Abstract
Aphelids are a poorly known group of parasitoids of algae that have raised considerable interest due to their pivotal phylogenetic position. Together with Cryptomycota and the highly derived Microsporidia, they have been recently re-classified as the Opisthosporidia, which constitute the sister group to the fungi within the Holomycota. Despite their huge diversity, as revealed by molecular environmental studies, and their phylogenetic interest, only three genera have been described (Aphelidium, Amoeboaphelidium, and Pseudaphelidium), from which 18S rRNA gene sequences exist only for Amoeboaphelidium and Aphelidium species. Here, we describe the life cycle and ultrastructure of a new representative of Aphelida, Paraphelidium tribonemae gen. et sp. nov., and provide the first 18S rRNA gene sequence obtained for this genus. Molecular phylogenetic analysis indicates that Paraphelidium is distantly related to both Aphelidium and Amoebaphelidium, highlighting the wide genetic diversity of aphelids. Paraphelidium tribonemae has amoeboflagellate zoospores, rhomboid mitochondrial cristae in zoospores, trophonts and plasmodia; lipid-microbody complex and dictyosomes. The amoeboid trophont uses pseudopodia to feed from the host cytoplasm. Though genetically distinct, the genus Paraphelidium is morphologically indistinguishable from other aphelid genera and has zoospores able to produce lamellipodia with subfilopodia like those of Amoeboaphelidium.
Keywords: Aphelids, parasitoids of algae, ultrastructure, molecular phylogeny, life cycle, ecology
Introduction
The aphelids are a divergent group of intracellular parasitoids of common species of eukaryotic phytoplankton. The three known genera have different ecological preferences: Aphelidium and Amoeboaphelidium occur in freshwater and Pseudaphelidium is found in marine environments. Although with only three described genera, the group is highly diverse, including many environmental sequences from diverse ecosystems (Karpov et al. 2013; 2014a). The phyla Microsporidia and Rozellida (Cryptomycota), together with the class Aphelidea, constitute the deepest branches of the Holomycota lineage, forming the so-called ARM-clade (Aphelidea-Rozellida-Microsporidia), which is sister to the classical fungi (Karpov et al. 2013, Torruella et al. 2015). Consequently, the taxonomy of the ARM clade has been reorganized, and a new superphylum Opisthosporidia with three phyla, Aphelida, Cryptomycota (Rozellida) and Microsporidia, has been proposed (Karpov et al. 2014a). We do not consider the Opisthosporidia to be true fungi. Not only does their phylogenetic position place them as sister to true fungi, but also several of their biological peculiarities do not conform the classical definition of fungi. The most remarkable of these is that, unlike osmotrophic fungi, the trophonts of Aphelida and Rozellosporidia (but not Microsporidia, which are extremely specialized derived parasites) engulf the host cytoplasm by phagocytosis, like amoebae (Gromov 2000; Karpov et al. 2014a).
Even though the aphelids seem to encompass a huge genetic diversity (Karpov et al. 2014b), only three strains of Amoeboaphelidium: A. protococcarum: X-5 CALU (Karpov et al. 2013), FD95 (Letcher et al. 2015), and A. occidentale FD01 (Letcher et al., 2013), and a strain of Aphelidium aff. melosirae P-1 CALU (Karpov et al., 2014b) have been investigated with molecular methods. Here, we report the morphological and molecular phylogenetic study of three strains that belong to a new genus and species Paraphelidium tribonemae, which grow in culture on the alga Tribonema gayanum Pasch.
Material and Methods
Isolation and cultivation of Paraphelidium tribonemae
The strains were isolated by M. Mamkaeva in 2014 from roadside ditches in the villages Krestcy (X-108, X-109) and Kurakino (X-103), Novgorod district, Russia and maintained in culture on Tribonema gayanum (strain 20 CALU) as the host. The culture of the host was grown on mineral medium (KNO3, 2 g L-1; KH2PO4, 0.3 g L-1; MgSO4, 0.15 g L-1; EDTA, 10 mg L-1; FeSO4, 5 mg L-1; NaBO3, 1.4 mg L-1; (NH4)6Mo7O2, 4.1 mg L-1; CaCl2, 0.6 mg L-1; ZnSO4, 0.1 mg L-1; CuSO4, 50 g L-1, Co(NO3)2, 20 g L-1) at room temperature in the presence of white light. After inoculation with the parasite, the cultures were incubated for 1 – 2 weeks to reach the maximum infection of host cells. Cells were then harvested and used directly for DNA extraction.
Light and transmission electron microscopy
Light and DIC microscopy observations of living cultures were carried out on a Zeiss Axioplan microscope equipped with black and white MRm Axiocam. For electron microscopy, we used the protocol published earlier (Karpov et al. 2014b). Ultrathin sections were prepared with a Leica Ultracut microtome and double stained. We observed sections on a JEM 1400 (Jeol) microscope equipped with an Olympus Veleta digital camera.
Molecular analyses
Approximately 2 ml of infected cultures were centrifuged and DNA extracted from pelleted cells with the DNA purification kit PowerSoil (MoBio) following the manufacturer’s instructions. To avoid amplifying an excess of host genes, the aphelid 18S rRNA gene was amplified by polymerase chain reaction with the fungi-like specific primers UF1 (5’-CGAATCGCATGGCCTTG) and AU4 (5’-RTCTCACTAAGCCATTC) (Kappe et al., 1996). Each PCR reaction was carried out in 25 µl of reaction buffer, containing 1 μl of the eluted DNA, 1.5 mM MgCl2, dNTPs (10 nmol each), 20 pmol of each primer, and 0.2 U TaqPlatinum DNApolymerase (Invitrogen). PCR reactions consisted of 2 min denaturation at 94 °C; 35 cycles of a denaturation step at 94 °C for 15 s, a 30 s annealing step at 50°C and an extension step at 72 °C for 2 min; and a final elongation step of 7 min at 72 °C. Negative controls without template DNA were used at all amplification steps. Because cultures were not axenic, we cloned the amplified 18S rRNA gene fragments using the Topo TA Cloning System (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Clone inserts were PCR-amplified using flanking vector primers; inserts of expected size (1,400 bp) were sequenced bidirectionally with vector primers (Beckman Coulter Genomics, Takeley, UK). Retrieved sequences were compared by BLAST to sequences in GenBank. For each strain, all of the clone sequences were identical.
Molecular phylogenetic analyses
The three Paraphelidium tribonemae 18S rDNA sequences (X-103, X-108, X-109) were aligned together with the sequences previously used in Karpov et al. 2014 and Letcher et al. 2015 using Mafft auto (Katoh, 2002). The multiple alignment was then manually trimmed to eliminate sites spuriously aligned. A total of 1,393 unambiguously aligned sites were used to reconstruct a phylogenetic tree applying Maximum Likelihood (ML) and Bayesian Inference (BI) methods. ML analyses were done with RAxML 8 (Stamatakis 2014). The best tree was obtained out of 500 best tree searches applying a GTR+G+I model of nucleotide substitution, taking into account a proportion of invariable sites, and a Gamma-shaped distribution of substitution rates with four rate categories. Bootstrap values were calculated using 500 non-parametric replicates with the same substitution model. BI analyses were carried out with MrBayes (Ronquist et al. 2012) applying the GTR+G+I model with four chains and 10,000,000 generations per run. After checking for convergence and eliminating the first 15,000 trees (burn-in), a consensus tree was constructed sampling every 100 trees. Paraphelidium tribonemae 18S rRNA sequences have been deposited in GenBank with the following accession numbers: strain X-108, KX576680; strain X-109, KX576681 and strain X-103, KX576682.
Results
Molecular phylogeny
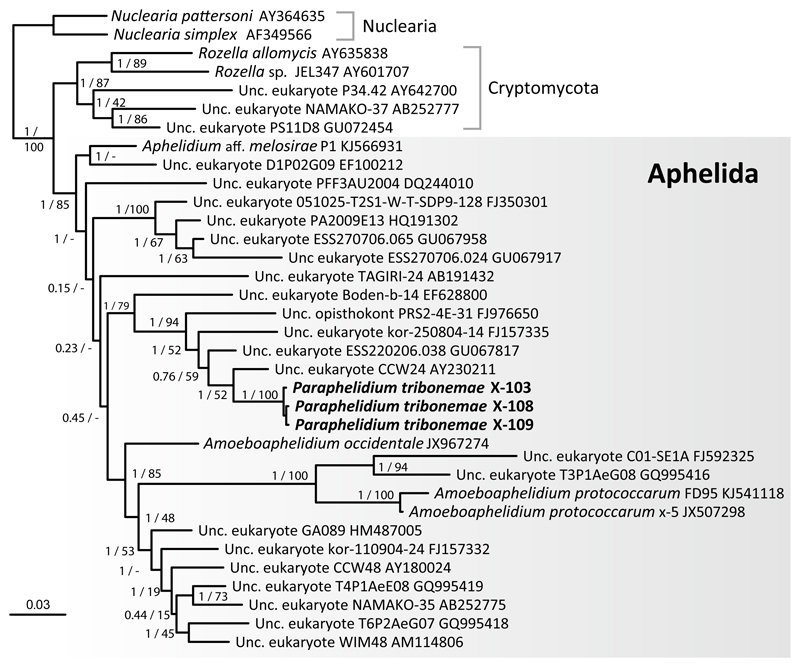
We amplified and sequenced near-full 18S rRNA genes for three morphologically similar aphelid strains, X-108, X-109 and X-103 (CCPP ZIN RAS), maintained in culture on the xanthophyte alga Tribonema gayanum (strain 20 CALU). The three strains share 99.8% pair-wise nucleotide identity but are only distantly related to 18S rRNA sequences from described aphelids. Thus, at the level of 18S rRNA gene, Paraphelidium tribonemae shares only 89% identity with Aphelidium aff. melosirae, 91% with Amoeboaphelidium occidentale and 87% with A. protococcarum. . We reconstructed maximum likelihood (ML) and Bayesian inference (BI) phylogenetic trees including the new 18S rDNA sequence of strains X-108, X-109 and X-103 and a selection of fungal sequences similar to the one previously used by Karpov et al. (2013). In our trees, all Aphelida strains branched within a strongly supported group (Fig. 1) (ML bootstrap proportion 100% and Bayesian posterior probability 1) that contained the three available Amoebaphelidium sequences (strain x-5 from Karpov et al. 2013 and strains FD01, FD95 from Letcher et al. 2013; 2015), and a strain of Aphelidium aff. melosirae P-1 CALU (Karpov et al., 2014b) as well as a large number of environmental 18S rDNA sequences. Because the 18S rRNA gene sequences of the three strains were nearly identical to each other, we consider these three strains to belong to the same species. Further, these strains share a 243 bp deletion that is not found in the closest 6 environmental sequences, which form a larger cluster with them (Fig.1). Given that these strains formed a distinct cluster distant from known aphelid genera, they deserve a new genus and species. Herein we characterize the morphology and biological peculiarities of the three strains X-108, X-109 and X-103 in greater detail and name the new taxon Paraphelidium tribonemae (see taxonomy section below).
Fig. 1. 18S rDNA-based Bayesian phylogenetic tree showing the position of Paraphelidium tribonemae strains X-103, X-108 and X-109.
The tree contains aphelid sequences, and some representatives of two outgroup lineages, Cryptomycota/Rozellids and nucleariids. The tree was inferred using 1,393 conserved positions. Statistical supports are shown at each node; Bayesian posterior probabilities are displayed on the left and Maximum Likelihood bootstrap values, on the right.
Life cycle
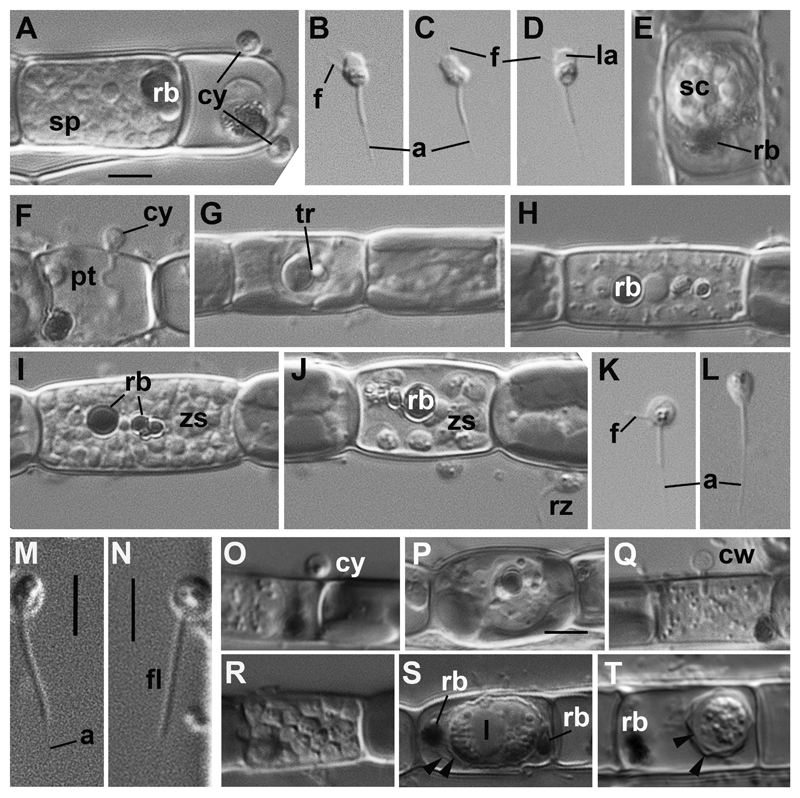
The life cycle of the three strains of Paraphelidium tribonemae was very similar to that of the genus Aphelidium, and encompassed several phases as follows (Fig. 2). The zoospore attaches to the host alga, and encysts (Fig. 2 A, O). The cyst germinates and penetrates the host cell wall with an infection tube (Fig. 2 F). A conspicuous enlarging vacuole pushes the contents of the cyst towards the interior of the host cell through the infection tube. The parasitoid becomes an intracellular phagotrophic amoeba which engulfs the host cytoplasm forming food vacuoles. The parasitoid continues to grow and forms an endobiotic plasmodium with one or two residual bodies while it totally consumes the cytoplasm of the host cell (Fig. 2 H,Q). A multinucleate plasmodium is formed with a large central vacuole and a residual excretion body. The mature plasmodium then divides into a number of uninucleated cells (Fig. 2 A, I, R). After maturation, the uniflagellated zoospores are released from the empty host cell, and then infect other host algal cells (Fig. 2 J).
Fig. 2. Main stages of the life cycle of Paraphelidium tribonemae observed in living material by differential interference contrast (DIC) microscopy.
A– E: strain X-108: A: cysts (cy) and sporangium (sp) with maturating zoospores at the Tribonema filament; B –D: amoeboid zoospores with anterior lamellipodium (la) and filopodia (f), and long acroneme (a); E: sporocyst (sp) ejecting a residual body (rb). F-L: strain X-109: cyst (cy) with penetration tube (pt); G: trophont (tr) in the host cell; H: plasmodium with residual body (rb) in the central vacuole; I: zoosporangium with mature zoospores (zs) and fragmented residual body (rb); J: zoospore releasing: inside the host (zs), and released (rz); K: amoeboid zoospore with filopodia (f); L: swimming zoospore. Both have long acroneme (a). M– T: strain X-103: M – N: zoospores on the surface of algal filament showing a flagellum (fl) with short acroneme (a); O: cyst (cy); P: trophont; Q: plasmodium with empty cyst wall (cw); R: sporangium with zoospores; S – stage of sporocyst formation with lipid globule (l) and rejecting residual bodies (rb) between 2 walls (arrowheads); T: mature sporocyst covered with inner and outer walls (arrowheads), residual body (rb) ejected. Arrowheads in B, C, D, K show filopodia. Scale bar = 4 μm (A-J,P,S); 3 μm (K-N); 5 μm (O,Q,R,T).
We also studied the ultrastructure at the main life cycle stages of strains X-108 and X-103 (Figs 3, 4) but found no consistent ultrastructural differences between these two strains.
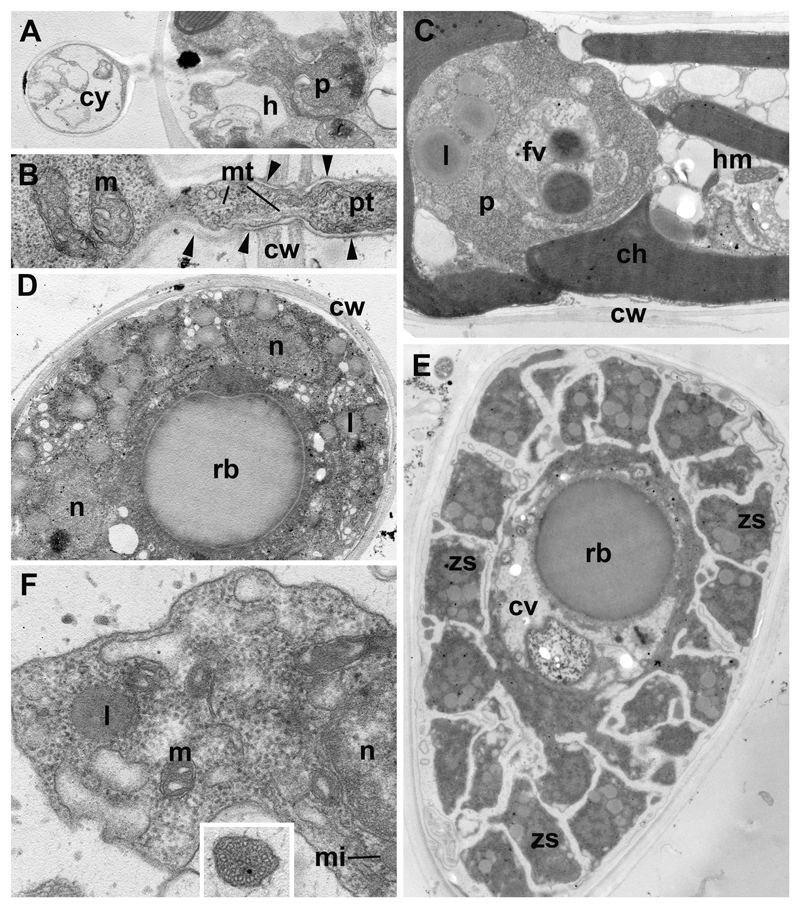
Fig. 3. Ultrastructure of Paraphelidium tribonemae (strain X-108).
A: parasite (p) recently penetrated the host (h) from the cyst (cy). B: contents of penetration tube (pt) during parasite migration from the cyst (left) to the host (right): cw-cell wall of the host, m-mitochondrion, mt-microtubules; arrowheads show the chitin wall of the cyst. C: young trophont (p) in the alga: ch-chloroplast, cw-algal cell wall, hm-host mitochondrion, fv-food vacuole in trophont, l-lipid globule of parasite. D: plasmodium in the algal cell wall (cw) with nuclei (n), lipids (l), central vacuole with residual body (rb). E: zoosporangium at the final stage of zoospore (zs) maturation with zoospores budding from cytoplasm surrounding central vacuole (cv) with residual body (rb). F: zoospore in sporangium at higher magnification: l-lipid globule, m-mitochondrium, mi-microbody, n-nucleus; insert: cross section of flagellum with complete set of microtubules. Scale bar = 2 µm (A-E); 250 nm (F),
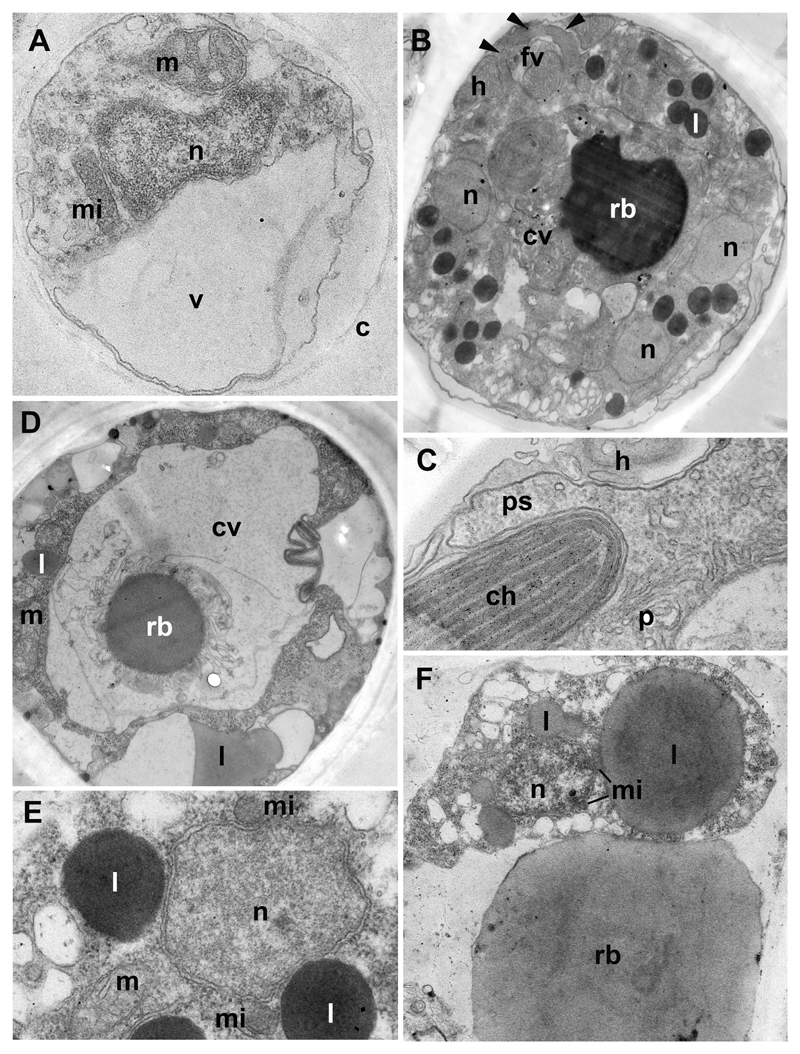
Fig. 4. Ultrastructure of Paraphelidium tribonemae (strain X-103).
A: structure of cyst covered with chitin wall (c), contains nucleus (n), mitochondrium (m), microbody (mi) and growing vacuole (v). B: late trophont with several nuclei (n), central vacuole (cv) with residual body (rb), lipids (l) and food vacuole (fv) after engulfing a portion of the host cytoplasm (h). Arrowheads mark enclosed pseudopodia. C: pseudopodium (ps) of parasite (p) around the host (h) chloroplast (ch). D: cross section of the plasmodium through the large central vacuole (cv) with residual body (rb); l-lipid droplets, m-mitochondrium. E: portion of plasmodium at higher magnification with nucleus (n) and associated microbodies (mi), lipids (l) and mitochondrium (m). F: zoospore attached to residual body (rb) in sporangium, note a microbody (mi) profiles associated with nucleus (n) and large lipid globule (l). Scale bar = 500 nm (A, C, E); 2 µm (B, D); 1.5 µm (F).
Zoospores
Generally, the most informative feature for aphelid taxonomy is considered to be the structure of zoospores and resting spores, or sporocysts (Gromov, 2000; Karpov et al., 2014a). The zoospores of all our strains are able to swim with a posterior flagellum, or to crawl on substrate, like amoebae, with filopodia. Strains X-108 and X-109 have similar zoospores; these are ovoid swimming cells ~2.5 µm long with an acronematic flagellum of ~7 µm including an acroneme of 3.5 µm (Fig. 2 B-D, K, L). In the vicinity of the host algal filament zoospores produce a broad anterior lamellipodium, approximately half of the cell long with few subfilopodia extruding in anterior and lateral directions (Fig. 2 B-D). Some filopodia may appear independently of the lamellipodium from the posterior half of the body. The lamellipodium is often visible in the strain X-108, and quite rare in the strain X-109. Zoospores of strain X-103 have similar cell length and appearance, but have a much shorter acroneme: about 1.3 µm instead of 3.5 µm in other strains in nearly the same total length of flagellum (7 – 7.5 µm) (Fig. 2 M, N).
The ultrastructure could only be studied for immature zoospores in the zoosporangium (Figs 3 E, F; 4 F). Zoospore maturation includes the development of the flagellum, which may have both the complete (Fig. 3 F, insert) and incomplete (not shown) set of microtubules in the axoneme. A flagellum grows at the zoospore distal end with respect to the center of the sporangium, which is often occupied by a central vacuole with residual body (Fig. 3 E). The thin cytoplasmic layer surrounding a central vacuole is the remnant of the plasmodium left after zoospore formation. Just after division, the immature zoospores are amoeboid with long cytoplasmic projections connecting the cells to each other and to the central portion of cytoplasm (Figs 3 E). Many lipid globules, probably containing a storage material supporting the metabolism of these dispersal stages, are associated with small microbodies which also adjoin the nucleus (Fig. 4 F). Mitochondria have rare flat to rhomboid cristae.
Cysts
Newly encysted zoospores and empty cyst walls of the three strains are indistinguishable at this stage (Fig. 2 A, F, O, Q). They are spherical and ~2.5 µm in diameter. After encystment, the parasitoid germinates through the host cell wall and reaches the host plasma membrane (Fig. 3 A, B). On the external side of the plasma membrane, the cyst produces a relatively thin wall which covers a penetration or infection tube. The length of the infection tube differs from cell to cell (Fig. 2 F, Q). The cyst normally contains a nucleus, a microbody, a mitochondrion with rhomboid cristae, lipid globules (not shown) and a growing vacuole (Fig. 4 A). Empty cysts remain attached to the host cells by their infection tubes for some time.
After injection of the cyst contents in the host, the parasitoid remnants, notably membranes and a thin layer of cytoplasm, remain in the cyst (Fig. 3 A). We propose that a vacuole pushes out the main part of the parasite cytoplasmic content through the penetration tube, and that the vacuole, with its contents and surrounding cytoplasm, persists within the space delimited by the cyst wall, being separated from the migrating cell content. The penetration tube contains microtubules (Fig. 3 B), implying that cytoplasmic organelles move into the host not only by vacuole pressure but also using microtubule-mediated mechanisms of intracellular transport.
Trophont and plasmodium
These stages, shown in Fig. 2 G and P for strains X-109 and X-103, are similar in all strains. The residual body appears rather early in the parasitoid trophic cell, which consumes first the central zone of the host cell and then engulfs the peripheral chloroplasts and mitochondria. Food vacuole formation (Fig. 4 B, C) and newly formed food vacuoles that have engulfed host organelles can be seen in young (Fig. 3 C) and old (Fig. 4 B) trophonts. The latter contain several nuclei, many lipid globules, mitochondria and large central vacuole with residual body (Fig. 4 B). This vacuole appears also in very young trophic cells (Fig. 3 C).
The plasmodium looks homogenous with a residual body in the center (Fig. 2 H). Before zoospore formation, a residual body may migrate to the cell periphery or remain at the cell center (Fig. 2 Q; 3 D; 4 D). Many plasmodia have a huge central vacuole, which looks nearly empty, and contains a comparatively small residual body (Fig. 4 D). Nuclei are associated with small microbodies and lipid globules (Fig. 4 E). This association can be also seen in the newly formed zoospores (Fig. 3 F; 4 F).
Zoosporangium
Sporangium formation is a short stage constrained by zoospore maturation. The mature plasmodium divides into zoospores more or less simultaneously; the borders between future zoospores become progressively visible (cf. Fig. 2 A, I, R). The number of zoospores in sporangia varies (20 – 50), but is limited by the dimension of host cell. The sporangial stage does not differ between strains.
Recently formed zoospores are densely packed within the space delimited by the host wall. Each mature zoospore develops a flagellum. Zoospores seem to be released from the host one by one, as we often see a few zoospores inside nearly empty host cells (Fig. 2 J).
Resting spore (sporocyst)
Resting spores or sporocysts are rare in young cultures of this parasitoid, but often occur in old cultures of all the strains. Spore formation seems to start with plasmodium constriction, which removes one or two residual bodies outside the cell and produces an inner wall (Fig. 2 E, S). At this stage residual bodies are in the space between inner and outer walls: the sporocyst is ellipsoid and contains a big central lipid globule surrounded by granular cytoplasm (Fig. 2 S). Mature spores are rounded, 8– 10 μm in diameter, and surrounded by two spore walls without residual bodies, which are rejected from the inter-wall space (Fig. 2 T). The outer wall may be slightly folded (Fig. 2 E).
Discussion
The freshwater aphelid strains X-108, X-109 and X-103 are closely related based on 18S rRNA molecular phylogenetic analysis: their sequences form a compact clade that is only distantly related to the sequence of Aphelidium aff. melosirae Scherffel 1925, and to three available sequences from the genus Amoeboaphelidium (Fig. 1). Although 18S rRNA gene sequences for representatives of Pseudaphelidium are not yet available, members of this genus are exclusively marine and parasitize a different host, hence, they most likely represent an independent genus. Based on our molecular phylogenetic analysis, we establish Paraphelidium as a separate genus for the cluster formed by X-108, X-109 and X-103.
Based exclusively on the morphology of the life cycle stages, Paraphelidium tribonemae is most similar to Aphelidium tribonemae Scherffel, 1925, as described by B.V. Gromov (1972): parasitoid of a yellow-green alga Tribonema; zoospores 2–3 μm in diameter; flagellum is about 6 – 8 μm long with long (1/3 – 1/2 of flagellum length) acroneme; zoospores can produce filopodia and move like amoebae with an immotile flagellum. Gromov observed the development of A. tribonemae in Tribonema gayanum and Botridiopsis intercedens Visch. et Pasch.
The original description of A. tribonemae is a bit different (Scherffel, 1925): cyst diameter is ~2 μm, zoospore body is 4 μm long with an 11 μm flagellum (measured from Figure 107 a, Taf. 3 in: Scherffel, 1925). The acroneme was not obviously present in Scherffel's pictures. According to Scherffel, A. tribonemae's zoospores become amoeboid when they encounter an obstacle, but he did not describe the pseudopodia shape. Resting spores have a diameter of 6 – 7 μm. The residual body is outside the spore, which has thick wall and restricted round body. Unlike in A. tribonemae the resting spore of Paraphelidium has two spore walls. Thus, we cannot consider our species as A. tribonemae Scherffel, 1925. Paraphelidium is more similar to A. tribonemae, as found and studied by Gromov (1972), but also has some minor differences, notably in the shape of zoospore pseudopodia. In this respect, the shape of pseudopodia in aphelids deserves some discussion.
Amoeboid activity is common for Amoeboaphelidium and Aphelidium zoospores (Gromov 2000; Karpov et al. 2013; 2014a, b; Letcher et al., 2013; 2015). Aphelidium can produce two types of pseudopodia: filopodia (A. chlorococcarum f. majus,) and a short anterior lobopodium (A. melosirae). In A. tribonemae sensu Gromov, 1972 and A. chlorococcarum f. majus filopodia appear at the anterior end of the zoospore (Gromov and Mamkaeva 1975), whereas the zoospores of A. tribonemae produce filopodia in all directions. However, whereas in both Aphelidium species filopodia project straight from the cell body, they appear from the lamellipodium in Paraphelidium. The latter character is also common for Amoeboaphelidium (Gromov and Mamkaeva 1970a; Karpov et al., 2013; Letcher et al. 2013; 2015), which forms a sister clade to Paraphelidium (Fig. 1), but zoospores in this genus have a pseudocilium instead of a normal flagellum and cannot swim.
We preliminarily suggest that the ability of zoospores to produce an anterior lamellipodium with subfilopodia is a feature of the Amoeboaphelidium + Paraphelidium clade. We cannot exclude, of course, that this character can be also found in Aphelidium, but up to now such evidence is missing.
Our results highlight once more the fact that similar protist morphologies can hide huge genetic divergences (cryptic species) and suggest that, as more sequences are available for protists traditionnally classified within single morphospecies, protist diversity will be unveiled to a much greater extent. This genetic diversity must underlie phenotypic differences other than morphology and ultrastructure.
Taxonomical description
Paraphelidium gen. nov. Karpov, Moreira et Lopez-Garcia
Zoospores swim with a posteriorly oriented flagellum or move like amoebae with an immobile flagellum. Amoeboid zoospore can produce short anterior lamellipodium with subfilopodia and separate filopodia. Mature resting spore (sporocyst) is ellipsoid and covered with two walls.
P. tribonemae Karpov, Moreira et Lopez-Garcia (Fig. 2)
Zoospores with body length of 2 – 2.5 µm, flagellum 7 µm in length with variable length of acroneme (1 – 3.5µm). Mature sporocyst is 8 – 10 µm diameter with big central globule.
Host: Tribonema gayanum.
Type: Fig. 2 A-E (strain X-108). Strain X-108 was collected and isolated by Maria Mamkaeva in 2014 from road ditch in the village Krestsi (58°15′17″ N, 32°30′41″ W) , Novgorod district of Russian Federation. Culture deposited in CCPP ZIN RAS collection under the No: X-108.
Comments. Morphologically similar strains were collected and isolated by Maria Mamkaeva in 2014 in the villages Krestci (X-109) and Zaborka (X-103), Novgorod district of Russian Federation. Cultures have been deposited in the CCPP ZIN RAS collection under No: X-109 and X-103.
Ultrastructure. The ultrastructure of Paraphelidium spp. was not studied in detail, but in general looks very similar to that of other aphelids: Pseudaphelidium drebesii (Schweikert and Schnepf 1997), Amoeboaphelidium spp. (Gromov and Mamkaeva 1970b; Karpov et al. 2013; Letcher et al. 2013; 2015) and Aphelidium spp. (Schnepf et al. 1971; Gromov and Mamkaeva, 1975; Karpov et al. 2014b). Trophic amoeba of P. tribonemae are unambiguously phagotrophic (Fig. 4).
Acknowledgments
Research leading to these results received funding from the Russian Foundation for Basic Research (projects No 15-29-02734), and the European Research Council under the European Union’s Seventh Framework Program ERC Grant Agreement 322669 “ProtistWorld”. The morphological study of strains X-103, X-108, and X-109, data analysis and the manuscript writing have been supported by the Russian Scientific Foundation grant no. 16-14-10302. We acknowledge funding from the Conseil régional Ile-de-France (SESAME program) that served to set up the Unicell facility. We also thank the Research Resource Center (RRC) for Molecular and Cell Technologies and Center for Culturing Collection of Microorganisms of Research park of St. Petersburg State University (SPbSU) for access to the EM facilities and long-term maintenance of cultures strains, respectively.
Literature Cited
- Gromov BV. Aphelidium tribonemae Scherffel parasitizing yellow green algae. Mikol Fitopatol. 1972;6:443–445. in Russian. [Google Scholar]
- Gromov BV. Algal parasites of the genera Aphelidium, Amoeboaphelidium and Pseudoaphelidium from the Cienkovski’s “Monadea” group as representatives of new class. Zool Zhurnal. 2000;79:517–525. in Russian. [Google Scholar]
- Gromov BV, Mamkaeva KA. Electron-microscopic investigations of development cycle and feeding behaviour of intercellular parasite of Chlorella, Amoebaphelidium chlorellavorum. Tsilogiya. 1970a;12:1191–1196. in Russian. [Google Scholar]
- Gromov BV, Mamkaeva KA. The fine structure of Amoeboaphilidium protococcarum – an endoparasite of green alga Scenedesmus. Arch Hydrobiol. 1970b;67:452–459. [Google Scholar]
- Gromov BV, Mamkaeva KA. Zoospore ultrastructure of Aphelidium chlorococcarum Fott. Mikol Fitopatol. 1975;9:190–193. in Russian. [Google Scholar]
- Kappe R, Fauser C, Okeke CN, Maiwald M. Universal fungus-specific primer systems and group-specific hybridization oligonucleotides for 18S rDNA. Mycoses. 1996;39:25–30. doi: 10.1111/j.1439-0507.1996.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Karpov SA, Mikhailov KV, Mirzaeva GS, Mirabdullaev IM, Mamkaeva KA, Titova NN, Aleoshin VV. Obligately phagotrophic aphelids turned out to branch with the earliest-diverging fungi. Protist. 2013;164:195–205. doi: 10.1016/j.protis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Karpov SA, Mamkaeva MA, Aleoshin VV, Nassonova ES, Lilje O, Gleason FH. Morphology, phylogeny and ecology of the aphelids (Aphelidea, Opisthokonta) with proposal of new superphylum Opisthosporidia. Front Microbiol. 2014a;5:112. doi: 10.3389/fmicb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov SA, Mamkaeva MA, Benzerara K, Moreira D, López-García P. Molecular phylogeny and ultrastructure of Aphelidium aff. melosirae (Aphelida, Opisthosporidia) Protist. 2014b;165:512–526. doi: 10.1016/j.protis.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher PM, Lopez S, Schmieder R, Lee PA, Behnke C, Powell MJ, McBride RC. Characterization of Amoeboaphelidium protococcarum, an algal parasite new to the cryptomycota isolated from an outdoor algal pond used for the production of biofuel. PLoS One. 2013;8:2. doi: 10.1371/journal.pone.0056232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher PM, Powell MJ, Lopez S, Lee PA, McBride RC. A new isolate of Amoeboaphelidium protococcarum, and Amoeboaphelidium occidentale, a new species in phylum Aphelida (Opisthosporidia) Mycologia. 2015;107:522–531. doi: 10.3852/14-064. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherffel A. Endophytische Phycomyceten-Parasiten der bacilleriaceen und einige neue Monadinen. Arch Protistenkd. 1925;52:1–141. [Google Scholar]
- Schnepf E, Hegewald E, Soeder CJ. Electronen-mikroskopische Beobachtungen an Parasiten aus Scenedesmus-Massenkulturen. 2. Uber Entwicklung und Parasit-Wirt-Kontakt von Aphelidium und virusartige Partikel im Cytoplasma infizierter Scenedesmus-Zellen. Acta Mikrobiol. 1971;75:209–229. [Google Scholar]
- Schweikert M, Schnepf E. Electron microscopical observations on Pseudoaphelidium drebesii Schweikert and Schnepf, a parasite of the centric diatom Thalassiosira punctigera. Protoplasma. 1997;199:113–123. [Google Scholar]
- Torruella G, de Mendoza A, Grau-Bove X, Anto M, Chaplin MA, del Campo J, Eme L, Perez-Cordon G, Whipps CM, Nichols KM, Paley R, et al. Phylogenomics Reveals Convergent Evolution of Lifestyles in Close Relatives of Animals and Fungi. Curr Biol. 2015;25:2404–2410. doi: 10.1016/j.cub.2015.07.053. [DOI] [PubMed] [Google Scholar]