctness of numbers, 4) presence of two hands, and 5) presence of two hands set to ten after eleven. Individual command and copy scores were combined to produce an overall Clock Drawing Test total score (0 – 10). This total score was selected for the current analysis rather than separate command and copy scores to maximize the range of possible performance while minimizing any potential ceiling effects. For further information on clock drawing administration and scoring criteria please refer to the ADNI-2 Procedures Manual: http://adni.loni.usc.edu/wp-content/uploads/2008/07/adni2-procedures-manual.pdf
Transformations and Normative Standardization
The distribution of each neuropsychological variable was examined for non-normality within the sample of robust normal control participants. Each variable was investigated using the ladder function in Stata version 12, which utilizes a chi-square test to determine if and what type of transformation is most appropriate (Tukey, 1977). Animal fluency; TMT, Part A; and TMT, Part B were identified with skew and kurtosis that would significantly benefit from application of the square-root, logarithm-10, and inverse square-root functions, respectively, to improve normality. The remaining five neuropsychological variables (i.e., CDT, MMSE pentagons, BNT, AVLT Recall, and AVLT Recognition) did not significantly benefit from any transformation and therefore retained their identity distributions.
Following application of transformations, standardized regression-based (SRB) formulas were used to generate normative data for each neuropsychological variable based on robust normal control performance. Age, education, and gender were included to account for potential demographic effects; beta coefficients, adjusted R2, and standard error of the estimates for each equation are available in Supplemental Table e-2.
These regression formulas were subsequently used to calculate the predicted performance of each MCI participant on all eight neuropsychological variables. This predicted score was then applied to obtain a z-score reflecting an MCI subject’s degree of impairment on each variable:
Distal Outcome Variables
Distal outcome variables of interest included demographics, ADNI diagnostic measures, biological and genetic markers, longitudinal clinical outcome, and ADNI phase at time of enrollment (ADNI-1, ADNI-GO, ADNI-2). Diagnostic measures used by ADNI to originally identify MCI included WMS-R LM-II score, CDR sum of boxes, MMSE, and the Functional Activities Questionnaire (FAQ). Biological markers were available on 52.4% of MCI (n=422) and 55.0% (n=143) of robust normal control participants; markers included CSF concentrations of total tau, hyperphosphorylated tau (p-tau181p), beta-Amyloid (Aβ1–42), and the ratio of p-tau181p to Aβ1–42. Subjects were classified according to CSF concentration thresholds (tau: >93 pg/mL; p-tau181p: >23 pg/mL; Aβ1–42: <192 pg/mL; p-tau181p/Aβ1–42 ratio: >0.10) previously established to maximize sensitivity and specificity of autopsy confirmed AD (Shaw et al., 2009). Apolipoprotein E (APOE) e4 allele frequency was accessible for 98.8% of MCI participants (n=796) and included in the current study as a genetic marker of AD. Longitudinal clinical outcome was available on 93.8% of MCI participants (n=756), with average follow-up of 28.7 months. Variables included type of clinical conversion (progression to dementia, remain stable MCI, or reversion to normal) and the associated number of months to conversion.
Statistical Analyses
Data preparation (descriptive statistics, regression, and formatting for import into MPlus), were conducted in SPSS version 22. The ladder command in Stata version 12 was utilized to determine the benefit of transformations on the normality of neuropsychological variables in robust normal controls. All multivariate analyses were performed in MPlus version 7.3.
Latent profile analysis (LPA) was conducted using SRB z-scores of the eight neuropsychological variables as indicators of class membership. Models with two to eight latent classes were evaluated and maximum likelihood estimation with robust standard errors was used in LPA model estimation. Unavailable MMSE pentagons (82% covariance coverage) were assumed missing at random (MAR) in the model. All LPA’s were initially performed with the default number of random starts, which were subsequently increased twice (100, 25; and 500, 100) to ensure reproduction of global maxima and protect against misidentification of an erroneous local maxima (Hipp & Bauer, 2006). In the current study, all LPA results were unchanged after increasing random starts.
Determination of the best-fitting LPA is an iterative process, comparing a model with k latent classes to k-1 classes until obtaining an optimal solution. Multiple indicators of model fit are useful to determine the best number of latent classes; however, LPA lacks a gold standard and requires consideration of these indices in conjunction with model parsimony and meaningful theoretical interpretation (Berlin, Williams & Parra, 2014; Roesch et al., 2010). The current study considered three comparative fit indices: Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC) and sample-size adjusted Bayesian Information Criterion (sBIC), with the smallest values indicating the best-fitting model. In addition, the Vuong-Lo-Mendell-Rubin adjusted Likelihood Ratio Test (VLMR-LRT) and the Bootstrap Likelihood Ratio Test (BLRT) were used to compare the model with k latent classes to the k-1 class solution; statistical significance (p<0.05) suggests the k class model is a better fit than k-1 classes. Additional statistics used to identify suitable model fit include entropy, an aggregate index of posterior probabilities that reflects the overall precision with which subjects were correctly classified (Berlin, Williams & Parra, 2014; Roesch et al., 2010); a scree-plot of each model’s log-likelihood, which can be a helpful exploratory diagnostic tool in optimal class determination (Nylund, Asparouhov & Muthén, 2007); and the number of classes containing <5% of the overall sample size, an indicator of potential data over-extraction (Berlin, Williams & Parra, 2014; Roesch, et al., 2010). Monte Carlo simulation studies using a variety of sample sizes suggest the sBIC, BLRT, and entropy are the most robust fit indices (Berlin, Williams & Parra, 2014; Nylund et al., 2007; Roesch et al., 2010; Tein, Coxe & Cham, 2013). Finally, LPA solutions were evaluated for model parsimony, data over-extraction, and meaningful theoretical interpretation based on previous research.
After selection of the optimal LPA, distal outcome variables were examined between latent classes within the structural equation modeling (SEM) framework. This method is preferential over subject assignment to most likely latent class membership and subsequent ANOVA comparisons; analyzing distal outcomes within SEM models classification uncertainty in statistical comparisons, generating accurate standard errors and reducing biased inferences (Asparouhov & Muthén, 2015; Bray et al, 2015). In the current study the 3-step BCH method (Bakk & Vermunt, 2015; Bolck, Croon, & Hagenaars, 2004; Vermunt, 2010) was employed for continuous distal outcome variables, while the DCAT command was utilized with categorical distal outcome variables (Lanza et al, 2013). The former uses a weighting procedure to account for classification error, while the latter treats distal outcomes as a form of covariate. Asparouhov & Muthén (2015) have demonstrated that these methods are the preferable approaches for continuous and categorical distal outcomes, respectively, due to their satisfactory estimation of standard error, resistance to class shifts, and minimal bias. MPlus performs parameter comparisons on all measures using the Wald chi-square test (Asparouhouv & Muthén, 2007) and statistical significance was set at α=0.005 to control for Type-I errors.
Results
Latent Profile Analysis
Two to eight latent class models were tested. Fit indices and descriptive characteristics for each model are provided in Table 2 and 3 respectively; an exploratory scree-plot of the associated log-likelihood values is available in Supplemental Figure e-1.
Table 2.
LPA Comparative Fit Indices & Likelihood Ratio Tests
| Number of Classes | AIC | BIC | sBIC | VLMR-LRT | BLRT |
|---|---|---|---|---|---|
| 2 | 20291.57 | 20408.87 | 20329.48 | p=0.0265 | p<0.0001 |
| 3* | 19982.63 | 20142.16 | 20034.19 | p=0.0152 | p<0.0001 |
| 4 | 19820.68 | 20022.44 | 19885.90 | p=0.2045 | p<0.0001 |
| 5 | 19710.91 | 19954.90 | 19789.77 | p=0.0622 | p<0.0001 |
| 6 | 19560.23 | 19846.45 | 19652.74 | p=0.1000 | p<0.0001 |
| 7 | 19502.07 | 19830.52 | 19608.23 | p=0.4687 | p<0.0001 |
| 8 | 19435.09 | 19805.77 | 19554.89 | p=0.3352 | p<0.0001 |
Chosen as Best Class Solution
Abbreviations: LPA = Latent Profile Analysis; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; sBIC = sample-size adjusted Bayesian Information Criterion; VLMR-LRT = Lo-Mendell-Rubin adjusted Likelihood Ratio Test; BLRT = Bootstrapped Likelihood Ratio Test
Table 3.
LPA Model Characteristics
| Number of Classes |
Final Log-Likelihood |
Entropy | Number of Classes <5% |
Smallest Class Size Percentage |
|---|---|---|---|---|
| 2 | −10120.782 | 0.671 | 0 | 39.95% |
| 3* | −9957.315 | 0.773 | 0 | 13.15% |
| 4 | −9867.341 | 0.823 | 0 | 5.83% |
| 5 | −9803.455 | 0.764 | 1 | 3.60% |
| 6 | −9719.116 | 0.771 | 1 | 3.85% |
| 7 | −9681.037 | 0.789 | 2 | 2.85% |
| 8 | −9638.548 | 0.781 | 2 | 2.48% |
Chosen as Best Class Solution
Abbreviations: LPA = Latent Profile Analysis
AIC, BIC, and sBIC comparative fit indices successively decreased with increasing latent classes; the BLRT showed a similar pattern, with k classes always a statistically significant fit compared to k-1 classes. These indices failed to clearly converge on an optimal solution, as this trend would presumably continue past eight latent classes and likely result in data over-fitting based on other indicators (Nylund et al., 2007).
An examination of the LPA log-likelihood scree plot revealed two elbow points, at the 3-and 6-class models. The VLMR-LRT suggested the 3-class solution as a significantly better fit than 2-classes. However, the 4-class solution (vs. 3-classes) did not result in statistically significant improvement in model fit via the VLMR-LRT. The VLMR-LRT remained non-significant for all subsequent class comparisons (e.g., 5- vs. 4-classes, etc.). Entropy was highest for the 4-class model, though satisfactory (Asparouhov & Muthén, 2014; Tein et al., 2013) and relatively equivalent for the 3- and 6-class solutions. The smallest class size for the 3-class solution was 13.2% of all MCI participants; LPA models with 5 or greater classes contained at least one class that was <5% of the overall sample. The 3-class LPA was selected as the optimal solution on the basis of fit indices (e.g., VLMR-LRT), satisfactory entropy, model parsimony, signs of possible data over-fitting with increasing latent classes, and meaningful neuropsychological interpretation of classes.
The final 3-class LPA grouped MCI participants into a “mixed” MCI class (n=106, 13.2%), an “amnestic” MCI class (n=455, 56.5%), and an “LPA-derived normal” class (n=245, 30.4%) based upon neuropsychological performance. Final class counts based on most likely class membership are presented in Table 4.
Table 4.
Final Class Counts and Proportions for Most Likely Class Membership of 3-Class LPA
| n | Proportion of Total MCI Sample | |
|---|---|---|
| Mixed MCI Class | 106 | 13.15% |
| Amnestic MCI Class | 455 | 56.45% |
| LPA-Derived Normal Class | 245 | 30.40% |
Abbreviations: LPA = Latent Profile Analysis; MCI = Mild Cognitive Impairment
Posterior probabilities for correct classification ranged from 0.40 to 1.00 for the mixed MCI class, 0.47 to 1.00 for the amnestic MCI class, and 0.50 to 1.00 for the LPA-derived normal class. Average posterior probability for most likely class membership across each class was satisfactory and is available in in Supplemental Table e-3.
Neuropsychological Measures
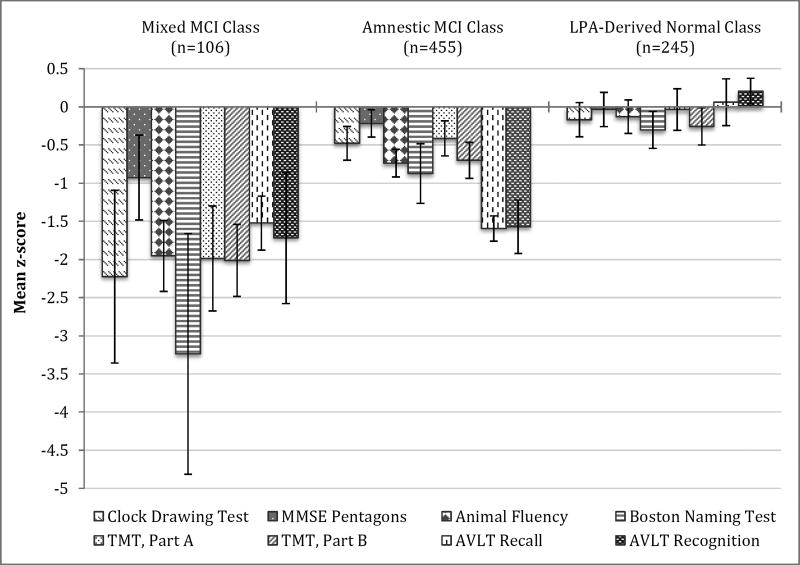
The mixed MCI class yielded a profile of neuropsychological impairment across all four cognitive domains, ranging from mild-to-moderate to severe deficits. However, performance on the MMSE pentagon test was only low average for the mixed MCI class. The amnestic MCI class demonstrated mild-to-moderate impairment on both measures of episodic memory and average to low average performance across all other cognitive domains. The LPA-derived normal class demonstrated average performance across all neuropsychological tests, despite their original MCI diagnosis. Neuropsychological performance of each class is presented in Figure 1.
Figure 1. Neuropsychological Performance for the Latent Profile Classes.
Error bars denote 99.5% confidence intervals.
Abbreviations: MCI = Mild Cognitive Impairment; LPA = Latent Profile Analysis; MMSE = Mini-Mental State Examination; TMT = Trail Making Test; AVLT = Rey Auditory Verbal Learning Test
Omnibus Wald tests suggested significant differences between classes on every neuropsychological variable (all p’s<0.001). Post-hoc comparisons indicated the mixed MCI class performed significantly worse (all p’s<0.001) than both the amnestic MCI and LPA-derived normal classes on all measures of visuoconstructional ability, language, and attention/executive functioning. However, on AVLT Recall and Recognition the mixed MCI class was only significantly worse compared to the LPA-derived normal class (p< 0.001). The amnestic MCI class produced significantly lower scores than the LPA-derived normal class on all tests of episodic memory and language, as well as TMT, Part B (p< 0.001). There was no statistical difference in performance between the two groups on measures of visuoconstructional ability or TMT, Part A. Differences in neuropsychological performance between classes are presented in Table 5.
Table 5.
Neuropsychological Performance of Latent Profile Classes
| Variable | Mixed MCI Class |
Amnestic MCI Class |
LPA-Derived Normal Class |
Omnibus Wald χ2Test (df) |
p-value |
|---|---|---|---|---|---|
| Visuoconstructional Ability | |||||
| MMSE Pentagons | −2.2262,3 | −0.4781 | −0.1691 | χ2(2)=17.829 | p<0.001 |
| (0.403) | (0.079) | (0.080) | |||
| Clock Drawing Test | −0.9272,3 | −0.2171 | −0.0351 | χ2(2)=27.685 | p<0.001 |
| (0.198) | (0.064) | (0.080) | |||
| Language | |||||
| Animal Fluency | −1.9532,3 | −0.7371,3 | −0.1301,2 | χ2(2)=100.948 | p<0.001 |
| (0.166) | (0.065) | (0.078) | |||
| BNT | −3.2382,3 | −0.8741,3 | −0.3021,2 | χ2(2)=49.202 | p<0.001 |
| (0.562) | (0.139) | (0.086) | |||
| Attention/Executive Function | |||||
| TMT, Part A | −1.9872,3 | −0.4131 | −0.0361 | χ2(2)=51.823 | p<0.001 |
| (0.245) | (0.082) | (0.097) | |||
| TMT, Part B | −2.0132,3 | −0.7021,3 | −0.2561,2 | χ2(2)=82.083 | p<0.001 |
| (0.168) | (0.084) | (0.087) | |||
| Episodic Memory | |||||
| AVLT Recall | −1.5243 | −1.5963 | 0.0611,2 | χ2(2)=395.429 | p<0.001 |
| (0.126) | (0.059) | (0.034) | |||
| AVLT Recognition | −1.7193 | −1.5723 | 0.2051,2 | χ2(2)=529.387 | p<0.001 |
| (0.306) | (0.125) | (0.060) | |||
Data summarized as mean (standard error) in Standardized Regression-Based z-scores. Numbered superscripts denote significant Wald χ2 test post-hoc differences at p<0.005 between each class and the class number indicated (1= Mixed MCI Class, 2= Amnestic MCI Class, 3= LPA-Derived Normal Class)
Abbreviations: MCI = Mild Cognitive Impairment; LPA = Latent Profile Analysis; χ2 = chi-square; df= degrees of freedom; MMSE = Mini-Mental State Examination; BNT = Boston Naming Test; TMT = Trail Making Test; AVLT = Rey Auditory Verbal Learning Test
Distal Outcomes Variables
Latent class differences on all distal outcome variables are presented in Table 6. Certain variables (e.g., CSF biomarkers, APOE allele, longitudinal outcomes) only included a subset of the total MCI sample; the distribution of these subsamples across latent classes is available in Supplemental Table e-4 for descriptive purposes.
Table 6.
Demographic, Diagnostic, Genetic, CSF Biomarker, Longitudinal, and ADNI Phase Differences Between Latent Profile Classes
| Variable | Mixed MCI Class |
Amnestic MCI Class |
LPA-Derived Normal Class |
Omnibus Wald χ2Test (df) |
p-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 73.72 | 74.01 | 73.79 | χ2(2)=0.165 | p=0.921 |
| (0.72) | (0.35) | (0.55) | |||
| Education | 15.77 | 15.82 | 16.25 | χ2(2)=3.158 | p=0.206 |
| (0.37) | (0.15) | (0.19) | |||
| Gender (%) | 42.1% F | 36.6% F | 45.4% F | χ2(2)=3.015 | p=0.222 |
| (5.6) | (2.5) | (4.0) | |||
| GDS | 1.85 | 1.54 | 1.75 | χ2(2)=3.690 | p=0.158 |
| (0.16) | (0.07) | (0.11) | |||
| Diagnostic Measures | |||||
| WMS-R LM II | 4.123 | 4.733 | 8.031,2 | χ2(2)=178.914 | p<0.001 |
| (0.359) | (0.18) | (0.19) | |||
| CDR Sum of Boxes | 2.02,3 | 1.581,3 | 1.171,2 | χ2(2)=63.624 | p<0.001 |
| (0.11) | (0.05) | (0.05) | |||
| Baseline MMSE | 26.342,3 | 27.411,3 | 28.431,2 | χ2(2)=105.151 | p<0.001 |
| (0.19) | (.10) | (0.11) | |||
| FAQ | 4.873 | 3.693 | 1.381,2 | χ2(2)=75.677 | p<0.001 |
| (0.54) | (0.23) | (0.20) | |||
| Genetic & CSF Biomarkers | |||||
| % APOE e4-positive | 61.3%3 | 57.8%3 | 34.5%1,2 | χ2(2)=30.014 | p<0.001 |
| (6.3) | (3.4) | (3.4) | |||
| % high total tau | 53.1%3 | 42.5%3 | 22.6%1,2 | χ2(2)=17.159 | p<0.001 |
| (9.2) | (3.9) | (3.6) | |||
| % high p-tau181p | 84.2%3 | 64.0%3 | 33.4%1,2 | χ2(2)=51.233 | p<0.001 |
| (6.4) | (4.4) | (4.3) | |||
| % low Aβ1–42 | 81.6%3 | 73.0%3 | 29.3%1,2 | χ2(2)=72.773 | p<0.001 |
| (6.9) | (3.6) | (4.3) | |||
| % high p-tau/Aβ ratio | 80.3%3 | 76.2%3 | 34.6%1,2 | χ2(2)=64.444 | p<0.001 |
| (6.9) | (3.8) | (4.3) | |||
| Longitudinal Outcome | |||||
| % progression to dementia | 60.0%2,3 | 38.3%1,3 | 5.8%1,2 | χ2(2)=133.050 | p<0.001 |
| (6.7) | (2.9) | (1.8) | |||
| Months until progression | 15.852,3 | 23.421 | 51.151 | χ2(2)=20.338 | p<0.001 |
| (1.58) | (1.46) | (11.25) | |||
| % reversion to normal | 1.2%3 | 0.6%3 | 11.2%1,2 | χ2(2)=21.037 | p<0.001 |
| (1.2) | (0.6) | (2.1) | |||
| Months until reversion | 10.32 | 18.11 | 21.45 | χ2(2)=8.619 | p=0.013 |
| (1.71) | (4.29) | (3.69) | |||
| % stable MCI | 38.8%2,3 | 61.1%1,3 | 83.0%1,2 | χ2(2)=65.159 | p<0.001 |
| (6.7) | (2.8) | (2.6) | |||
| Amount of total follow-up (in months) | 27.74 | 29.23 | 28.02 | χ2(2)=0.403 | p=0.818 |
| (2.37) | (1.23) | (1.79) | |||
| Phase | |||||
| % from ADNI1 | 64.3%3 | 53.7%3 | 27.4%1,2 | χ2(2)=51.420 | p<0.001 |
| (5.2) | (2.8) | (3.2) | |||
| % from ADNIGO | 0.0%2,3 | 9.9%1,3 | 28.5%1,2 | χ2(2)=125.303 | p<0.001 |
| (0.0) | (1.8) | (3.1) | |||
| % from ADNI2 | 35.7% | 36.4% | 44.1% | χ2(2)=3.915 | p=0.141 |
| (5.2) | (2.5) | (3.4) | |||
Data summarized as mean or percent of class and (standard error) unless otherwise noted. Numbered superscripts denote significant Wald χ2 test post-hoc differences at p<0.005 between each class and the class number indicated (1=Mixed MCI Class, 2=Amnestic MCI Class, 3=LPA-Derived Normal Class)
Abbreviations: MCI = Mild Cognitive Impairment; LPA = Latent Profile Analysis; χ2 = chi-square; df= degrees of freedom; GDS = Geriatric Depression Scale; WMS-R LM II = Weschler Memory Scale-Revised Logical Memory II subtest; CDR = Clinical Demetia Rating Scale; MMSE = Mini-Mental State Examination; FAQ = Functional Activities Questionnaire; CSF = Cerebrospinal Fluid; APOE = Apolipoprotein; RCI = Reliable Change Index; ADNI1 = Alzheimer’s Disease Neuroimaging Initiative Phase 1; ADNIGO = Alzheimer’s Disease Neuroimaging Initiative Phase GO; ADNI2 = Alzheimer’s Disease Neuroimaging Initiative Phase
Omnibus Wald tests indicated no significant differences between classes on the demographic variables of age, education, gender, or Geriatric Depression Scale (GDS). Significant omnibus differences (all p’s<0.001) were noted between classes on all ADNI diagnostic measures (i.e., WMS-R LM-II, CDR Sum of Boxes, MMSE, and FAQ). The LPA-Derived normal class performed significantly better on all ADNI diagnostic measures than both the mixed and amnestic MCI class. The amnestic MCI class produced a significantly higher MMSE score and lower CDR Sum of Boxes tally than the mixed MCI class, though no differences between the two classes were noted on WMS-R LM-II or the FAQ.
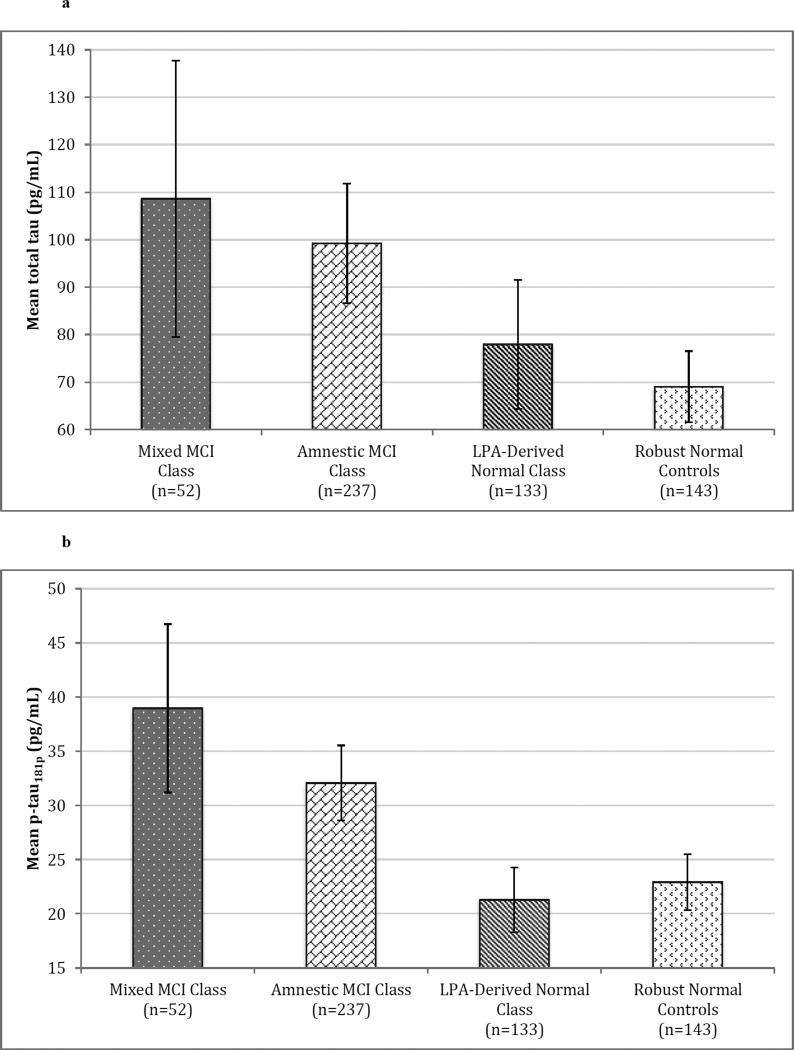
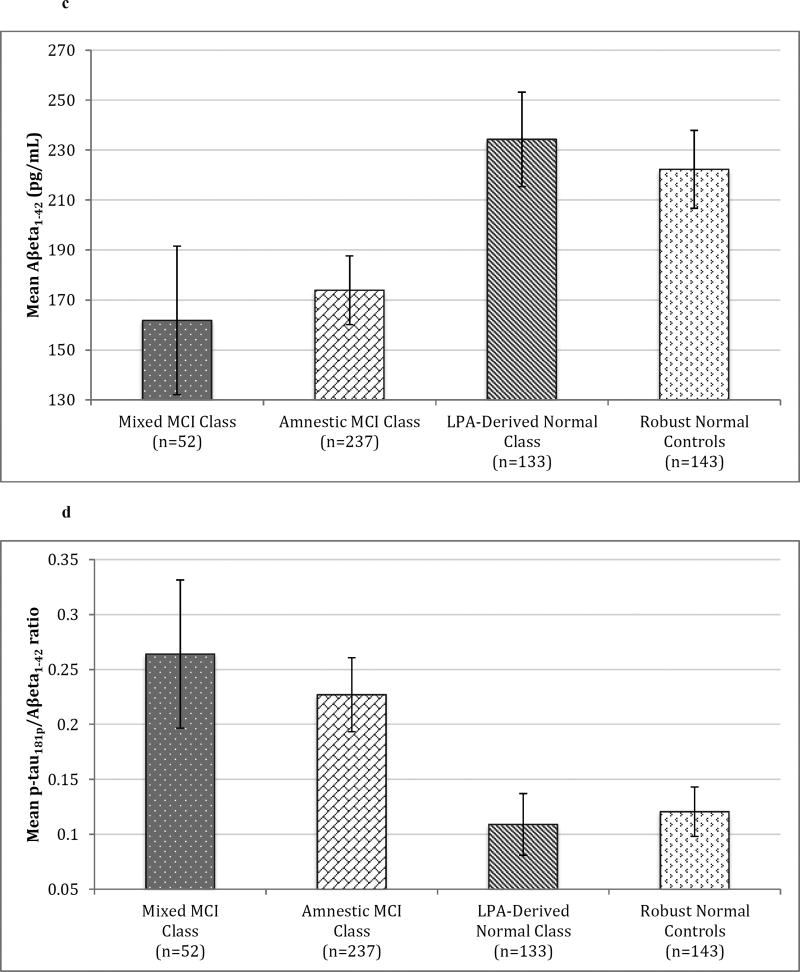
Significant omnibus differences were also present for all genetic and CSF biomarkers (all p’s<0.001) available on a subset of the overall sample. A significantly lower proportion of the LPA-derived normal class had the APOE e4 allele than both other classes (all p’s<0.001); the mixed MCI and amnestic MCI classes did not differ. A similar pattern emerged for CSF biomarkers: both MCI classes contained a significantly higher percentage of subjects with AD-positive CSF biomarkers (i.e., high total tau, high p-tau181p, low AB1–42, and high p-tau181p/AB1–42 ratio) than the LPA-derived normal class (all p’s<0.003), while the amnestic and mixed MCI classes did not differ. Identical results were obtained upon examination of mean CSF biomarker concentrations, with the exception that post-hoc total tau levels were only a nonsignificant trend between the LPA-derived normal and the mixed MCI class (p=0.007). This trend is due to our use of α=0.005 significance level to adjust for multiple comparisons and increased variability of total tau in the mixed class, which produced a larger standard error than the other classes. Mean CSF biomarker concentrations between all latent classes as well as robust normal controls are presented in Figure 2. Additionally, the LPA-derived normal class did not differ from robust normal controls on any of the CSF biomarker concentrations (all p’s>0.101).
Figure 2. Mean CSF Biomarker Concentrations of Latent Profile Classes and Robust Normal Controls.
Error bars denote 99.5% confidence intervals. 2a) Mean total tau (pg/mL).2b) Mean p-tau181p (pg/mL). 2c) Mean Aβeta1–42 (pg/mL). 2d) Mean ratio of p-tau181p to Aβeta1–42.
Abbreviations: CSF = Cerebrospinal fluid; MCI = Mild Cognitive Impairment; LPA = Latent Profile Analysis
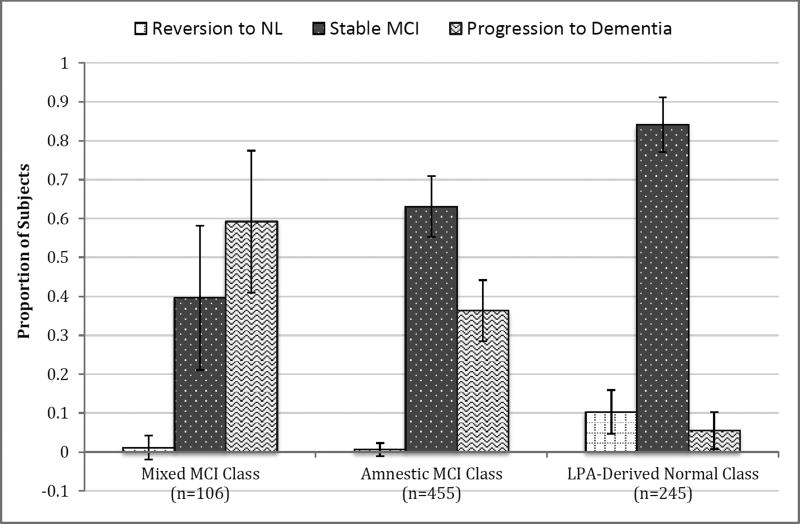
With respect to longitudinal outcomes, there was no significant difference between latent classes in amount of available follow-up. However, omnibus differences were noted among the proportion of individuals who progressed to AD diagnoses, reverted to normal, and remained as stable MCI. In particular, a significantly smaller percentage of the LPA-derived normal class progressed to AD than the other classes (all p’s<0.001). A larger proportion of the LPA-derived normal class also reverted to normal or remained as stable MCI than both other classes (all p’s<0.001). Compared to the mixed MCI class, the amnestic MCI class had a significantly smaller proportion of individuals who progressed to AD but larger percentage who remained stable (all p’s<0.003); no difference was noted in reversion to normal. Furthermore, the mixed MCI class progressed to AD more quickly than both other classes (all p’s<0.002); no difference was observed in progression time between the amnestic MCI and LPA-derived normal classes. Clinical progression rates for LPA classes are presented in Figure 3.
Figure 3. Progression and Reversion Rates of Latent Profile Classes.
Error bars denote 99.5% confidence intervals.
Abbreviations: NL = Normal; MCI = Mild Cognitive Impairment; LPA = Latent Profile Analysis
Upon investigation of ADNI enrollment phase, there was no difference in the proportion of individuals recruited during Phase 2. However, significant omnibus differences were noted between the classes for both Phase 1 and ADNI GO. A significantly smaller percentage of the LPA-derived normal class was enrolled during Phase 1 than both other classes (all p’s< 0.001); no difference was observed between the mixed and amnestic MCI classes. The opposite trend emerged for ADNI GO, such that a significantly larger percentage of the LPA-derived normal class was enrolled during this phase than both other classes (all p’s< 0.001). The amnestic MCI class also had a significantly larger proportion of participants recruited during ADNI GO than the mixed MCI class (p< 0.001), as the latter enrolled no individuals in this phase.
Discussion
We employed LPA across four cognitive domains (visuoconstructional ability, language, attention/executive function, and episodic memory) to identify unique, empirically-derived MCI subgroups within ADNI. In contrast to past neuropsychological research in ADNI, tests of visuoconstructional ability were included to better capture aspects of visuospatial functioning in statistically-defined MCI subtypes. The optimal solution contained three classes: a mixed MCI, an amnestic MCI, and LPA-derived normal class. Contrary to our expectations, a unique MCI subtype characterized by predominant visuoconstructional deficits did not emerge in the 3-class LPA. Several reasons might explain the absence, including the neuropsychological measures chosen, psychometric properties of scoring systems, selected latent model, and MCI diagnostic criteria used by ADNI.
Visuospatial assessments available in ADNI were unfortunately limited to visuoconstructional tasks, which are multi-factorial and require integration of visuoperceptual, organizational, and motor skills (Ahmed et al., 2016). Thus, low scores on clock drawing and MMSE pentagons may reflect a combination of visuospatial and executive functioning difficulties rather than “pure” visuospatial impairment. Additionally, the psychometric properties of these visuoconstructional measures were non-normally distributed and did not benefit from transformations, likely contributing to our results. Post-hoc examination of frequency distributions revealed that 86.5% of the robust normal controls and 80.1% of the total MCI sample produced two or fewer MMSE pentagon errors, suggesting the task or scoring system may not sensitively discriminate between normal and mildly impaired individuals.
Another possible factor contributing to our results is the initial ADNI diagnosis of MCI. ADNI inclusion criteria are heavily weighted towards verbal episodic memory to target preclinical AD, while previous research has demonstrated early, differential visuospatial/constructional impairment most frequently in individuals with non-amnestic MCI (Clark et al., 2013; Ferman et al., 2013; Molano et al, 2010). Thus, one might argue that ADNI’s reliance on a single memory score to determine MCI potentially biases the prevalence of non-amnestic deficits in ADNI. However, visuoconstructional impairment is not captured by verbal memory assessment and, along with other non-amnestic domains, remains uncharacterized with ADNI’s diagnostic criteria. In fact, past work (Bondi et al., 2014; Edmonds et al., 2015) has demonstrated considerable heterogeneity in ADNI neuropsychological profiles despite the vast majority of individuals receiving a conventional “amnestic MCI” diagnosis. Furthermore, recent research also indicates that the “pure” AD pathology targeted by ADNI is less common than multiple underlying neuropathologies (Schneider et al., 2009; Wilson et al., 2013; Zlokovic, 2011), providing further support for using comprehensive neuropsychological assessment to classify MCI across multiple cognitive domains.
Unsurprisingly, results of the current study are similar to the cluster subgroups found by Edmonds et al. (2015), who reported analogous amnestic MCI (34.9%), dysexecutive MCI (12.5%), and cluster-derived normal (34.2%) subtypes. Although our amnestic MCI class was much larger (56.5%), the LPA-derived normal class was comparable in size (30.4%). The mixed MCI class (13.2%) appears to correspond to the dysexecutive MCI group in Edmonds et al. (2015), with analogous size and performance. However, Edmonds et al. (2015) also found a fourth dysnomic/amnestic MCI subtype. There are two possibilities explaining its absence in our study: 1) The statistical algorithms underlying LPA, which converged on a different solution, and 2) inclusion of visuoconstructional assessment, which revealed more robust impairment in a subset of dysnomic individuals. These subjects may have been reclassified as mixed MCI in our LPA and the remaining dysnomic subjects, lacking adequate differentiation in their scores, were folded into the amnestic class. Additionally, our 3-class solution was very consistent with another ADNI cluster analysis by Bondi et al. (2014) using conventional Petersen/Winblad MCI criteria. They also found three MCI subgroups (amnestic: 56.4%, dysexecutive/mixed: 12.3%, and “false positive” normal: 31.3%) of almost identical size and cognitive profile, along with similar genetic/CSF biomarker associations and longitudinal outcomes.
Overall, the current research appears very consistent with previous findings and further underscores the problem using single test scores, cognitive screening measures, and subjective rating scales in MCI diagnosis. ADNI’s MCI criteria led to “false-positive” diagnoses in approximately a third of the sample; this class performed within-normal limits on all neuropsychological measures, had a lower proportion of AD-positive CSF and genetic biomarkers, and better longitudinal outcomes compared to the other MCI classes, similar to past results (Bondi et al., 2014; Edmonds et al., 2015). Although our amnestic and mixed MCI classes demonstrated unique neuropsychological profiles, the groups only differed in total MMSE score and rate of conversion to AD among all CSF/genetic biomarker, ADNI diagnostic, and longitudinal outcomes. These results raise the possibility that the amnestic and mixed MCI classes may represent stages of disease progression. A recent analysis of cortical atrophy patterns among Edmond et al’s. (2015) cluster-defined MCI subtypes supports such speculation, as the authors demonstrated distinct but overlapping profiles of cortical thinning consistent with their neuropsychological performance (Edmonds et al., 2016). Taken together, our results advocate for comprehensive neuropsychological assessment in the clinical and research diagnosis of MCI, which has been shown to improve MCI classification, associations with AD biomarkers, and longitudinal outcomes (Bondi et al., 2014). Additionally, the neuropsychological profiles derived from our LPA may provide useful prototypic models of performance for clinicians attempting to identify varying levels of disease burden and risk of progression to AD in non-demented individuals.
Despite the majority of similarities, a few notable differences were present in this study compared to past work (Bondi et al., 2014; Edmonds et al., 2015). Most importantly, the LPA-derived normal class yielded a rate of dementia progression (5.8%) that is almost half of Edmonds et al.’s (2015) finding (10.7%) as well as Bondi et al.’s (2014) results (9.3%). This significant result suggests our LPA methods further improved classification accuracy and are preferential to cluster analysis in future classification research. Another unique finding in our study was the disproportionate representation of the LPA-derived normal class by ADNI phase; fewer such individuals were enrolled during ADNI-1 than other classes, though significantly more were recruited in ADNI-GO. This shift likely reflects ADNI-GO’s efforts to focus on “early” MCI (Aisen et al., 2010). However, without the incorporation of comprehensive neuropsychological assessment to inform diagnosis, ADNI may have unintentionally recruited cognitively normal individuals erroneously identified as “early” MCI. Such misclassification has considerable implications for MCI research, where inaccurate diagnosis will increase the likelihood of Type-II errors, attenuate effects sizes, and reduce the efficacy of pharmacologic interventions.
Strengths of the current study include its large sample size, neuropsychological representation of four major cognitive domains including visuoconstructional assessment, availability of longitudinal clinical follow-up, CSF AD-biomarkers, APOE e4 genotyping, and the use of a robust normal control group to standardize performance. Additionally, LPA is a novel statistical technique in the MCI classification literature; previous studies have employed traditional cluster analysis (Clark et al., 2013; Delano-Wood et al., 2009; Edmonds et al. 2015; Libon et al., 2010), although it has been used in neuropsychological studies of dementia (Libon et al., 2014), elderly normal (Hayden et al., 2014), and subjective cognitive complaint populations (Köhler et al., 2013). Limitations of our study include the lack of a diverse set of visuospatial measures in ADNI (i.e., no available tests of visuoperceptual relationships, block construction, etc.), the multi-factorial nature of visuoconstructional assessment, the non-normative distributions of some neuropsychological tests and the use of transformations on select variables to normalize distributions in the robust normal control group, and lack of clear convergence among LPA fit indices on a best-fitting model. Furthermore, cross-sectional studies are unable to properly answer questions regarding the longitudinal patterns of cognitive decline in MCI subtypes. Future research should utilize longitudinal multivariate methods such as latent transition analysis (Collins & Lanza, 2013) and growth mixture modeling (Berlin, Parra & Williams, 2014) to better understand the stability and trajectory of MCI classes over time in conjunction with biomarker, neuroimaging, and genetic data.
Supplementary Material
Acknowledgments
Study funding: This study was supported by National Institutes of Health grants R01 AG049810 (M.W.B.) and K24 AG026431 (M.W.B.). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Supplemental Data, electronic file name: Eppig_LPASupplementalMaterial
Disclosure: The authors report no disclosures.
References
- Ahmed S, Brennan L, Eppig J, Price CC, Lamar M, Delano-Wood L, Jak A. Visuoconstructional impairment in subtypes of mild cognitive impairment. Applied Neuropsychology: Adult. 2016;23(1):43–52. doi: 10.1080/23279095.2014.1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Jack CR. Clinical core of the Alzheimer’s Disease Neuroimaging Initiative: Progress and plans. Alzheimer’s & Dementia. 2010;6(3):239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Disease Neuroimaging Initiative. ADNI 2 Procedures Manual. 2008 http://adni.loni.usc.edu/wp-content/uploads/2008/07/adni2-procedures-manual.pdf.
- Asparouhouv T, Muthén B. Online technical appendix. Los Angeles, CA: Muthén & Muthén; 2007. Wald test of mean equality for potential latent class predictors in mixture modeling. Available for download at https://www.statmodel.com/download/MeanTest1.pdf. [Google Scholar]
- Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Structural Equation Modeling: A Multidisciplinary Journal. 2014;21(3):329–341. doi: 10.1080/10705511.2014.915181. [DOI] [Google Scholar]
- Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Using the BCH method in Mplus to estimate a distal outcome model and an arbitrary secondary model. Mplus Web Notes. 2015;21(2):1–22. Available for download at https://www.statmodel.com/examples/webnotes/webnote21.pdf. [Google Scholar]
- Bakk Z, Vermunt JK. Robustness of stepwise latent class modeling with continuous distal outcomes. Structural Equation Modeling: A Multidisciplinary Journal. 2016;23(1):20–31. doi: 10.1080/10705511.2014.955104. [DOI] [Google Scholar]
- Berlin KS, Parra GR, Williams NA. An introduction to latent variable mixture modeling (part 2): Longitudinal latent class growth analysis and growth mixture models. Journal of Pediatric Psychology. 2014;39(2):188–203. doi: 10.1093/jpepsy/jst085. [DOI] [PubMed] [Google Scholar]
- Berlin KS, Williams NA, Parra GR. An introduction to latent variable mixture modeling (part 1): Overview and cross-sectional latent class and latent profile analyses. Journal of Pediatric Psychology. 2014;39(2):174–187. doi: 10.1093/jpepsy/jst084. [DOI] [PubMed] [Google Scholar]
- Bolck A, Croon M, Hagenaars J. Estimating latent structure models with categorical variables: One-step versus three-step estimators. Political Analysis. 2004;12(1):3–27. doi: 10.1093/pan/mph001. [DOI] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Salmon DP. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease. 2014;42(1):275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray BC, Lanza ST, Tan X. Eliminating bias in classify-analyze approaches for latent class analysis. Structural equation modeling: a multidisciplinary journal. 2015;22(1):1–11. doi: 10.1080/10705511.2014.935265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Bondi MW. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society. 2013;19(06):635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL, Muthén B. Relating latent class analysis results to variables not included in the analysis. 2009 Submitted for publication. Available for download at https://www.statmodel.com/download/relatinglca.pdf.
- Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. The Lancet Neurology. 2012;11(2):170–178. doi: 10.1016/S1474-4422(11)70289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Schott JM, Rabinovici GD, Boeve BF, Cappa SF, Dickerson BC, Mendez MF. Shining a light on posterior cortical atrophy. Alzheimer’s & Dementia. 2013;9(4):463–465. doi: 10.1016/j.jalz.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Sacco J, Abeles NORM, Jak AJ, Libon DJ, Bozoki A. Heterogeneity in mild cognitive impairment: Differences in neuropsychological profile and associated white matter lesion pathology. J Int Neuropsychol Soc. 2009;15(6):906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR Alzheimer’s Disease Neuroimaging Initiative. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & Dementia. 2015;11(4):415–424. doi: 10.1016/j.jalz.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L Alzheimer’s Disease Neuroimaging Initiative. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology. 2016;87(20):2108–2116. doi: 10.1212/WNL.0000000000003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Boeve BF, Graff-Radford NR, Lucas JA, Knopman DS, Dickson DW. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. The Clinical Neuropsychologist. 2006;20(4):623–636. doi: 10.1080/13854040500376831. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Kantarci K, Boeve BF, Pankratz VS, Dickson DW, Pedraza O. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013;81(23):2032–2038. doi: 10.1212/01.wnl.0000436942.55281.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L, Dexter LE. Visuospatial ability in cortical dementia. Journal of clinical and experimental neuropsychology. 1991;13(5):677–690. doi: 10.1080/01688639108401082. doi: http://dx.doi.org/10.1080/01688639108401082. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS. Visuospatial dysfunction in the neurodegenerative diseases. Frontiers in bioscience: A journal and virtual library. 2003;8:e428–436. doi: 10.2741/1143. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Grossi D, Trojano L. Constructional and visuospatial disorders. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 4. Amsterdam, Netherlands: Elsevier Science, B.V; 2001. p. 99.p. 120. [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, Thal LJ. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22(6):729–737. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Kuchibhatla M, Romero HR, Plassman BL, Burke JR, Browndyke JN, Welsh-Bohmer KA. Pre-clinical cognitive phenotypes for Alzheimer disease: a latent profile approach. The American Journal of Geriatric Psychiatry. 2014;22(11):1364–1374. doi: 10.1016/j.jagp.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JR, Bauer DJ. Local solutions in the estimation of growth mixture models. Psychological methods. 2006;11:36–53. doi: 10.1037/1082-989X.11.1.36. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Cosentino SA, Ball SK, Bogdanoff B, Leopold N, Kaplan E, Libon DJ. Errors produced on the mini-mental state examination and neuropsychological test performance in Alzheimer’s disease, ischemic vascular dementia, and Parkinson’s disease. The Journal of neuropsychiatry and clinical neurosciences. 2002;14(3):311–320. doi: 10.1176/jnp.14.3.311. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65(8):1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL. Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy. Alzheimer disease and associated disorders. 2009;23(4):365–370. doi: 10.1097/WAD.0b013e3181b5065d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S, Hamel R, Sistermans N, Koene T, Pijnenburg YA, van der Flier WM, Ramakers I. Progression to dementia in memory clinic patients without dementia A latent profile analysis. Neurology. 2013;81(15):1342–1349. doi: 10.1212/WNL.0b013e3182a82536. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Tan X, Bray BC. Latent class analysis with distal outcomes: A flexible model-based approach. Structural equation modeling: a multidisciplinary journal. 2013;20(1):1–26. doi: 10.1080/10705511.2013.742377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Libon DJ, Drabick DA, Giovannetti T, Price CC, Bondi MW, Eppig J, Nation DA. Neuropsychological syndromes associated with Alzheimer’s/vascular dementia: a latent class analysis. Journal of Alzheimer’s Disease. 2014;42(3):999–1014. doi: 10.3233/JAD-132147. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Eppig J, Wicas G, Lamar M, Lippa C, Wambach DM. The heterogeneity of mild cognitive impairment: A neuropsychological analysis. Journal of the International Neuropsychological Society. 2010;16(01):84–93. doi: 10.1017/S1355617709990993. [DOI] [PubMed] [Google Scholar]
- Magidson J, Vermunt J. Latent class models for clustering: A comparison with K-means. Canadian Journal of Marketing Research. 2002;20(1):36–43. doi: 10.1.1.128.9157. [Google Scholar]
- Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment Getting lost between aging and AD. Neurology. 2003;60(5):802–808. doi: 10.1212/01.WNL.0000049471.76799.DE. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Lennox G. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB) Report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/WNL.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Kantarci K. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133(2):540–556. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B. Latent variable analysis. In: Kaplan D, editor. The Sage handbook of quantitative methodology for the social sciences. Thousand Oaks, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Nielson KA, Cummings BJ, Cotman CW. Constructional apraxia in Alzheimer’s disease correlates with neuritic neuropathology in occipital cortex. Brain research. 1996;741(1):284–293. doi: 10.1016/S0006-8993(96)00983-3. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling. 2007;14(4):535–569. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of internal medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Trojanowski JQ. Alzheimer’s disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of neurology. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: A commentary on Nooner et al., Pears et al., and looking beyond. Child abuse & neglect. 2010;34(3):155–160. doi: 10.1016/j.chiabu.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett D. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of neurology. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao A, Liang L, Yuan C, Bian Y. A latent class analysis of bullies, victims and aggressive victims in chinese adolescence: relations with social and school adjustments. PloS one. 2014;9(4):e95290. doi: 10.1371/journal.pone.0095290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of neurology. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tein JY, Coxe S, Cham H. Statistical power to detect the correct number of classes in latent profile analysis. Structural equation modeling: a multidisciplinary journal. 2013;20(4):640–657. doi: 10.1080/10705511.2013.824781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley Publishing Company; 1977. [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Morris JC. The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimer’s & Dementia. 2013;9(5):e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA neurology. 2013;70(11):1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Arai H. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of internal medicine. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.