Abstract
Obinutuzumab is a humanized, type II anti-CD20 monoclonal antibody designed for strong induction of direct cell death and antibody-dependent cell-mediated cytotoxicity. The Phase III GADOLIN trial tested the clinical efficacy of obinutuzumab plus bendamustine followed by obinutuzumab monotherapy in rituximab-refractory indolent non-Hodgkin lymphoma versus treatment with bendamustine alone. It demonstrated significantly longer progression-free survival for the obinutuzumab-containing regimen in this difficult to treat patient group. Based on the results of this trial, US FDA approval was most recently granted for obinutuzumab in the treatment of follicular lymphoma that has relapsed after or was refractory to a rituximab-containing regimen. This article summarizes the available data on chemistry, pharmacokinetics, clinical efficacy and safety of obinutuzumab in the treatment of indolent non-Hodgkin lymphoma.
KEYWORDS : anti-CD20 monoclonal antibody, efficacy, indolent lymphoma, obinutuzumab, rituximab-refractoriness, safety
Indolent B-cell non-Hodgkin lymphoma (NHL) is a heterogeneous group of B-cell-derived lymphoproliferative disorders which tend to grow slowly and cause few clinical symptoms. The most common indolent lymphoma is follicular lymphoma (about 22% of all diagnosed NHL according to the WHO), followed by small lymphocytic lymphoma (about 6%), marginal zone B-cell lymphoma (about 6%) and lymphoplasmocytic lymphoma (about 1%). The majority of patients are diagnosed at the advanced stages III and IV. In these stages a ‘watch and wait’ strategy is indicated as long as the patients are asymptomatic and have a low tumor burden [1]. For patients developing a high tumor burden and/or clinical symptoms, chemoimmunotherapy using a combination of the anti-CD20 monoclonal antibody rituximab with chemotherapy [2–4] followed by maintenance therapy with rituximab given every 2 months for a maximum of 2 years [5,6] is the most commonly used first-line therapy in medically fit patients. Chemotherapeutic protocols combined with rituximab are mainly bendamustine, CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CVP (cyclophosphamide, doxorubicin and prednisone) [7,8].
Though periods of prolonged progression-free survival (PFS) can be obtained after first-line treatment with modern treatment regimens, the clinical course of indolent lymphoma is hallmarked by relapses. PFS periods tend to become shorter with multiple relapses. Over the course of the disease, the majority of patients become resistant to treatment with rituximab, meaning that these patients show no response to rituximab or have a response duration of less than 6 months to a previous rituximab-containing therapy. Patients with resistance to rituximab have limited options for further treatment and their prognosis is poor.
Chemoimmunotherapy is also the treatment of choice for chronic lymphocytic leukemia (CLL). Rituximab is approved for the treatment of CLL when used in combination with chemotherapy based upon the results of the German CLL Study Group led CLL8 trial (NCT00281918) [9]. This trial examined the use of rituximab in combination with fludarabine and cyclophosphamide (FCR) and demonstrated a survival advantage of this regimen over FC alone [10,11]. The CLL10 trial of the German CLL Study Group (NCT00769522) [12] compared FCR with bendamustine plus rituximab (BR) in medically fir patients. Its results suggested that elderly fit patients (≥65 years) might benefit from treatment with BR due to a better safety profile of this regimen. Though enrolling only medically fit patients with a score ≤6 on the Cumulative Illness Rating scale (CIRS), 67.8% of patients had severe neutropenia and 26.8% had severe infections [13]. So treatment with FCR or BR is too aggressive for many CLL patients, the majority of whom are elderly and have significant comorbidities with a CIRS score >6. Phase II data demonstrated excellent results using chlorambucil in combination with rituximab for the treatment of CLL in elderly and comorbid patients [14,15]. The CLL11 trial (NCT01010061) [16] of the German CLL Study Group demonstrated that the anti-CD20 monoclonal antibody obinutuzumab was superior to rituximab when each was combined with chlorambucil. Obinutuzumab plus chlorambucil had a favorable safety profile (up to 35% of patients with severe neutropenia and up to 12% with severe infections) [17,18]. The results of this trial led to the licensing of obinutuzumab for the treatment of CLL.
Obinutuzumab is a glycoengineered anti-CD20 monoclonal antibody with enhanced functional activities in the induction of direct cell death and antibody-dependent cell-mediated cytotoxicity [19]. Recently presented data from the interim analysis of the GADOLIN trial (NCT01059630) [20], which tested efficacy of obinutuzumab in combination with bendamustine in rituximab-refractory indolent lymphoma, documented prolonged PFS for patients treated with obinutuzumab and bendamustine compared with bendamustine monotherapy [21]. Obinutuzumab is currently only approved for its use in combination with chlorambucil to treat patients with previously untreated CLL, but results from the GADOLIN trial might lead to a fast approval of obinutuzumab in the treatment of relapsed, rituximab-refractory indolent lymphoma.
Overview of the market
CD20 antigen is an attractive target for treatment with monoclonal antibodies as it is expressed by most B-cell lymphoproliferative disorders. It is also expressed by healthy B cells after their early pro-B phase. Healthy long-lived plasma cells loose expression of CD20 and are therefore not targeted by anti-CD20 monoclonal antibodies [22].
Rituximab was the first monoclonal antibody against CD20 to obtain drug approval by the US FDA in November 1997 and by the European Agency for the Evaluation of Medicinal Products in June 1998. Since then it revolutionized the treatment of B-cell lymphoproliferative disorders, as the mainly immune-mediated mechanisms of action by rituximab improved survival rates in indolent [2–4,22–24] and aggressive lymphoma [25–27] substantially.
Combination of chemotherapy with rituximab in first-line therapy has the potential to induce long-term remissions, as can be seen from results of the PRIMA trial (NCT00140582) [28], where almost 60% of patients treated with chemoimmunotherapy followed by rituximab maintenance remained free of disease progression after 6 years of observation [6]. Despite these improvements, advanced indolent lymphoma (stages III and IV) remains incurable and an unmet clinical need clearly is the treatment of patients with rituximab-refractory indolent lymphoma, since few other treatment options are currently approved.
FDA approval for treatment of relapsed/refractory indolent lymphoma was granted to the radioimmunotherapeutic antibody Yttrium-90 ibritumomab tiuxetan (2002), the chemotherapy drug bendamustine (2008) and the PI3K inhibitor idelalisib (2014 for follicular and small lymphocytic lymphoma) [29,30].
The numbers of investigational drugs recently increased vastly. Among the most promising substances for treatment of relapsed indolent lymphoma are ibrutinib [31,32], venetoclax [33] and lenalidomide [34–37]. Though these drugs might have the potential to help overcome treatment resistance in significant numbers of indolent lymphoma patients, they will most likely be part of combination therapies integrating immune-mediated mechanisms of action such as monoclonal antibodies.
Monoclonal antibodies against other targets than CD20 were developed. Anti-CD19 monoclonal antibodies show efficacy when they are conjugated to either a cytotoxic agent, such as in SAR3419 [38,39], or another antibody fragment, such as in the bispecific T-cell-engaging antibody blinatumomab [40]. Otlertuzumab as anti-CD37 monoclonal antibody [41,42] and lucatumumab as anti-CD40 monoclonal antibody showed modest clinical activity [43,44]. These antibodies remain investigational and CD20 antigen still remains by far the most successful target up to now.
Anti-CD20 antibodies have been optimized to have a higher binding affinity toward CD20 and/or to have enhanced functional activities aiming for improved clinical efficacy. Ofatumumab was designed to bind to a novel, membrane-proximal epitope thereby leading to a stronger induction of complement-dependent cytotoxicity (CDC) compared with rituximab [45]. But despite its higher binding affinity to CD20, ofatumumab did not show superiority over rituximab as single agent in a Phase III clinical trial for follicular lymphoma (HOMER trial, NCT01200589 [46]), as was announced by Genmab® in November 2015.
Results from the GADOLIN trial [20], in which patients with rituximab-refractory indolent lymphoma were either treated with a combination of obinutuzumab and bendamustine followed by obinutuzumab maintenance or with bendamustine alone, showed that obinutuzumab has clinical efficacy in this difficult to treat patient group. Patients treated with obinutuzumab had a benefit in PFS [21] and based on this trial, FDA approval was recently granted for obinutuzumab in the treatment of follicular lymphoma that has relapsed after or was refractory to a rituximab-containing regimen. Results for a direct comparison between the clinical efficacy of obinutuzumab and rituximab in combination with chemotherapy in first-line treatment of indolent lymphoma are not yet available. The GALLIUM trial, NCT01332968 [47] addresses this question and first results can be expected in 2017.
Following FDA approval of obinutuzumab for follicular lymphoma in February this year, it is the aim of this review to summarize the pharmacology of obinutuzumab as well as its clinical efficacy and safety in the treatment of indolent lymphoma and to evaluate its future role in the treatment of this lymphoma entity. An overview over the design of clinical trials evaluating obinutuzumab in the treatment of indolent NHL is given in Table 1. Literature search was performed in PubMed using the search term ‘obinutuzumab’ (latest search 16 February 2016).
Table 1. . Summary of clinical trials evaluating obinutuzumab in the treatment of indolent non-Hodgkin lymphoma.
| Trial | Phase | Treatment | Treated patients (n) | Patient population | Ref. |
|---|---|---|---|---|---|
| GAUGUIN | I | Obinutuzumab as single agent Flat dose in a dose-escalating fashion (up to 2000 mg) Up to 8 × 21-day cycles |
21 | Relapsed/refractory lymphoma (indolent lymphoma [n = 16], MCL [n = 4], DLBCL [n = 1]) | [48,49] |
| II | Obinutuzumab as single agent in up to 8 × 21-day cycles | Relapsed/refractory indolent NHL | [48,50] | ||
| Two-dose regimens: – Low dose: 400 mg days 1 + 8 of cycle 1; 400 mg day 1 of cycles 2–8 |
18 |
||||
| |
|
– High dose: 1600 mg days 1 + 8 of cycle 1; 800 mg day 1 of cycles 2–8 |
22 |
|
|
| GAUSS | I | Obinutuzumab as single agent flat dose in a dose-escalating fashion (up to 2000 mg) Up to 4 weekly infusions followed by maintenance treatment every 3 months for up to 2 years in case of response |
22 | Relapsed/refractory lymphoma (indolent lymphoma [n = 12], CLL [n = 5], DLBCL [n = 3], MCL [n = 1], transformed lymphoma [n = 1]) | [51,52] |
| II | Obinutuzumab as single agent 1000 mg weekly for 4 weeks followed by maintenance treatment every 2 months for up to 2 years in case of response vs |
87 | Relapsed indolent NHL | [51,53] | |
| |
|
Rituximab as single agent 375 mg/m2 weekly for 4 weeks followed by maintenance treatment every 2 months for up to 2 years in case of response |
87 |
|
|
| GAUDI | Ib | Obinutuzumab plus chemotherapy (CHOP or FC) | Relapsed/refractory follicular lymphoma | [54,55] | |
| Two-dose regimens: – Low dose: 400 mg days 1 + 8 of cycle 1; 400 mg day 1 of cycles 2–8 plus CHOP or FC |
28 |
||||
| |
|
– High dose: 1600 mg days 1 + 8 of cycle 1; 800 mg day 1 of cycles 2–8 plus CHOP or FC |
28 |
|
|
| GADOLIN | III | Bendamustine 90 mg/m2 days 1 + 2 for 6 × 28-day cycles plus obinutuzumab 1000 mg days 1, 8 and 15 in cycle 1 and day 1 in cycles 2–6 followed by maintenance (1000 mg every 2 months in case of response for up to 2 years) vs |
194 | Rituximab-refractory indolent NHL | [20,21] |
| |
|
Bendamustine 120 mg/m2 days 1 + 2 for 6 × 28-day cycles |
198 |
|
|
| GALLIUM | III | Chemotherapy plus obinutuzumab 1000 mg days 1, 8 and 15 in cycle 1 and day 1 in cycles 2–6 followed by maintenance in responders vs |
n.a. | Untreated advanced indolent NHL | [47] |
| Chemotherapy plus rituximab 375 mg/m2 day 1 of each cycle followed by maintenance in responders | n.a. |
CHOP: Cyclophosphamide, doxorubicin, vincristine, and prednisone; CLL: Chronic lymphocytic leukemia; DLBCL: Diffuse large B-cell lymphoma; FC: Fludarabine/cyclophosphamide; MCL: Mantle cell lymphoma; n.a.: Not annotated; NHL: Non-Hodgkin lymphoma.
Introduction to the compound
Obinutuzumab is an anti-CD20 monoclonal antibody that was designed by GlycArt Biotechnology AG and then further developed by Genentech Inc., F Hoffmann-La Roche Ltd, Biogen Idec Inc., and Chugai Pharmaceutical Co Ltd. It is a humanized antibody based on a murine IgG1-κ antibody [19].
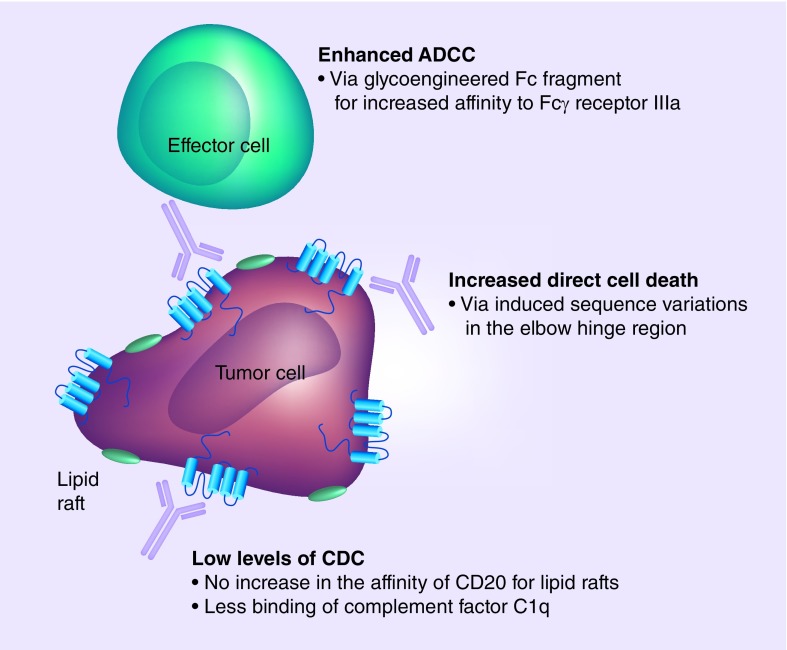
The underlying mechanisms of actions of monoclonal antibodies are comprised of direct cell death, CDC and antibody-dependent cell-mediated cytotoxicity via Fcγ receptor expressing cells such as natural killer cells and macrophages [56]. How much these different mechanisms precisely contribute to the mechanism of action of each individual monoclonal antibody, is not completely understood, but generally, two types of anti-CD20 monoclonal antibodies can be distinguished: type I antibodies which lead to a strong binding of the complement factor C1q and thereby to a potent induction of CDC, whereas direct cell death plays a subordinate role. Type II antibodies, which are less capable of C1q binding with the result of low levels of CDC, but which can potently induce direct cell death [56–58].
Obinutuzumab was designed as a type II antibody. Type II CD20 binding was achieved by the introduction of sequence variations into the variable part of the antibody. The most potent induction of direct cell death was achieved by a variation in the elbow hinge region of the antibody (valine instead of leucine at Kabat position 11) [19]. The mechanisms behind obinutuzumab-induced direct cell death were shown to be independent of BCL-2 and caspases and therefore nonapoptotic. Instead, cell death was shown to be dependent on actin reorganization, permeabilization of the lysosomal membrane and release of lysosomal hydrolases into the cytosol [59]. This was in line with findings for the type II anti-CD20 antibody tositumomab [60].
In addition to the variable region, the Fc segment of the antibody was also engineered. Obinutuzumab was produced in Chinese hamster ovary cells, which overexpress two recombinant glycosylation enzymes (β-1,4-N-acetyl-glucosaminyltransferase III and Golgi α-mannosidase II) [19]. Both enzymes lead to a reduction of fucose attached to the Fc segments, which increases the affinity of the antibody to Fcγ receptor III [61], an activating Fc receptor displayed by immune effector cells, such as natural killer cells and macrophages (Figure 1).
Figure 1. . Mechanisms of action of obinutuzumab.
Whereas the ability to induce ADCC and direct cell death is enhanced, obinutuzumab is less capable to induce CDC compared with type I anti-CD20 monoclonal antibodies such as rituximab and ofatumumab.
ADCC: Antibody-dependent cell-mediated cytotoxicity; CDC: Complement-dependent cytotoxicity.
Pharmacology
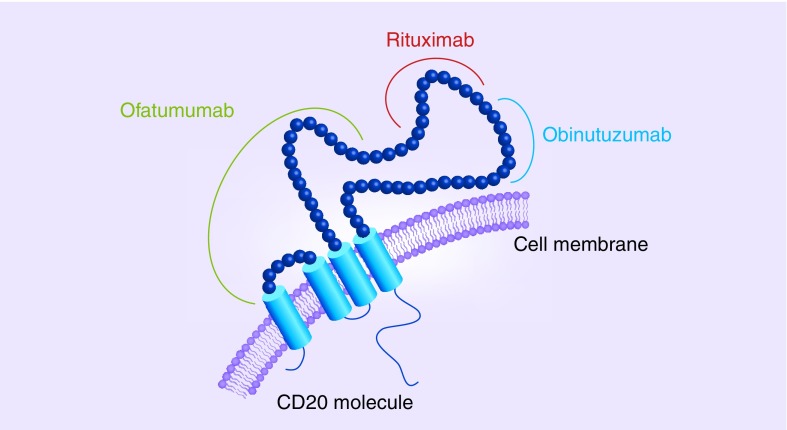
Obinutuzumab selectively binds to the extracellular domain of the human CD20 antigen, an integral membrane protein expressed by B cells. CD20 has two extracellular portions – one small loop from positions 72–80 and a larger loop from positions 142 to 182. Both, rituximab and obinutuzumab bind to the larger extracellular loop, but they have distinct, though overlapping epitopes (core binding site of rituximab is position 171, core binding site of obinutuzumab is position 176) and different binding orientations [62]. These differences in binding sites might in addition to the varied elbow hinge region of obinutuzumab and its glycoengineered Fc segment contribute to the different cellular responses upon binding of the two different antibodies (Figure 2).
Figure 2. . CD20 binding sites for obinutuzumab, rituximab and ofatumumab.
To evaluate safety and tolerability of obinutuzumab as monotherapy, two Phase I trials were conducted, a subset of the GAUGUIN trial (NCT00517530) [48] and the GAUSS trial (NCT00576758) [51]. Both trials included heavily pretreated patients (median of 5, respectively four prior therapies) with relapsed/refractory CD20+ indolent or aggressive lymphoma. Their results were published head to head [49,52]. In both dose-escalation trials obinutuzumab was given at a maximum single flat dose of 2000 mg. At this maximum single dose, no dose-limiting toxicities were observed in either of the trials.
• Both trial tested different schedules of drug applications
The Phase I part preceding the GAUGUIN trial [48] used an every 3-week schedule (maximum of eight cycles) and obinutuzumab was administered on day 1 (50–1600 mg) and day 8 (100–2000 mg) during the first cycle to quickly elevate serum levels and on day 1 (100–2000 mg) during the subsequent cycles. The Phase I part preceding the GAUSS trial [51] used a weekly schedule (100–2000 mg, maximum of four applications) during induction followed by maintenance therapy in case of nonprogression with obinutuzumab applications (200–2000 mg) every 3 months for a maximum of 2 years.
A clear association between dose and response could not be observed both clinical trials [48,51], as responses were seen in all dose-escalation cohorts. So, dose selection for subsequent Phase II clinical trials could not only be based on response and toxicity.
The Phase II component of the GAUGUIN trial [48] therefore compared two different dose regimens within 21-day cycles: a low-dose cohort with 400 mg for all infusions and a high-dose cohort with 1600 mg on days 1 and 8 of the first cycle and 800 mg on day 1 of all subsequent cycles [50,63]. Within this Phase II part including 100 patients (40 patients with indolent lymphoma, 40 patients with aggressive lymphoma and 20 patients with CLL), clinical variables were tested for their influence on pharmacokinetics. Patients’ age, sex and bodyweight were only of minor influence at the most. However, CD20 antigen mass (B-cell count before treatment, disease stage and tumor size) affected obinutuzumab serum concentrations in the low-dose cohort, whereas these factors had little impact on serum concentrations in the high-risk cohort. This suggested that, contrary to the high-dose cohort, target saturation was not reached in the low-dose cohort. This was further supported by the fact that steady-state serum concentrations were quickly reached by cycle two in the high-dose cohort, whereas serum concentrations rose steadily over the course of the treatment in the low-dose cohort [64]. Responses were observed more frequently in the high-dose cohort [50] indicating a trend for a dose–response relationship [64]. No correlation was seen between toxicity and dose [50,63–64].
In the Phase II component of the GAUSS trial [51] a flat dose of 1000 mg was used for all infusions. This decision was based on pharmacokinetic data obtained during the Phase I part that was similar to results from the GAUGUIN trial [48], as higher dose cohorts with a cumulative dose of at least 2000 mg after the second infusion had early steady-state serum concentrations indicating target saturation. During maintenance therapy, serum concentrations of obinutuzumab remained constant over time; in patients not receiving maintenance therapy, serum concentrations decreased rapidly [52].
Phase III clinical trials were designed to use a flat obinutuzumab dose of 1000 mg with infusions given on days 1, 8 and 15 during the first cycle, in order to quickly reach steady-state serum concentrations, and on day 1 during the subsequent cycles. Pharmacokinetic model-based simulations showed that this regimen resulted in serum concentrations similar to the high-dose regimen tested in the GAUGUIN trial [48].
Clinical efficacy
• Phase II clinical trials
Two Phase I clinical trials have shown promising clinical efficacy in indolent lymphoma. The Phase I part of the GAUGUIN trial [48] included 21 patients with relapsed NHL, of which 16 had an indolent lymphoma subtype (n = 13 follicular lymphoma, n = 1 small lymphocytic leukemia, n = 1 lymphoplasmacytic lymphoma, n = 1 Waldenström macroglobulinemia). The response rate at the end of treatment was 33%. The best overall response rate (ORR) assessed during follow-up was 43% in this trial. All nine responding patients had follicular lymphoma. So the ORR for this entity was 69% including five patients with complete remission (CR) [49].
The Phase I part of the GAUSS trial [51] included 22 patients with relapsed NHL, of which 12 had an indolent lymphoma subtype (n = 10 follicular lymphoma, n = 2 small lymphocytic lymphoma). Of these 12 patients five responded to therapy (one patient with CR), four had stable disease and only three were nonresponders [52].
Based on this promising response data, both trials were expanded by a Phase II part.
The GAUGUIN trial [48] continued with 18 relapsed indolent NHL patients being assigned to a low-dose cohort and 22 patients being assigned to a high-dose cohort. In the low-dose cohort the best ORR was 33%, in the high-dose cohort the best ORR was 64% including 23% CR/unconfirmed CR (CRu). Focusing on patients with follicular lymphoma (n = 20), the best ORR was 60% [50].
The GAUSS trial [51] continued with 175 relapsed, indolent NHL patients who previously responded to a rituximab containing treatment regimen. Patients were randomly assigned to four weekly infusions of obinutuzumab or rituximab followed by maintenance therapy with the assigned antibody. Of the 175 patients 149 had follicular lymphoma, 12 had small lymphocytic lymphoma, 11 had marginal zone B-cell lymphoma and three had lymphoplasmocytic lymphoma. Histologic subtypes were equally randomized to both treatment arms. At the end of induction, a nonstatistically significant trend toward higher ORR with obinutuzumab was observed (44.6 vs 33.3%; difference, p = 0.08) with nine patients achieving CR/CRu with obinutuzumab and four patients with rituximab. On completion of maintenance the ORR was 66.2% in the obinutuzumab arm and 64.0% in the rituximab arm with a higher CR/CRu rate with obinutuzumab (41.9 vs 22.7%; p = 0.006). However, when response was assessed by an independent review facility, CR/CRu rates did not show a statistically significant difference (37.8% with obinutuzumab vs 26.7% with rituximab; p = 0.07) [53].
In addition, efficacy of low-dose and high-dose obinutuzumab in combination with chemotherapy (CHOP or FC) in relapsed/refractory follicular lymphoma was evaluated in the GAUDI trial (NCT00825149) [54]. The results were favorable with 86 to 100% ORR in the four different treatment arms and a CR rate of 45% over all treatment arms [55].
Based on these results two Phase III trials were designed: the GADOLIN trial [20] testing the efficacy of obinutuzumab in rituximab-refractory indolent lymphoma and the GALLIUM trial [47] testing obinutuzumab in the setting of untreated, advanced indolent NHL in combination with investigator's choice chemotherapy.
• Phase III clinical trials
By the time of this review no Phase III clinical trial evaluating the efficacy of obinutuzumab in the treatment of indolent lymphoma had been fully published. Results from the interim analysis of the GADOLIN [20] trial have been presented at the annual meeting of the American Society of Clinical Oncology in 2015 [21].
The GADOLIN trial [20] was an open label study of bendamustine with or without obinutuzumab in the treatment of patients with CD20+ rituximab-refractory indolent lymphoma, in other words patients who did either not respond to rituximab or relapsed early after having received rituximab (median of two prior therapies). Patients with follicular lymphoma, marginal zone lymphoma and small lymphocytic lymphoma were included. In the standard arm (B arm, n = 198 patients treated) up to six cycles of bendamustine were given at a dose of 120 mg/m2 on days 1 and 2 of a 28-day cycle. In the experimental arm (GB arm, n = 194 patients treated) obinutuzumab was added to this dose of chemotherapy and given at a dose of 1000 mg on days 1, 8 and 15 during the first cycle and on day 1 during the subsequent cycles. Patients in the GB arm who had nonprogressing disease after six cycles of therapy received obinutuzumab maintenance (1000 mg every 2 months for up to 2 years), whereas patients in the B arm were observed. At the time point of interim staging, the median observation time was 20 months for the B arm and 22 months for the GB arm. Response was assessed by an independent radiology facility as primary endpoint. No differences between the two treatment arms were seen for the overall response rates (63.0% in the B arm vs 69.1% in the GB arm) and for the CR rates at the end of induction (12.2% for the Barm vs 11.2% for the GB arm). But results for PFS were clearly favoring the GB arm. In the B arm the median PFS was 14.9 months, whereas in the GB arm the median PFS was not reached after an observation time of 22 months (hazard ratio [HR]: 0.55; 95% CI: 0.40–0.74; p = 0.00011). Preliminary data on OS (median OS not reached in either arm at interim analysis) suggested no difference between the two treatment arms [21]. By these results, the GADOLIN trial provides the first randomized evidence of a benefit for treatment with obinutuzumab in rituximab-refractory, indolent NHL.
The GADOLIN trial [20] included 321 randomized patients who were diagnosed with follicular lymphoma. Within this trial minimal residual disease (MRD) levels were analyzed in patients with follicular lymphoma by t(14;18) and/or Ig variable domain allele-specific real-time PCR in peripheral blood or bone marrow in order to estimate the depth of response. Altogether, baseline samples from 128 patients, who completed induction therapy at the clinical cut-off date, were available. These samples were required to have a detectable clonal marker and the real-time PCR assays done on them had to fulfill the sensitivity criteria (assays were designed with a sensitivity of 10-5). MRD negativity was defined as an MRD level ≤10-4. At mid of induction 64 samples were available and at end of induction 93 samples were available to perform the analyses. Results favored a deeper response in patients treated in the GB arm compared with patients treated in the B arm: at mid of induction 77% achieved MRD negativity in the GB arm versus 40% in the B arm (p = 0.0029; Pearson's Chi-square test) and at end of induction 82% achieved MRD negativity in the GB arm versus 43% in the B arm (p < 0.0001). These results suggest that obinutuzumab contributes significantly to the depth of response to bendamustine [65].
For indolent lymphoma, the role of obinutuzumab in first-line treatment compared with rituximab is addressed in the GALLIUM Phase III clinical trial [47]. This trial was designed for patients with untreated advanced indolent NHL and combined rituximab or obinutuzumab with investigator's choice chemotherapy (CHOP, CVP or bendamustine). In the comparator arm, rituximab 375 mg/m2 of body surface area was given on day 1 of a 21-day or 28-day chemotherapy cycle. In the experimental arm, obinutuzumab 1000 mg was given on days 1, 8 and 15 of the first chemotherapy cycle and on day 1 on the subsequent chemotherapy cycle. Up to six cycles of therapy were given and patients achieving response (PR or CR) went on to maintenance therapy continuing with their randomized antibody, which was given every 2 months until disease progression for a maximum of 2 years. Results from this trial are eagerly awaited and first results are expected for 2016.
The CLL11 trial [16] evaluated the role of obinutuzumab in first-line treatment of CLL when combined with chlorambucil to treat elderly and/or comorbid patients. A total of 781 patients were enrolled on this trial, who either had a CIRS score higher than 6 or an estimated creatinine of 30–69 ml per minute. Patients were randomized to treatment with chlorambucil alone at an oral dose of 0.5 mg/kg of bodyweight on days 1 and 15 of an 28-day cycle, to treatment with chlorambucil plus rituximab (rituximab given at a dose of 375 mg/m2 of body surface area on day 1 of cycle 1 and 500 mg/m2 of body surface area on day 1 of the subsequent cycles) or to treatment with chlorambucil plus obinutuzumab (obinutuzumab given at a dose of 1000 mg on days 1, 8 and 15 of cycle 1 and on day 1 of the subsequent cycles). Up to six therapy cycles were given in each therapy arm. A head-to-head comparison of obinutuzumab/chlorambucil with rituximab/chlorambucil revealed a prolonged PFS for the obinutuzumab arm (HR: 0.39; 95% CI: 0.31–0.49; p < 0.001), higher rates of CR with obinutuzumab (20.7 vs 7.0% in the rituximab/chlorambucil arm) as well as higher rates of molecular response with obinutuzumab. MRD negativity was achieved in a considerable number of patients treated with obinutuzumab/chlorambucil in peripheral blood (37.7 vs 3.3% in patients treated with rituximab/chlorambucil; p < 0.001) and in the bone marrow (19.5 vs 2.6% in patients treated with rituximab/chlorambucil; p < 0.001) [18]. To what extent the differences in dose and schedule of rituximab and obinutuzumab administration influence survival differences remains unclear. However, in combination with FCR higher rituximab doses could not improve response and survival rates [66].
Postmarketing surveillance
So far, no new relevant aspects were reported in the postmarketing surveillance.
Safety & tolerability
Overall, obinutuzumab has an acceptable safety profile with mainly grades 1 and 2 adverse events (AEs) and more severe AEs (grades 3–5) being reported within clinical trials [21,49–50,52–53,55,63]. In all cited trials, events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 guidelines [67].
The Phase I part of the GAUGUIN trial [48] included 21 patients [49], the Phase I part of the GAUSS trial [51] included 22 patients [52] and the Phase Ib GAUDI trial [54] included 56 patients [55], who were all included into the safety analysis. Both Phase I trials revealed no dose-limiting toxicities up to a dose of 2000 mg [49,52]. The Phase II part of the GAUGUIN trial included 40 patients diagnosed with relapsed/refractory indolent lymphoma [50] and 40 patients with relapsed/refractory aggressive lymphoma or mantle cell lymphoma [63] and the Phase II part of the GAUSS trial included 87 patients in the obinutuzumab arm [53] with available safety data. In addition, safety data for 194 patients treated with obinutuzumab plus bendamustine within the GADOLIN trial [20] was presented at the American Society of Clinical Oncology meeting in 2015 [21].
The three major safety events were infusion-related reactions (IRRs), hematologic AEs, primarily neutropenia and infections.
IRRs were the most common AEs throughout all clinical trials listed above. They comprised of various symptoms such as hypotension or hypertension, tachycardia, dyspnea, pyrexia, chills, nausea, vomiting, diarrhea, flushing and headache coinciding with drug infusion. In CLL the occurrence of IRR with obinutuzumab was recently associated with the release of cytokines, in particular IL-6 and IL-8 [68] and a rapid reduction of CLL cell numbers in the peripheral blood, thereby showing similarities to IRRs after rituximab infusion, which were linked to the release of IL-6, IL-8 and TNF-α and rapid blood tumor clearance [69,70]. In line with this, serum levels of IL-6, IL-8 and IL-10 as well as TNF-α and IFN-γ were found to be elevated during the first cycle of therapy given in the GAUSS trial [51]. Greater cytokine increases were found with obinutuzumab than with rituximab. During subsequent infusions cytokine levels remained close to baseline [53].
IRRs mainly occurred during the first infusion and almost always resolved with slowing or interrupting the infusion and/or upon steroid administration [49,52,55]. The listed trials describe incidences of IRRs between 64 [55] and 86% [49], the majority of reactions being described as grades 1 and 2 [49–50,52–53,55,63]. A relation between obinutuzumab dose and intensity of the IRR was not observed. The Phase II part of the GAUSS trial [51] provided a head-to-head comparison between obinutuzumab and rituximab for the treatment of relapsed indolent lymphoma. The overall incidence of IRR was higher in the obinutuzumab arm (74%) compared with the rituximab arm (51%), likewise the incidence of grades 3 and 4 IRRs (11% in the obinutuzumab arm vs 5% in the rituximab arm) [53]. This is in line with data from the CLL11 trial, where grades 3 and 4 IRRs were described to occur in 20% of patients during the first obinutuzumab infusion, but were not described to occur during subsequent infusions [17]. However, 7% of patients in the obinutuzumab plus chlorambucil arm permanently discontinued therapy because of IRR [17]. To prevent IRR, premedication with acetaminophen, an antihistamine and a glucocorticoid is now recommended to be given prior to the first infusion [71], though experiences from the CLL11 suggest an only moderate effect of prophylactic measures on the frequency of IRRs [17]. Since cardiac and respiratory distress can occur in the context of IRR, patients with pre-existing cardiac and pulmonary conditions should be treated with extra care.
Neutropenia was the most common hematologic AE. In monotherapy grade 3/4 neutropenia occurred infrequently with an incidence of 10% (Phase I) [49] and 14% (Phase II for indolent NHL, only in the high-dose arm) [50] in the GAUGUIN trial [48] and 23% (Phase I) [52] and 3% (Phase II) [53] in the GAUSS trial [51]. As to be expected the incidence of grade 3/4 neutropenia was higher in combination with chemotherapy. The incidence was 29–64% [55] in the GAUDI trial [54] and 33% in the GB arm of the GADOLIN trial [20], thereby not differing much from the incidence in the B arm (26.3%) [21].
Grade 3/4 infections occurred rarely and were not observed in the Phase I clinical trials [49,52]. The incidence was 10% [50] in the Phase II part of the GAUGUIN trial [48] and 4% during obinutuzumab maintenance [53] in the GAUSS trial [51]. In combination with CHOP and FC (GAUDI trial [54]) the incidence rose to 21% [55]. In combination with bendamustine (GADOLIN trial [20]) the incidence was below 3%, thereby lower than the incidence in the bendamustine only arm [21].
With the approval of obinutuzumab two black box warnings were released: for the risk of hepatitis B virus (HBV) reactivation and for the risk of progressive multifocal leukoencephalopathy (PML).
HBV reactivations, which can result in fulminant hepatitis with fatal outcome, are already known to occur in patients treated with anti-CD20 monoclonal antibodies including obinutuzumab [72]. After treatment with rituximab-CHOP, patients being seropositive for hepatitis B surface antigen (HBsAg) have a 59–80% risk for HBV reactivation and patients with resolved HBV infection are still at a 9–24% risk for HBV reactivation [73]. Therefore, all patients being considered for anti-CD20 monoclonal antibody treatment including obinutuzumab need to be screened before initiation of therapy by measuring HBsAg, antibodies against hepatitis B core antigen (anti-HBc) and antibodies against HBsAg (anti-HBs). To prevent the development of hepatitis due to HBV reactivation after anti-CD20 monoclonal antibody treatment, antiviral prophylaxis is recommended for HbsAg-positive patients and/or patients in whom HBV DNA is detectable. In patients with resolved HBV infection, HBV DNA should be regularly monitored and an antiviral drug is given once HBV DNA becomes detectable over the course of treatment.
PML – a severe and often fatal demyelinating disease of the CNS – has been reported to occur in patients being treated with obinutuzumab for relapsed/refractory follicular lymphoma [72]. PML is caused by a reactivation of the John Cunningham (JC) virus, a human polyomavirus residing in latent form in up to 86% of the adult population [74]. The patient who was affected by PML after obinutuzumab treatment, was enrolled on the GAUDI trial [54] and had confirmed presence of JC virus in the cerebrospinal fluid [72]. Long-lasting B-cell depletion is known to be associated with reactivation of JC virus, since a rare incidence of PML (about one case per 25,000 individuals) was reported after rituximab treatment [75]. Conclusions on the incidence of PML with obinutuzumab treatment can yet not be drawn. By the time of the first documented case of PML with obinutuzumab over 1200 patients were treated with this drug. In theory, the incidence of PML could be higher with obinutuzumab compared with rituximab, as obinutuzumab is more potent in terms of B-cell depletion than rituximab.
The overall tolerability of obinutuzumab by patients can be assumed to be acceptable. The GADOLIN trial [20] assessed health-related quality of patients’ life by ‘The functional assessment of cancer treatment – lymphoma’ questionnaire. Compared with patients treated with bendamustine alone, more patients treated with obinutuzumab and bendamustine in this trial reported a meaningful improvement in their health-related quality of life, suggesting that improved PFS rates in this trial were not at the expense of an increase in treatment-related toxicity [76].
Regulatory affairs
Obinutuzumab was approved by the FDA in November 2013 and by the EMA in May 2014 for its use in combination with chlorambucil to treat patients with previously untreated CLL. This approval was based on the results of the CLL11 trial [16], which demonstrated superiority of obinutuzumab/chlorambucil over rituximab/chlorambucil including CRs with MRD negativity in the obinutuzumab/chlorambucil arm [17]. Upon the results of this trial obinutuzumab was granted a breakthrough therapy designation on request of the sponsor. This designation could be granted, as preliminary clinical evidence indicates the drug may offer a substantial improvement over available therapies for patients with serious diseases. Obinutuzumab was also granted priority review because the drug demonstrated the potential to be a significant improvement in safety and efficacy. Obinutuzumab's brand name is ‘Gazyva’ in the USA and ‘Gazyvaro’ in Europe. In February 2016, the FDA also granted approval for obinutuzumab in the treatment of rituximab-refractory follicular lymphoma in combination with bendamustine. This approval was based on results of the interim analysis of the GADOLIN trial [20].
Conclusion
Obinutuzumab presents with a favorable benefit–risk profile when used as a single agent as well as to its use in combination with different chemotherapeutic protocols. Based on results of the interim analysis of the GADOLIN trial, obinutuzumab was shown to have clinically relevant impact on PFS although longer follow-up will be required to determine if obinutuzumab also impacts the overall survival in this patient group. First results from the GALLIUM trial for obinutuzumab's use in first-line treatment of indolent lymphoma are eagerly awaited and may lead to the approval of obinutuzumab in first-line treatment of indolent lymphoma.
EXECUTIVE SUMMARY.
Mechanisms of action
Obinutuzumab is a humanized, type II anti-CD20 monoclonal antibody.
Obinutuzumab has sequence variation in its hinge region to enhance its induction of direct cell death.
Obinutuzumab has a glycoengineered Fc segment leading to a reduced fucose content, thereby enhancing antibody-dependent cell-mediated cytotoxicity.
Pharmacokinetic properties
A dose schedule of 1000 mg on days 1, 8 and 15 of the first cycle and day 1 during the subsequent cycles followed by a maintenance dose of 1000 mg every 3 months leads to quick and durable CD20 saturation.
Clinical efficacy
Obinutuzumab has shown single-agent activity in patients with relapsed/refractory non-Hodgkin lymphoma.
Treatment with obinutuzumab plus bendamustine significantly improves progression-free survival compared with treatment with bendamustine alone in patients with rituximab-refractory indolent lymphoma.
The ongoing Phase III trial GALLIUM will provide important information regarding efficacy of obinutuzumab combined with chemotherapy in first-line treatment of indolent lymphoma.
Safety & tolerability
Obinutuzumab has been found to be generally well tolerated, with a favorable benefit–risk profile, both as a single agent and in combination with chemotherapeutic regimens.
The most common adverse events (AEs) associated with obinutuzumab have been infusion-related reactions, but these were mainly grade 1/2 AEs, they mainly occurred during the first infusion and almost always resolved with slowing or interrupting the infusion and/or upon steroid administration.
Hematologic AEs and infections did not exceed the expected frequencies for monotherapy and combination therapies with anti-CD20 monoclonal antibodies.
Drug interactions
No significant drug–drug interactions have been reported.
Dosage & administration
The currently approved dose schedule proved to be safe and effective in the treatment of rituximab-refractory indolent lymphoma.
The safety and efficacy of the currently approved dose schedule in first-line treatment of indolent lymphoma is currently being evaluated.
Footnotes
Disclosure
In addition to the peer-review process, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the author at their discretion and based on scientific or editorial merit only. The author maintained full control over the manuscript, including content, wording and conclusions.
Financial & competing interests disclosures
JG Gribben has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, AbbVie, TG Therapeutics, and A2 for advisory boards and speaking. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hiddemann W, Cheson BD. How we manage follicular lymphoma. Leukemia. 2014;28(7):1388–1395. doi: 10.1038/leu.2014.91. [DOI] [PubMed] [Google Scholar]
- 2.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J. Clin. Oncol. 2008;26(28):4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 4.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112(13):4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 5.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a Phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 6.Salles G, Seymour JF, Feugier P, et al. 2013 Annual Meeting of the American Society of Hematology. New Orleans, LA, USA: 7–10 December 2013. Updated 6 year follow-up of the PRIMA study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline immunochemotherapy. Presented at. Abstract 509. [Google Scholar]
- 7.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, Phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 8.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://clinicaltrials.gov/ct2/show/NCT00281918 Clinical Trials Database: NCT00281918.
- 10.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, Phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 12.https://clinicaltrials.gov/ct2/show/NCT00769522 Clinical Trials Database: NCT00769522.
- 13.Eichhorst B, Fink AM, Busch R, et al. 2014 Annual Meeting of the American Society of Hematology. San Francisco, CA, USA: 6–9 December 2014. Frontline chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) shows superior efficacy in comparison to bendamustine (B) and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): final analysis of an international, randomized study of the German CLL Study Group (GCLLSG) (CLL10 Study) Presented at. Abstract 19. [Google Scholar]
- 14.Hillmen P, Gribben JG, Follows GA, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: final analysis of an open-label Phase II study. J. Clin. Oncol. 2014;32(12):1236–1241. doi: 10.1200/JCO.2013.49.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foà R, Del Giudice I, Cuneo A, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am. J. Hematol. 2014;89(5):480–486. doi: 10.1002/ajh.23668. [DOI] [PubMed] [Google Scholar]
- 16.https://clinicaltrials.gov/ct2/show/NCT01010061 Clinical Trials Database: NCT01010061.
- 17.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014;370(12):1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]; • Efficacy data from this Phase III study (CLL11) supported the accelerated approval of obinutuzumab in combination with chlorambucil for first-line treatment of chronic lymphocytic leukemia in elderly and/or comorbid patients.
- 18.Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. 2015;29(7):1602–1604. doi: 10.1038/leu.2015.14. [DOI] [PubMed] [Google Scholar]
- 19.Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Preclinical study that elucidates the development and mechanisms of action of obinutuzumab.
- 20.https://clinicaltrials.gov/ct2/show/NCT01059630 Clinical Trials Database: NCT01059630.
- 21.Sehn LH, Chua NS, Mayer J, et al. 2015 Annual Meeting of the American Society of Clinical Oncology. Chicago, IL, USA: 29 May–2 June 2015. GADOLIN: primary results from a Phase III study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Presented at. Abstract 8502. [Google Scholar]; •• Randomized Phase III study (GADOLIN) which demonstrated that obinutuzumab has clinical efficacy in rituximab-refractory indolent non-Hodgkin lymphoma (NHL).
- 22.Hoyer BF, Manz RA, Radbruch A, Hiepe F. Long-lived plasma cells and their contribution to autoimmunity. Ann. NY Acad. Sci. 2005;1050:124–133. doi: 10.1196/annals.1313.014. [DOI] [PubMed] [Google Scholar]
- 23.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 24.Herold M, Scholz CW, Rothmann F, Hirt C, Lakner V, Naumann R. Long-term follow-up of rituximab plus first-line mitoxantrone, chlorambucil, prednisolone and interferon-alpha as maintenance therapy in follicular lymphoma. J. Cancer Res. Clin. Oncol. 2015;141(9):1689–1695. doi: 10.1007/s00432-015-1963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 26.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J. Clin. Oncol. 2005;23(22):5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 27.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 28.https://clinicaltrials.gov/ct2/show/NCT00140582 Clinical Trials Database: NCT00140582.
- 29.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 2014;370(11):1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22):3406–3413. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, Phase 1b study. Lancet Oncol. 2014;15(9):1019–1026. doi: 10.1016/S1470-2045(14)70311-0. [DOI] [PubMed] [Google Scholar]
- 32.Maddocks K, Christian B, Jaglowski S, et al. A Phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125(2):242–248. doi: 10.1182/blood-2014-08-597914. [DOI] [PubMed] [Google Scholar]
- 33.Gerecitano JF, Roberts AW, Seymour JF, et al. 2015 Annual Meeting of the American Society of Hematology. Orlando, FL, USA: 5–8 December 2015. A Phase 1 study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma. Presented at. Abstract 254. [Google Scholar]
- 34.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J. Clin. Oncol. 2009;27(32):5404–5409. doi: 10.1200/JCO.2008.21.1169. [DOI] [PubMed] [Google Scholar]
- 35.Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br. J. Haematol. 2014;165(3):375–381. doi: 10.1111/bjh.12755. [DOI] [PubMed] [Google Scholar]
- 36.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, Phase 2 trial. Lancet Oncol. 2014;15(12):1311–1318. doi: 10.1016/S1470-2045(14)70455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab resistance in patients with indolent B-cell and mantle cell lymphomas. Clin. Cancer Res. 2015;21(8):1835–1842. doi: 10.1158/1078-0432.CCR-14-2221. [DOI] [PubMed] [Google Scholar]
- 38.Younes A, Kim S, Romaguera J, et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J. Clin. Oncol. 2012;30(22):2776–2782. doi: 10.1200/JCO.2011.39.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribrag V, Dupuis J, Tilly H, et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2014;20(1):213–220. doi: 10.1158/1078-0432.CCR-13-0580. [DOI] [PubMed] [Google Scholar]
- 40.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 41.Byrd JC, Pagel JM, Awan FT, et al. A Phase 1 study evaluating the safety and tolerability of otlertuzumab, an anti-CD37 mono-specific ADAPTIR therapeutic protein in chronic lymphocytic leukemia. Blood. 2014;123(9):1302–1308. doi: 10.1182/blood-2013-07-512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagel JM, Spurgeon SE, Byrd JC, et al. Otlertuzumab (TRU-016), an anti-CD37 monospecific ADAPTIR™ therapeutic protein, for relapsed or refractory NHL patients. Br. J. Haematol. 2015;168(1):38–45. doi: 10.1111/bjh.13099. [DOI] [PubMed] [Google Scholar]
- 43.Byrd JC, Kipps TJ, Flinn IW, et al. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk. Lymphoma. 2012;53(11):2136–2142. doi: 10.3109/10428194.2012.681655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanale M, Assouline S, Kuruvilla J, et al. Phase IA/II, multicentre, open-label study of the CD40 antagonistic monoclonal antibody lucatumumab in adult patients with advanced non-Hodgkin or Hodgkin lymphoma. Br. J. Haematol. 2014;164(2):258–265. doi: 10.1111/bjh.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta IV, Jewell RC. Ofatumumab the first human anti-CD20 monoclonal antibody for the treatment of B cell hematologic malignancies. Ann. NY Acad. Sci. 2012;1263:43–56. doi: 10.1111/j.1749-6632.2012.06661.x. [DOI] [PubMed] [Google Scholar]
- 46.https://clinicaltrials.gov/ct2/show/NCT01200589 Clinical Trials Database: NCT01200589.
- 47.https://clinicaltrials.gov/ct2/show/NCT01332968 Clinical Trials Database: NCT01332968.
- 48.https://clinicaltrials.gov/ct2/show/NCT00517530 Clinical Trials Database: NCT00517530.
- 49.Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–5132. doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 50.Salles GA, Morschhauser F, Solal-Céligny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the Phase II GAUGUIN study. J. Clin. Oncol. 2013;31(23):2920–2926. doi: 10.1200/JCO.2012.46.9718. [DOI] [PubMed] [Google Scholar]; •• Phase II clinical trial providing safety data of single-agent obinutuzumab in patients with relapsed/refractory indolent lymphoma.
- 51.https://clinicaltrials.gov/ct2/show/NCT00576758 Clinical Trials Database: NCT00576758.
- 52.Sehn LH, Assouline SE, Stewart DA, et al. A Phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–5125. doi: 10.1182/blood-2012-02-408773. [DOI] [PubMed] [Google Scholar]; •• Data from this Phase I/II study in patients with relapsed/refractory indolent NHL provided safety data of single-agent obinutuzumab in patients with relapsed/refractory indolent NHL and informed the design of the Phase III GADOLIN trial, which is evaluating obinutuzumab plus bendamustine in the treatment of rituximab-refractory indolent NHL.
- 53.Sehn LH, Goy A, Offner FC, et al. Randomized Phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J. Clin. Oncol. 2015;33(30):3467–3474. doi: 10.1200/JCO.2014.59.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Data from this Phase I/II study in patients with relapsed/refractory indolent NHL provided safety data of single-agent obinutuzumab in patients with relapsed/refractory indolent NHL and informed the design of the Phase III GADOLIN trial, which is evaluating obinutuzumab plus bendamustine in the treatment of rituximab-refractory indolent NHL.
- 54.https://clinicaltrials.gov/ct2/show/NCT00825149 Clinical Trials Database: NCT00825149.
- 55.Radford J, Davies A, Cartron G, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000) Blood. 2013;122(7):1137–1143. doi: 10.1182/blood-2013-01-481341. [DOI] [PubMed] [Google Scholar]
- 56.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 2007;44(16):3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 57.Chan HT, Hughes D, French RR, et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 2003;63(17):5480–5489. [PubMed] [Google Scholar]
- 58.Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103(7):2738–2743. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 59.Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosomemediated cell death in B-cell malignancies. Blood. 2011;117(17):4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ivanov A, Beers SA, Walshe CA, et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J. Clin. Invest. 2009;119(8):2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 1999;17(2):176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 62.Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118(2):358–367. doi: 10.1182/blood-2010-09-305847. [DOI] [PubMed] [Google Scholar]
- 63.Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large B-cell lymphoma or mantle-cell lymphoma: results from the Phase II GAUGUIN study. J. Clin. Oncol. 2013;31(23):2912–2919. doi: 10.1200/JCO.2012.46.9585. [DOI] [PubMed] [Google Scholar]
- 64.Cartron G, Hourcade-Potelleret F, Morschhauser F, et al. Rationale for optimal obinutuzumab/GA101 dosing regimen in B-cell non-Hodgkin lymphoma. Haematologica. 2016;101(2):226–234. doi: 10.3324/haematol.2015.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pott C, Belada D, Danesi N, et al. 2015 Annual Meeting of the American Society of Hematology. Orlando, FL, USA: 5–8 December 2015. Analysis of minimal residual disease in follicular lymphoma patients in gadolin, a Phase III study of obinutuzumab plus bendamustine versus bendamustine in relapsed/refractory indolent non-Hodgkin lymphoma. Presented at. Abstract 397. [Google Scholar]
- 66.Short NJ, Keating MJ, Wierda WG, et al. Fludarabine, cyclophosphamide, and multiple-dose rituximab as frontline therapy for chronic lymphocytic leukemia. Cancer. 2015;121(21):3869–3876. doi: 10.1002/cncr.29605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 68.Freeman CL, Morschhauser F, Sehn L, et al. Cytokine release in patients with CLL treated with obinutuzumab and possible relationship with infusion-related reactions. Blood. 2015;126(24):2646–2649. doi: 10.1182/blood-2015-09-670802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94(7):2217–2224. [PubMed] [Google Scholar]
- 70.Byrd JC, Waselenko JK, Maneatis TJ, et al. Rituximab therapy in hematologic malignancy patients with circulating blood tumor cells: association with increased infusion-related side effects and rapid blood tumor clearance. J. Clin. Oncol. 1999;17(3):791–795. doi: 10.1200/JCO.1999.17.3.791. [DOI] [PubMed] [Google Scholar]
- 71.Gazyva® (obinutuzumab) full Prescribing Information. Genentech; South San Francisco, CA, USA: 2015. www.gene.com/download/pdf/gazyva_prescribing.pdf [Google Scholar]
- 72.Gazyvaro® (obinutuzumab) CHMP assessment report. F Hoffmann-La Roche AG; Basel, Switzerland: 2014. www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500171596 [Google Scholar]
- 73.Kusumoto S, Tobinai K. Screening for and management of hepatitis B virus reactivation in patients treated with anti-B-cell therapy. Hematology Am. Soc. Hematol. Educ. Program. 2014;2014(1):576–583. doi: 10.1182/asheducation-2014.1.576. [DOI] [PubMed] [Google Scholar]
- 74.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch. Neurol. 2011;68(9):1156–1164. doi: 10.1001/archneurol.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheson BD, Trask PC, Gribben J, et al. 2015 Annual Meeting of the American Society of Hematology. Orlando, FL, USA: 5–8 December 2015. primary results of the health-related quality of life assessment from the Phase III gadolin study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory, indolent non-Hodgkin lymphoma. Presented at. Abstract 1532. [Google Scholar]