Abstract
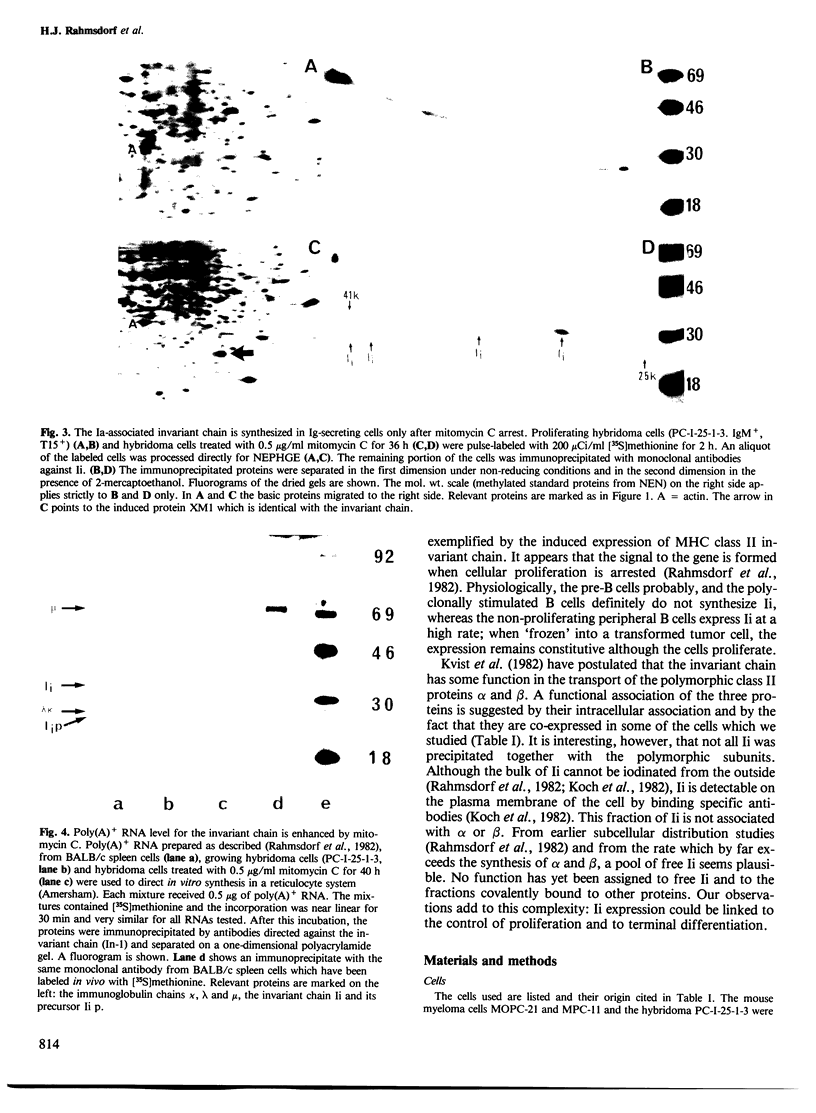
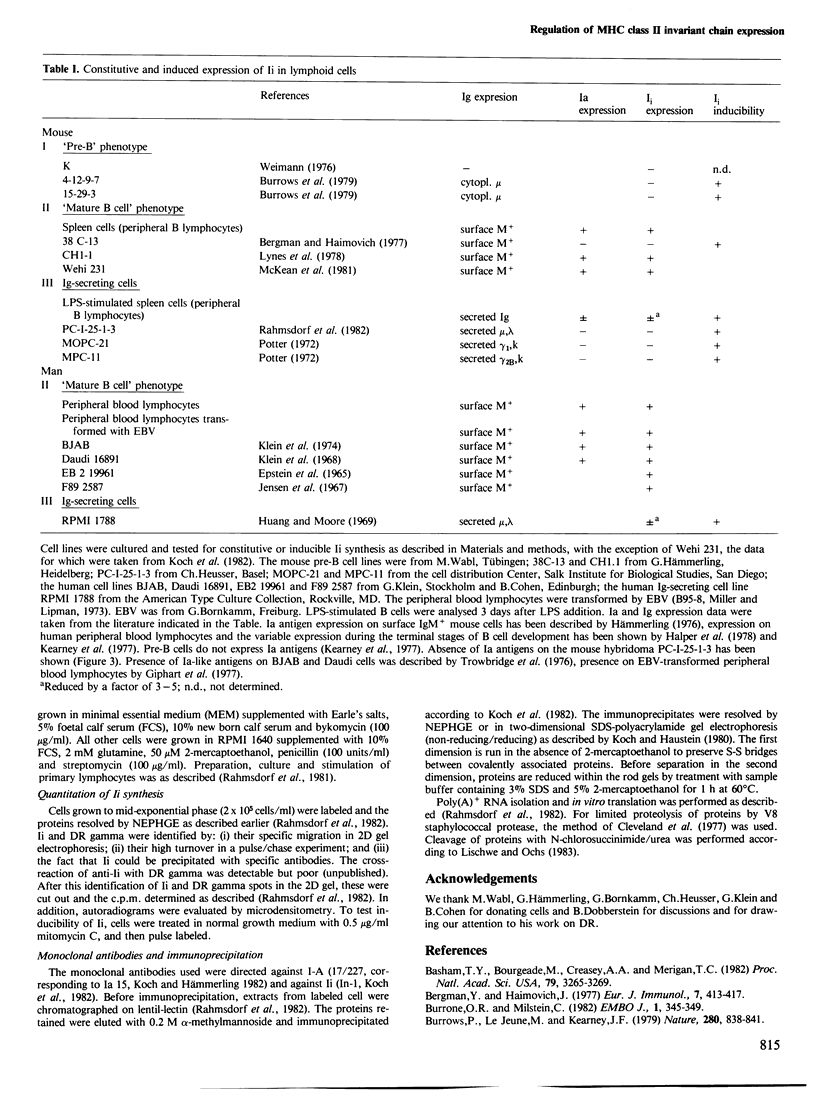
The expression of the H2 Ia-associated invariant chain (Ii) has been determined by pulse labeling cells with [35S]methionine and resolving the proteins. Expression is maximal in noncycling peripheral B lymphocytes and is reduced upon maturation of B lymphocytes to plasma cells. B cell-derived cell lines behave correspondingly: IgM+ non-secretor cell lines synthesize Ii while plasmocytoma cells do not. Pre-B cell lines are also negative. In all Ii-negative cell lines, the synthesis of Ii is selectively induced by treating the cells with inhibitors of replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basham T. Y., Bourgeade M. F., Creasey A. A., Merigan T. C. Interferon increases HLA synthesis in melanoma cells: interferon-resistant and -sensitive cell lines. Proc Natl Acad Sci U S A. 1982 May;79(10):3265–3269. doi: 10.1073/pnas.79.10.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Y., Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977 Jul;7(7):413–417. doi: 10.1002/eji.1830070702. [DOI] [PubMed] [Google Scholar]
- Burrone O. R., Milstein C. Control of HLA-A,B,C synthesis and expression in interferon-treated cells. EMBO J. 1982;1(3):345–349. doi: 10.1002/j.1460-2075.1982.tb01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Day C. E., Jones P. P. The gene encoding the Ia antigen-associated invariant chain (Ii) is not linked to the H-2 complex. Nature. 1983 Mar 10;302(5904):157–159. doi: 10.1038/302157a0. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Barr Y. M., Achong B. G. Studies with Burkitt's lymphoma. Wistar Inst Symp Monogr. 1965 Sep;4:69–82. [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giphart M. J., Kaufman J. F., Fuks A., Albrechtsen D., Solheim B. G., Bruning J. W., Strominger J. L. HLA-D associated alloantisera react with molecules similar to Ia antigens. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3533–3536. doi: 10.1073/pnas.74.8.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W., Walker I. D. Allelic forms of beta 2-microglobulin in the mouse. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7395–7399. doi: 10.1073/pnas.77.12.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Halper J., Fu S. M., Wang C. Y., Winchester R., Kunkel H. G. Patterns of expression of human "Ia-like" antigens during the terminal stages of B cell development. J Immunol. 1978 May;120(5):1480–1484. [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Goodenow R. Genes of the major histocompatibility complex. Cell. 1982 Apr;28(4):685–687. doi: 10.1016/0092-8674(82)90046-0. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Moore G. E. Chromosomes of 14 hematopoietic cell lines derived from peripheral blood of persons with and without chromosome anomalies. J Natl Cancer Inst. 1969 Nov;43(5):1119–1128. [PubMed] [Google Scholar]
- Hämmerling G. J. Tissue distribution of Ia antigens and their expression on lymphocyte subpopulations. Transplant Rev. 1976;30:64–82. [PubMed] [Google Scholar]
- Jensen E. M., Korol W., Dittmar S. L., Medrek T. J. Virus containing lymphocyte cultures from cancer patients. J Natl Cancer Inst. 1967 Oct;39(4):745–754. [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Cooper M. D., Klein J., Abney E. R., Parkhouse R. M., Lawton A. R. Ontogeny of Ia and IgD on IgM-bearing B lymphocytes in mice. J Exp Med. 1977 Jul 1;146(1):297–301. doi: 10.1084/jem.146.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Koch N., Haustein D. The detection of associated erythrocyte membrane proteins by cross-linking and surface radiolabelling. Hoppe Seylers Z Physiol Chem. 1980;361(6):885–894. doi: 10.1515/bchm2.1980.361.1.885. [DOI] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. Structure of Ia antigens: identification of dimeric complexes formed by the invariant chain. J Immunol. 1982 Mar;128(3):1155–1158. [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J., Szymura J., Wabl M. R. Ia associated Ii chain is not encoded by chromosome 17 of the mouse. Immunogenetics. 1982;16(6):603–606. doi: 10.1007/BF00372029. [DOI] [PubMed] [Google Scholar]
- Koch N., Koch S., Hämmerling G. J. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 1982 Oct 14;299(5884):644–645. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Lynes M. A., Lanier L. L., Babcock G. F., Wettstein P. J., Haughton G. Antigen-induced murine B cell lymphomas. I. Induction and characterization of CH1 and CH2. J Immunol. 1978 Dec;121(6):2352–2357. [PubMed] [Google Scholar]
- McKean D. J., Infante A. J., Nilson A., Kimoto M., Fathman C. G., Walker E., Warner N. Major histocompatibility complex-restricted antigen presentation to antigen-reactive T cells by B lymphocyte tumor cells. J Exp Med. 1981 Nov 1;154(5):1419–1431. doi: 10.1084/jem.154.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosic J. P., Nilson A., Hämmerling G. J., McKean D. J. Biochemical characterization of Ia antigens. I. Characterization of the 31K polypeptide associated with I-A subregion Ia antigens. J Immunol. 1980 Oct;125(4):1463–1469. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Mallick U., Ponta H., Herrlich P. A B-lymphocyte-specific high-turnover protein: constitutive expression in resting B cells and induction of synthesis in proliferating cells. Cell. 1982 Jun;29(2):459–468. doi: 10.1016/0092-8674(82)90162-3. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Ponta H., Bächle M., Mallick U., Weibezahn K. F., Herrlich P. Differentiated cells from BALB/c mice differ in their radiosensitivity. Exp Cell Res. 1981 Nov;136(1):111–117. doi: 10.1016/0014-4827(81)90042-2. [DOI] [PubMed] [Google Scholar]
- Rask L., Lindblom J. B., Peterson P. A. Subunit structure of H-2 alloantigens. Nature. 1974 Jun 28;249(460):833–834. doi: 10.1038/249833a0. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Graf L., Sege K. Two allelic forms of mouse beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1167–1170. doi: 10.1073/pnas.78.2.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld G., Meruelo D., McDevitt H. O., Merigan T. C. Effect of type I and type II interferons on murine thymocyte surface antigen expression: induction or selection? Cell Immunol. 1981 Jan 15;57(2):427–439. doi: 10.1016/0008-8749(81)90101-5. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. Surface molecules of cultured human lymphoid cells. Eur J Immunol. 1976 Nov;6(11):777–782. doi: 10.1002/eji.1830061105. [DOI] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- Weimann B. J. Induction of immunoglobulin synthesis in Abelson murine leukemia virus-transformed mouse lymphoma cells in culture. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):163–164. doi: 10.1101/sqb.1977.041.01.021. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]