Abstract
Bilateral adrenal hemorrhage is a rare condition, which is burdened by potentially life-threatening consequences related to the development of acute adrenal insufficiency. Despite treatment with stress-dose glucocorticoids, a mortality rate of 15% has been reported, which varies according to the severity of underlying predisposing illness and could be much more higher if the adrenal insufficiency is not promptly recognized. An early diagnosis is crucial, though, because of nonspecific clinical and laboratory findings, adrenal hemorrhage is rarely suspected. Therefore, imaging has a pivotal role for the diagnosis of this uncommon condition but, despite adrenal hematomas characteristically appear round or oval with peripheral fat stranding, their initial presentation could be ambiguous. The authors describe a case of postoperative bilateral adrenal hemorrhage initially presenting at computed tomography scan as thickening of both glands surrounded by fat stranding, which led to close monitoring of adrenal function before unequivocal hemorrhage developed.
Keywords: Adrenal hemorrhage, Computed tomography, Postoperative, Glucocorticoids
Introduction
Bilateral adrenal hemorrhage is a life-threatening condition leading to adrenal crisis and potentially to death if not early treated [1], [2]. The exact pathogenesis is unknown but is thought to be related to the physiologic increase of vascularization of adrenal glands in response to stressful events [2], [3]. Vascular congestion may occasionally cause hemorrhage, very rare bilateral. Several conditions determine such stress response, among which the most important risk factors seem to be postoperative status, thromboembolic disease, and coagulopathy [3]. Most of patients presenting with adrenal hemorrhage are already affected by severe conditions; therefore, it is difficult to recognize signs and symptoms of an incoming adrenal crisis [2], [3], [4], [5], [6]. When suspected, the diagnosis is confirmed whit adrenocorticotropic hormone (ACTH) test, which however is not always feasible in unstable patients, and computed tomography (CT), which shows the adrenal injury. CT is considered pivotal to early diagnose adrenal hemorrhage and start a prompt corticosteroid replacement therapy [2], [5], [7].
Case report
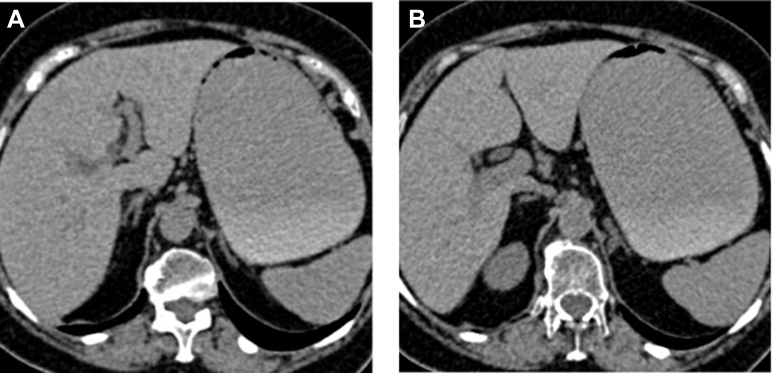
A 76-year-old woman underwent a pancreaticoduodenectomy with Whipple procedure for a malignant ampulloma. On the fifth postoperative day, the patient developed an acute epigastric pain with nausea, vomiting, and pyrexia (38.6°C) suggestive of postoperative complications. Therefore, a contrast-enhanced CT scan of the abdomen was performed and revealed nonspecific inflammatory stranding surrounding mildly enlarged adrenal glands (Fig. 1), which appeared normal at preoperative CT examination (Fig. 2). No anastomotic leak or other postsurgical complications were detected. Despite leukocyte count was within normal range, clinical suspect of systemic infection led to begin empiric antibiotic therapy, waiting for specific bacterial cultures. However, the patient continued suffering from epigastric pain, and in addition, on the 13th postoperative day, she presented with hemoglobin drop (120 g/dL), hypotension, hypovolemia, and tachycardia (130 bpm) with subtle electrolyte abnormalities (sodium 126 mmol/L; potassium 6.9 mmol/L) and low serum cortisol level (5 mcg/dL) with ACTH >300 ng/L, suggestive of adrenal crisis.
Fig. 1.
Computed tomography, axial image, portal phase showed bilateral (A: right; B: left) mild adrenal enlargement (stars) with surrounding fat stranding (arrows).
Fig. 2.
Unenhanced computed tomography (preoperative), axial image, showed normal right (A) and left (B) adrenal glands.
A new CT scan revealed bilateral mainly hypodense ovoid adrenal masses, which did not change after contrast administration, in keeping with organizing adrenal hemorrhages (Fig 3). Consequently, diagnosis of adrenocortical insufficiency secondary to bilateral adrenal hemorrhage related to postsurgical stress was made. Medical treatment, with intravenous hydrocortisone (initially, an intravenous bolus of 100-mg hydrocortisone followed by 200 mg over 48-hour continuous infusion) and correction of hypovolemia with isotonic saline (1000-mL isotonic saline within the first hour), resulted in a rapid improvement in her electrolyte imbalance with satisfactory outcome and no further abdominal symptoms. The patient made a good recovery and was discharged after 2 weeks on a reducing dose of steroids. After 6 months, the patient did not report any episode of adrenal insufficiency and a magnetic resonance imaging showed normal appearance of adrenal glands (Fig. 4).
Fig. 3.
Computed tomography, axial image, portal phase showed ovoid mainly hypodense right (A) and left (B) adrenal masses (25-30 UH) (arrows) suggestive for organizing hemorrhage.
Fig. 4.
MRI, axial image, at 6-month follow-up, showed normal volume of both right (A) and left (B) adrenal glands (arrows). MRI, magnetic resonance imaging.
Discussion
Bilateral adrenal hemorrhage is a rare condition with an estimated incidence of 0.14%-1.8% based on postmortem studies [1]. This infrequent clinical entity is burdened by potentially life-threatening consequences related to the development of acute adrenal insufficiency, when at least 90% of glands are injured [2]. Despite treatment with stress-dose glucocorticoids, some patients with adrenal hemorrhage may die because of underlying disease or diseases associated with the adrenal hemorrhage itself. Overall, a mortality rate of 15% has been reported; however, it varies according to the severity of the underlying predisposing illness and could be much more higher if the adrenal insufficiency is not promptly diagnosed [1], [4], [5].
The exact pathophysiology of bilateral adrenal hemorrhage is still uncertain. Cortisol has many important metabolic and endocrine functions, during stressful events in particular [6]. The stress-induced adrenal hemorrhage is hypothesized to be an exacerbation of the physiological combination of increased arterial blood flow to the adrenal gland and slow drainage by relatively few venules and a single adrenal vein, leading to intraglandular vascular congestion and possible subsequent hemorrhage [2], [3]. This dramatic event may manifest because of several predisposing factors, among which stress caused by surgery, severe illness, overwhelming sepsis, burns, hemorrhagic diatheses (e.g., anticoagulant use, thrombocytopenia), thromboembolic disease (including antiphospholipid antibody syndrome) are the main nontraumatic ones [2], [3], [4], [7], [8], [9].
Because of nonspecific clinical and laboratory findings, adrenal hemorrhage is rarely suspected. The most frequently described signs are abdominal pain, vomit, fever, weakness, severe hypotension and altered conscious state, which often overlap with other concurrent severe illness making difficult the diagnosis [3], [4], [5], [6]. Fever is the most common physical sign occurring in about 70% of cases [2]. Hypotension is not often seen before the development of dramatic hypotension and shock [2], [3].
Given these difficulties, in the past, primary adrenal insufficiency secondary to adrenal hemorrhage was almost exclusively diagnosed on postmortem examination [3], [9]. Even in the recent literature, several authors describe cases of misdiagnosed adrenal crisis especially in patients in their early postoperative period. Actually, likewise in our case, abdominal pain, fever, and hypotension are frequently misinterpreted as sepsis related to an anastomotic dehiscence [10], [11].
Similarly, laboratory findings are not invariably present. Hyponatremia, hyperkalemia, and hypoglycemia are found in both adrenal insufficiency and other conditions [3], [4], [5], [6], [8]. Although the combination of low serum sodium and high serum potassium is suggestive of adrenal insufficiency in the appropriate clinical setting, their absence should not exclude this diagnosis [3]. The hormonal diagnosis is confirmed by serum cortisol and plasma ACTH. In acutely conditions, the combination of increased plasma ACTH and low or even low-normal (ie, <13 mcg/dL) serum cortisol levels is highly suggestive of glucocorticoid deficiency due to primary adrenal insufficiency. The short adrenal stimulation test confirms the diagnosis of adrenal insufficiency; however, it could not be performed in acute setting. In these cases, basal cortisol and ACTH levels are enough to start glucocorticoid replacement therapy [2], [3], [9], [12].
The recent literature widely describes the crucial role of imaging, CT in particular, to confirm the diagnosis of adrenal hemorrhage. However, although adrenal hematomas are most frequently described as round or oval masses involving the glands, there are also other possible appearances. For example, an organized adrenal hematoma may appear with hypodense fluid central area with or without calcifications [2], [5], [7]. In these cases, particularly when unilateral, differential diagnosis with bleeding malignancies could be difficult. Actually, both benign and malignant adrenal conditions may present with massive bleeding or evolve in partially fluid lesions. A careful follow-up should demonstrate the typical decrease of volume of idiopathic hemorrhage in contrast with tumors, whereas in some cases, resection could be necessary [2], [7]. Furthermore, adrenal hemorrhage is suspected when there is ill-defined soft-tissue stranding around adrenal gland, being the consequence of infiltration of blood through the retroperitoneal fat [5]. Another possible pattern of presentation is thickening of adrenal glands, as it was described by Sacerdote et al. and Tan et al. These latter report cases of thickened adrenal glands, which only later manifested as unequivocal hematomas [8]. They interpreted this finding as an early stage of adrenal injury, supporting the current hypothesis of vascular congestion within the adrenal glands as an early stage of nontraumatic adrenal hemorrhage [5], [8]. Likewise, in our case, first CT evaluation demonstrated diffuse slight thickening of both adrenal glands with periadrenal fat stranding, which was misinterpreted. Actually, several authors refer the specific finding of adrenal enlargement in oncologic patients to malignancy-induced hyperplasia [13]. However, in the case we describe, preoperative CT examination showed normal glands, whereas they were found enlarged only after surgery, when the patient already presented with initial signs of adrenal insufficiency.
In cases like this, a continuous monitoring of hormone levels and imaging follow-up is justified to assess the straightforward development of adrenal hemorrhage as soon as possible and enable prompt steroid replacement therapy [14].
In the acute phase, the preferred treatment is hydrocortisone (initially 100-mg intravenous bolus followed by 200-300 mg over 24-48 hours as continuous infusion) and isotonic saline (1000 mL within the first hour) [12]. After the acute adrenal hemorrhagic event, long-term glucocorticoid replacement with or without mineralocorticoid replacement therapy may be necessary, based on the results of adrenal function test [3], [4].
In conclusion, in spite of ambiguous initial presentation of adrenal insufficiency, particularly when it overlaps manifestation of concurrent severe illness or possible postsurgery complications, an early diagnosis is crucial to avoid catastrophic consequences of adrenal crisis. Therefore, this diagnosis requires a high index of clinical suspicion and the rapid confirmation with CT of the abdomen. It is essential to identify early signs of adrenal congestion, such as gland thickening with surrounding fat stranding, to undertake a careful monitoring of the patient and eventually prompt replacement glucocorticoid therapy.
Footnotes
Competing Interests: The authors have declared that no competing interest exists.
References
- 1.Kovacs K., Lam Y., Pater J. Bilateral massive adrenal hemorrhage: assessment of putative risk factors by the case-control method. Medicine (Baltimore) 2001;80:45–53. doi: 10.1097/00005792-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kawashima A., Sandler C.M., Ernst R.D., Takahashi N., Roubidoux M.A., Goldman S.M. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949–963. doi: 10.1148/radiographics.19.4.g99jl13949. [DOI] [PubMed] [Google Scholar]
- 3.Rao R.H., Vagnucci A.H., Amico J.A. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. 1989;110(3):227–235. doi: 10.7326/0003-4819-110-3-227. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberger L.H., Smith P.W., Sawyer R.G., Hanks J.B., Adams R.B., Hedrick T.L. Bilateral adrenal hemorrhage: the unrecognized cause of hemodynamic collapse associated with heparin-induced thrombocytopenia. Critical Care Medicine. 2011;39:833–838. doi: 10.1097/CCM.0b013e318206d0eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacerdote M.G., Johnson P.T., Fishman E.K. CT of the adrenal gland: the many faces of adrenal hemorrhage. Emerg Radiol. 2012;19(1):53–60. doi: 10.1007/s10140-011-0989-9. [DOI] [PubMed] [Google Scholar]
- 6.Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol. 2015;172(3):R115–R124. doi: 10.1530/EJE-14-0824. [DOI] [PubMed] [Google Scholar]
- 7.Jordan E., Poder L., Courtier J., Sai V., Jung A., Coakley F.V. Imaging of nontraumatic adrenal hemorrhage. AJR Am J Roentgenol. 2012;199(1):W91–W98. doi: 10.2214/AJR.11.7973. [DOI] [PubMed] [Google Scholar]
- 8.Tan G.X., Sutherland T. Adrenal congestion preceding adrenal hemorrhage on CT imaging: a case series. Abdom Radiol (NY) 2016;41(2):303–310. doi: 10.1007/s00261-015-0575-9. [DOI] [PubMed] [Google Scholar]
- 9.Siu S.C., Kitzman D.W., Sheedy P.F., 2nd, Northcutt R.C. Adrenal insufficiency from bilateral adrenal hemorrhage. Mayo Clin Proc. 1990;65(5):664–670. doi: 10.1016/s0025-6196(12)65129-5. [DOI] [PubMed] [Google Scholar]
- 10.McNicol R.E., Bradley A., Griffin J., Duncan G., Eriksen C.A., Guthrie G.J. Post-operative bilateral adrenal haemorrhage: a case report. Int J Surg Case Rep. 2014;5(12):1145–1147. doi: 10.1016/j.ijscr.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peel N., Whitelaw S.C. Bilateral adrenal haemorrhage following right hemicolectomy. Int J Colorectal Dis. 2011;26(5):681–682. doi: 10.1007/s00384-010-1035-1. [DOI] [PubMed] [Google Scholar]
- 12.Bornstein S.R., Allolio B., Arlt W., Barthel A., Don-Wauchope A., Hammer G.D. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahdev A., Reznek R.H. The indeterminate adrenal mass in patients with cancer. Cancer Imaging. 2007;7:S100–S109. doi: 10.1102/1470-7330.2007.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrugia F.A., Martikos G., Surgeon C., Tzanetis P., Misiakos E., Zavras N. Radiology of the adrenal incidentalomas. Review of the literature. Endocr Regul. 2017;51(1):35–51. doi: 10.1515/enr-2017-0005. [DOI] [PubMed] [Google Scholar]