Abstract
Solid variant aneurysmal bone cyst is a rare benign bone lesion, representing a small fraction of all aneurysmal bone cysts. The imaging appearance and histologic features may overlap with other benign and malignant neoplasms, posing a diagnostic dilemma for clinicians, pathologists, and radiologists. We present a case of solid variant aneurysmal bone cyst of the distal fibula and review the radiologic and histologic features important for diagnosis.
Keywords: Solid ABC, Imaging characteristics, Histologic correlation
Introduction
Solid variant aneurysmal bone cyst (S-ABC) is a rare benign bone lesion, representing a small fraction of all aneurysmal bone cysts. Although S-ABC has historically been considered a reactive lesion, more recent studies suggest a chromosomal translocation. The imaging appearance and histologic features of S-ABC including solid internal enhancing architecture may overlap with other benign and malignant neoplasms such as giant-cell tumor (GCT) and telangiectatic osteosarcoma, posing a diagnostic dilemma for clinicians, pathologists, and radiologists [1], [2].
Case report
A 34-year-old woman with no pertinent past personal or family medical history presented with progressively worsening distal left leg pain over the past 5 weeks. She denied any history of trauma. Physical examination was notable for tenderness along the lateral aspect of the distal left leg. There was no palpable abnormality, swelling, or erythema appreciated at physical examination. Laboratory studies were noncontributory. Diagnostic radiographs were initially obtained following by advanced imaging examinations.
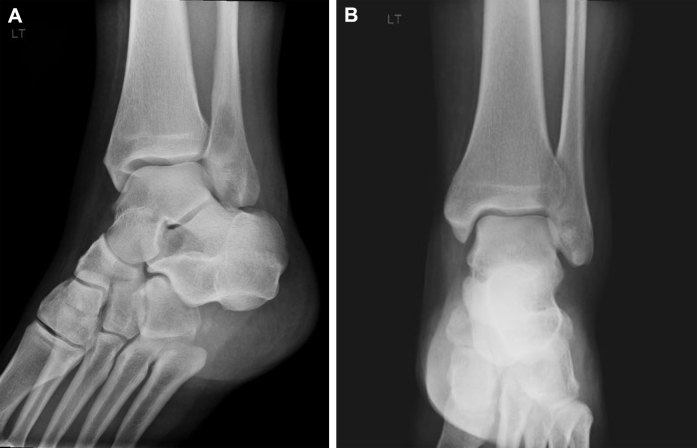
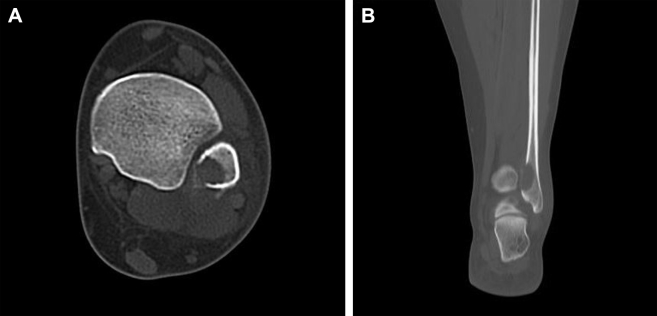
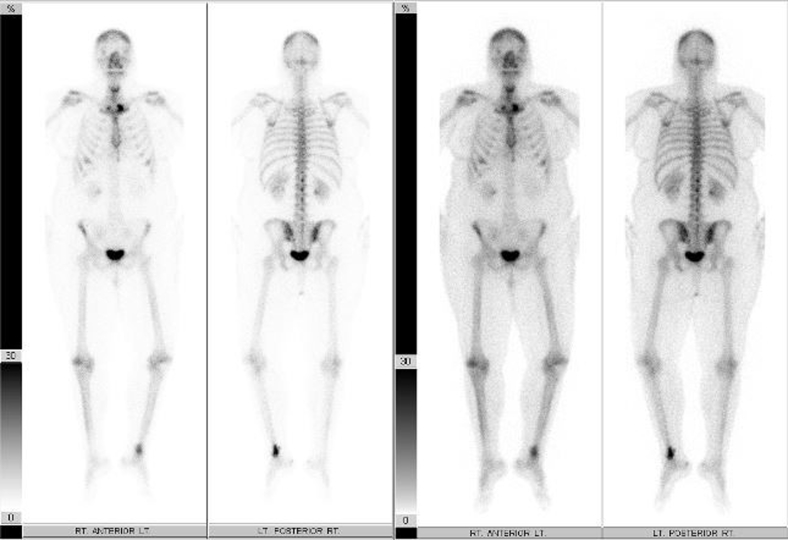
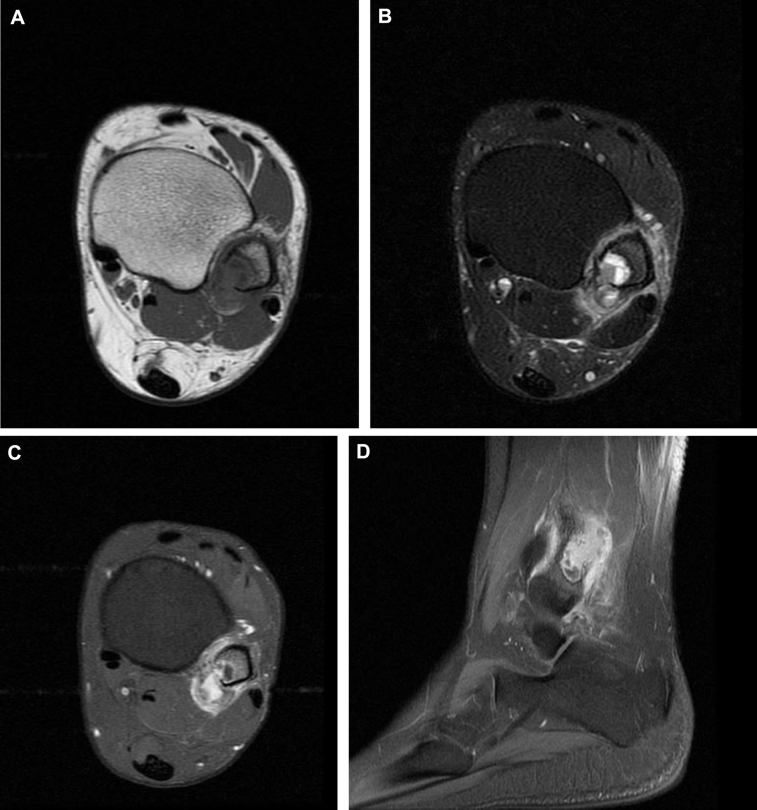
Radiographs revealed a well-demarcated geographic osteolytic lesion without marginal sclerosis involving the distal fibular metaphysis. Medial cortical disruption was suspected (Fig. 1), and therefore, cross-sectional imaging was recommended. Computed tomography confirmed posteromedial cortical discontinuity with extension into the adjacent soft tissues. There was no suspicious periosteal reaction or internal matrix appreciated (Fig. 2). Technetium 99m-HDP bone scan demonstrated a solitary focus of increased osteoblastic activity involving the distal left lower extremity (Fig. 3). Magnetic resonance imaging (MRI) demonstrated an aggressive-appearing mixed solid and cystic enhancing metaphyseal lesion with posterior cortical destruction, soft-tissue extension of disease, and surrounding inflammatory changes. The lesion was predominantly isointense to skeletal muscle on T1-weighted imaging with solid and cystic components and peritumoral edema on T2-weighted imaging. Following intravenous gadolinium administration, the lesion demonstrated avid enhancement of solid nodular components (Fig. 4). These findings raised concern for telangiectatic osteosarcoma, and therefore, the patient was referred for tissue sampling.
Fig. 1.
Oblique (A) and anteroposterior (AP) (B) radiographs of the left ankle demonstrate a geographic lytic lesion without marginal sclerosis involving the distal fibular metaphysis with possible cortical disruption.
Fig. 2.
Axial (A) and coronal (B) unenhanced CT images demonstrate the lytic bone lesion without any internal mineralization and confirm posteromedial cortical breakthrough with extra-osseous extension of disease. CT, computed tomography.
Fig. 3.
Technetium-99m hydroxymethylene diphosphonate (Tc99m-HDP) whole-body bone scan demonstrates increased osteoblastic activity in the distal left leg.
Fig. 4.
Axial T1-weighted (A), fat-suppressed T2-weighted (B), axial (C), and sagittal (D) contrast-enhanced magnetic resonance images demonstrate a solid and cystic distal fibular lesion with cortical breakthrough, an extra-osseous mass, and surrounding inflammatory changes. Note T2-weighted hyperintense nonenhancing cystic areas, and moderately T2-weighted hyperintense enhancing solid nodular elements.
Ultrasound-guided core needle biopsy was performed revealing proliferation of mononuclear cells among numerous macrophages and large osteoclast-like giant cells with associated osteoid formation consistent with a giant-cell rich neoplasm. There was no pleomorphism, atypia, or necrosis identified. The pathologic differential diagnosis included atypical GCT, aneurysmal bone cyst, and nonossifying fibroma, but telangiectatic and giant-cell rich osteosarcoma could not be excluded, though felt to be less likely given absence of significant pleomorphism and atypical mitoses. For this reason, a more definitive open surgical biopsy was performed. Immunohistochemical studies performed on this specimen revealed cells positive for p63 and negative for p53 and AE1/AE3/CAM. Molecular testing demonstrated a rearrangement of the USP6 (17p13) gene region resulting in a final diagnosis of solid variant aneurysmal bone cyst.
With a diagnosis of S-ABC, the patient returned to the operating room for curettage of the entire tumor and bone graft augmentation. To date, the patient remains asymptomatic and without evidence of local tumor recurrence.
Discussion
Conventional aneurysmal bone cyst (ABC) is a benign cystic lesion of bone composed of blood-filled spaces separated by connective tissue septa. They may arise as a primary or secondary lesion when associated with other bone tumors such as GCT for example. The much rarer solid variant was first described by Sanerkin et al. [3] in 1983, thought to be a reactive response to intraosseous hemorrhage [4]. The term S-ABC has been used interchangeably with a histologically similar entity, giant-cell reparative granuloma. Giant-cell reparative granuloma is commonly used to describe lesions involving the short tubular bones of the hands and feet or craniofacial skeleton, while lesions occurring within the appendicular long bones are referred to as S-ABC. Both demonstrate similar histologic features and have generally been considered to be reactive and nonneoplastic [1]. However, recent studies suggest the pathogenesis may relate to chromosomal translocations involving the USP6 gene on chromosome 17p13 [5]. Presentation is typically in the second or third decade with pain and swelling being common symptoms. There is a slight female predominance of 1.5:1 [6]. Most reported cases of S-ABC describe an eccentric cortically based lytic bone lesion involving the metaphysis or diaphysis of long bone. Differential considerations include ABC, GCT, and telangiectatic osteosarcoma.
Radiographically, S-ABC typically presents as an eccentric expansile lytic bone lesion with a narrow zone of transition. Similar to ABC, some degree of partial or circumferential marginal sclerosis is often present, whereas there is a notable absence of internal mineralization or suspicious periosteal reaction [7]. In contrast, GCT tends to occur in a slightly older patient population, typically does not demonstrate marginal sclerosis, and often extends to the subchondral end of the host bone. As with other subtypes of osteosarcoma, telangiectatic osteosarcoma typically presents with less well-defined margins, some degree of radiodense osteoid formation and irregular periosteal reaction on diagnostic radiographs [1].
Although the radiographic appearance of S-ABC may be similar to ABC, MRI may allow for differentiation. At MRI, S-ABC typically demonstrates slightly hypointense signal on T1-weighted imaging with a more heterogeneous, predominantly hyperintense appearance on T2-weighted imaging. Following contrast administration, these lesions are mostly solid and may or may not demonstrate scattered nonenhancing cystic areas. These cystic regions, when present, typically lack internal septations commonly identified within conventional ABC. S-ABC most often demonstrates diffuse uniform intravenous contrast enhancement as opposed to a peripheral enhancement pattern often seen in ABC [6]. Fluid-fluid levels are also less commonly seen in S-ABC as compared to ABC. Surrounding bone marrow and soft-tissue edema has been reported in approximately 50% of cases [1], although the etiology remains unclear, possibly related to associated cortical fractures.
S-ABC is histologically characterized by fibroblastic proliferation without cellular or nuclear pleomorphism. Other features include areas of osteoclast-like giant cells, foci of calcifying fibromyxoid tissue, osteoblastic differentiation with osteoid production, and aneurysmal sinusoids [3], [8]. S-ABC typically lacks the cavernous spaces seen in ABC [6]. Lack of cellular atypia is important in excluding malignancy such as osteosarcoma [9]. Helpful histologic features differentiating S-ABC from GCT include the abundance of fibromyxoid tissue and presence of osteoid seen with S-ABC [7]. In addition, although GCT typically demonstrates a uniform distribution of giant cells, S-ABC more often presents with scattered or clustered giant cells [10]. In addition to immunohistochemical features and chromosomal abnormality present in this case, Takechi et al. report a case of S-ABC of the tibia which expressed cyclooxygenase-2 in specimen spindle and giant cells [8].
Curettage and bone augmentation is the treatment of choice for conventional ABC. Although the treatment of S-ABC varies slightly due to an increased risk of local recurrence compared with ABC, these lesions have been previously treated with curettage/adjuvant therapy with augmentation and/or surgical resection [8], [11].
Conclusion
S-ABC is a rare variant of benign aneurysmal bone cyst often demonstrating confounding imaging characteristics including a nonaggressive radiographic appearance but solid internal architecture and avid contrast enhancement at MRI contributing to diagnostic challenges. Furthermore, the presence of solid and cystic elements, cortical disruption, and extra-osseous extension as seen in this case may raise concern for telangiectatic osteosarcoma. Finally, some histologic features of S-ABC overlap with other solid bone tumors. As such, it is important for radiologists and pathologists to recognize the imaging, histologic, and chromosomal findings discussed previously to arrive at the correct diagnosis and to reliably exclude other primary bone neoplasms.
References
- 1.Ilaslan H., Sundaram M., Krishnan U.K. Solid variant of aneurysmal bone cysts in long tubular bones: giant cell reparative granuloma. Am J Roentgen. 2003;180(6):1681–1687. doi: 10.2214/ajr.180.6.1801681. [DOI] [PubMed] [Google Scholar]
- 2.Savardekar A.R., Patra D., Chatterjee D., Ahuja C.K., Salunke P. Solid variant of aneurysmal bone cyst presenting as a giant cervical mass: a clinical, radiological, histopathological dilemma. Surg Neurol Int. 2015;6(Suppl 4):S182–S185. doi: 10.4103/2152-7806.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanerkin N.G., Mott M.G., Roylance J. An unusual intraosseous lesion with fibroblastic, osteoclastic, osteoblastic, aneurysmal and fibromyxoid elements. “Solid” variant of aneurysmal bone cyst. Cancer. 1983;51(12):2278–2286. doi: 10.1002/1097-0142(19830615)51:12<2278::aid-cncr2820511219>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Sato K., Sugiura H., Yamamura S., Takahashi M., Nagasaka T., Fukatsu T. Solid variant of an aneurysmal bone cyst (giant cell reparative granuloma) of the 3rd lumbar vertebra. Nagoya J Med Sci. 1996;59:159–165. [PubMed] [Google Scholar]
- 5.Agaram N.P., LeLoarer F.V., Zhang L., Hwang S., Athanasian E.A., Hameed M. USP6 gene rearrangements occur preferentially in giant cell reparative granulomas of the hands and feet but not in gnathic location. Hum Pathol. 2014;45(6):1147–1152. doi: 10.1016/j.humpath.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Shamy G., Relyea K., Adesina A., Whitehead W.E., Curry D.J., Luerssen T.G. Solid variant of aneurysmal bone cyst of the thoracic spine: a case report. J Med Case Reports. 2011;5(1):261. doi: 10.1186/1752-1947-5-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi T., Dorfman H. Giant cell reparative granuloma: a comparative clinicopathologic study of lesions in gnathic and extragnathic sites. Int J Surg Pathol. 2001;9(3):189–200. doi: 10.1177/106689690100900304. [DOI] [PubMed] [Google Scholar]
- 8.Takechi R., Yanagawa T., Shinozaki T., Fukuda T., Takagishi K. Solid variant of aneurysmal bone cyst in the tibia treated with simple curettage without bone graft: a case report. World J Surg Oncol. 2012;10:45. doi: 10.1186/1477-7819-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karampalis C., Lenthall R., Boszczyk B. Solid variant of aneurysmal bone cyst on the cervical spine of a child: case report, differential diagnosis and treatment rationale. Eur Spine J. 2013;22:523–531. doi: 10.1007/s00586-012-2548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lekka J.A., Gavresea T.V., Stanc-Giannakopoulos G.A., Demertzis N.S. Solid variant of aneurysmal bone cyst of the heel: a case report. J Med Case Reports. 2011;5:145. doi: 10.1186/1752-1947-5-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda Y., Tsuneyoshi M., Shinohara N. “Solid” variant of aneurysmal bone cyst (extragnathic giant cell reparative granuloma) in the axial skeleton and long bones. A study of its morphologic spectrum and distinction from allied giant cell lesions. Cancer. 1992;70:2642–2649. doi: 10.1002/1097-0142(19921201)70:11<2642::aid-cncr2820701113>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]