Abstract
Drug hypersensitivity involves the activation of T-cells in an HLA allele-restricted manner. Since the majority of individuals who carry HLA risk alleles do not develop hypersensitivity, other parameters must control development of the drug-specific T-cell response. Thus, we have utilized a T-cell priming assay and nitroso sulfamethoxazole (SMX-NO) as a model antigen to investigate (1) the activation of specific T-cell receptor (TCR)Vβ subtypes, (2) the impact of PD-1, CTLA4 and TIM-3 co-inhibitory signalling on activation of naïve and memory T-cells and (3) the ability of Tregs to prevent responses. An expansion of the TCR repertoire was observed for nine different Vβ subtypes, while spectratyping revealed that SMX-NO-specific T-cell responses are controlled by public TCRs present in all individuals alongside private TCR repertoires specific to each individual. We proceeded to evaluate the extent to which the activation of these TCR Vβ-restricted antigen-specific T-cell responses is governed by regulatory signals. Blockade of PDL-1/CTLA4 signalling dampened activation of SMX-NO-specific naïve and memory T-cells, while blockade of TIM-3 produced no effect. PD-1, CTLA4, and TIM-3 displayed discrete expression profiles during drug-induced T-cell activation and expression of each receptor was enhanced on dividing T-cells. As these receptors are also expressed on Tregs, Treg-mediated suppression of SMX-NO-induced T-cell activation was investigated. Tregs significantly dampened the priming of T-cells. In conclusion, our findings demonstrate that distinct TCR Vβ subtypes, dysregulation of co-inhibitory signalling pathways and dysfunctional Tregs may influence predisposition to hypersensitivity.
Introduction
Drug hapten-specific T-cell responses are detectable in patients that present with hypersensitivity reactions affecting skin and internal organs. It is important to emphasize that reactions do not develop in all patients; they are idiosyncratic in nature with a prevalence of between 1 in 10,000-100,000 individuals (1). Recent studies focusing on mechanisms of β-lactam hypersensitivity have shown that (1) a threshold level of antigenic drug-protein adduct is exceeded in all patients exposed to a therapeutic drug course (2) and (2) all individuals have T-cells within their repertoire that can be activated with drugs (3). Thus, it is now important to investigate the immunological parameters that determine whether the formation of protein adducts in vivo will result in a drug-specific T-cell response and tissue injury.
In recent years, progress in this field has centred on the association of multiple drugs with specific human leukocyte antigen (HLA) alleles. However, with the exception of HLA-B*57:01-restricted abacavir and HLA-B*15:02-restricted carbamazepine (CBZ) hypersensitivity (4–6), the majority of individuals who carry known HLA risk alleles do not develop hypersensitivity when exposed to a culprit drug. Indeed, T cell stimulation can be influenced by a multitude of factors which can be divided into signals 1 and 2. Signal 1 refers to the interaction of a T-cell receptor (TCR) with a corresponding peptide-HLA complex. Whether the expression of specific TCRs influences susceptibility to drug hypersensitivity remains largely unexplored. A recent study reported that CBZ hypersensitivity only occurs in individuals who express both a particular HLA variant and a specific TCR Vβ (7). However, this is not the case for abacavir hypersensitivity (8). While signal 1 is required for T-cell signalling, signal 2 determines whether this ultimately translates into T cell activation and thus it is the nature of both signals that ultimately determines the unique T-cell activation threshold for an individual ultimately whether a response ensues. Signal 2 is composed of both co-stimulatory and co-inhibitory pathways that signal simultaneously to regulate T cell activation in a complex balancing act between tolerance and activation. We have shown that blockade of the Programmed Death-1 (PD-1) pathway via PDL-1, but not PDL-2, enhances the priming of naïve T-cells to drug-antigens. Although PD-1 is an important immune checkpoint, complex interplay between pathways means that it is crucial to elucidate the role of additional co-signalling pathways and how they interact to effectively analyse the role of regulatory signalling during T-cell activation. Additionally, as it has recently been reported that certain reactions may be caused by a drug-antigen stimulating pre-existing memory T-cells (9), it is critical to assess the role of regulatory pathways during both primary and secondary T-cell responses.
Similar to the PD-1-PD-L1 interaction, Cytotoxic T-lymphocyte Associated Protein-4 (CTLA4) represents a critical checkpoint in T-cell regulation as the individual knockdown of these receptors leads to overwhelming lymphoproliferation in mice, ultimately resulting in death (10–12). CTLA4 has two ligands, CD80 and CD86, which it shares with the co-stimulatory receptor CD28 and thus these opposing pathways act to competitively inhibit one another (13). While there is a wide range of other co-inhibitory pathways, the role of the lesser known receptor T-cell immunoglobulin- and mucin-domain protein-3 (TIM-3), which mediates its function through binding to galectin-9 leading to the death of predominantly Th1-specific T-cells, is particularly interesting. This is because activation of TIM-3 has been reported to act synergistically alongside PD-1 on tumour-infiltrating lymphocytes, inferring that together these pathways represent a formidable immunological barrier to T-cell activation (14).
Aside from direct signalling between dendritic cells (DC) and effector T-cells, co-inhibitory pathways are also utilised by CD25+ T regulatory cells (Tregs). This subset of T helper cells function to suppress effector T cell responses and are physiologically important for the establishment of feto-maternal tolerance and dampening T-cell responses after antigen clearance (15). Recent research suggests that dysfunctional Tregs may have a role in specific drug hypersensitivity reactions with Takahashi’s group reporting that Tregs are functionally defective at the cutaneous sites of toxic epidermal necrolysis (TEN). Furthermore, models of toxic epidermal necrolysis (TEN) found that Tregs can prevent TEN-associated epidermal injury (16, 17) and so susceptibility in patients may be linked to a defect in Tregs, potentially through abnormal co-inhibitory signalling. Indeed, polymorphisms in co-inhibitory pathways are associated with the dysregulation of T-cell activation and the onset of T-cell-induced autoimmune diseases (18–21). However, how these receptors affect drug antigen-specific T-cell activation remains unknown.
As single genetic associations cannot fully account for the majority of drug-induced hypersensitivities, it is becoming increasingly apparent that the aetiologies of such reactions are likely multifaceted meaning the presence of a sole risk factor will not alone predispose an individual to develop hypersensitivity. Therefore, it is critical to examine how different elements of signal 1 and signal 2 interact during T cell priming to a specific drug and to characterise the phenotype of the T-cells responsible for eliciting drug hypersensitivity reactions. Using the chemically-defined model drug antigen nitroso sulfamethoxazole (SMX-NO) and an in vitro priming assay that utilises T-cells and autologous DCs from healthy individuals (22), we have investigated the effects of the above mentioned parameters on drug antigen-induced T-cell activation. Specifically, we (1) probed for the preferential use of specific TCR Vβ subtypes, (2) expanded on our previous PD-1 study by comparing the role of PD-1-PD-L1 signalling during the activation of naïve and memory T-cells from healthy donors, (3) investigated CTLA4 and TIM-3 co-inhibitory signalling, and (4) quantified the suppressive capacity of CD25+ Tregs.
Materials and Methods
Human subjects
120 ml of venous blood was donated by SMX-naïve human donors, all of whom had given informed written consent to partake in this study approved by the Liverpool local research ethics committee. A previous lack of sensitisation in donors who later produced SMX-responsive T-cells was confirmed by negative lymphocyte transformation test in response to SMX (1-1.5mM) and SMX-NO (20-40uM). This was observed despite a significant response following exposure to tetanus toxoid (positive control).
Cell separation
PBMC were separated from whole blood using Lymphoprep (Axis-shield, Dundee) and density gradient separation. Distinct cell populations were isolated using magnetic bead separation columns performed in line with the manufacturer’s instructions (Miltenyi Biotec, Bisley, U.K.). Briefly, total PBMC were subject to positive selection for CD14+ monocytes, after which the non-CD14+ population was incubated with an anti-T-cell antibody cocktail to negatively select for CD3+ pan T-cells. Lastly, pan T-cells were subject to two further consecutive positive selection steps; CD25+ selection for isolation of T regulatory cells, and CD45RO+ selection on the CD25- population for the isolation of memory T-cells (CD45RO+) from the remaining naïve (CD45RO-) T-cell population. 2 x 106 naïve T-cells from donors selected for TCR Vβ spectratyping analysis were frozen in RNAlater (stored at -80°C) while all other cells were frozen in 10% DMSO and stored at -150°C before use.
In vitro T-cell priming assay
To generate DCs, CD14+ monocytes were cultured (3-5 x 106/well; 6-well plate; total volume, 6 ml; 37°C/5% CO2) for 8 days in R9 medium (RPMI 1640, 100 µg/ml penicillin, 100 U/ml streptomycin, 25 µg/ml transferrin, 10% human AB serum [Innovative Research], 25 mM HEPES buffer, 2 mM L-glutamine) supplemented with GM-CSF and IL-4 (800 U/ml) [PeproTech Ltd]. To induce DC maturation, 25 ng/ml TNF-α [PeproTech Ltd] and 1 µg/ml lipopolysaccharide [Sigma-Aldrich Ltd, E.coli 0111:B4] were added on the penultimate day of culture. Naïve or memory CD3+ T-cells, with Tregs removed, were cultured (2.5 x 106/well; 24 well plate; total volume, 2 ml) with autologous mature DCs (0.8 x 105/well) and SMX-NO (50 µM) for 8 days. To specific wells, human targeted anti-PD-L1, anti-CTLA4, or anti-TIM-3 antibodies (Biolegend, London, UK; anti-PD-L1, 5 µg/ml; anti-CTLA4, 10 µg/ml; anti-TIM-3, 7.5 µg/ml) were added prior to the addition of SMX-NO and incubated for ≥ 30 min (37°C/5% CO2). Dose ranging studies were performed around those directed by the supplier to ascertain optimal antibody concentrations. Alternatively, specific quantities of autologous CD25+ Tregs were added to individual wells. Experiments were repeated a minimum of three times.
T-cell readouts
SMX-NO-primed naïve or memory T-cells were harvested and then plated (1 x 106/well; 96 well plate; total volume, 200 µl) with autologous mature DCs (4 x 103/well) and SMX-NO (20-50 µM) for assessment of proliferative capacity by [3H] thymidine incorporation (triplicate cultures) or CFSE-incorporation, or through analysis of cytokine/cytolytic molecule secretion using ELISpot (duplicate cultures) after incubations of 48, 72, and 96 h, respectively (37°C/5% CO2). Phytohemagglutinin (PHA; 20 μg/ml) was used as a positive control. The secretion of IFN-γ, IL-13, and granzyme B was visualised by following the ELISpot procedure provided by the manufacturer (Mabtech, Nacka Strand, Sweden). [3H] thymidine (0.5 µCi/well) was added to the proliferation plate, which was then subject to a further 16 hr incubation before analysis of incorporated radioactivity using a Microbeta Trilux 1450 LSC beta counter (PerkinElmer, Cambridge, U.K.). CFSE-analysis was conducted on a FACS Canto II flow cytometer (BD Bioscience, Oxford, U.K.) using a previously established protocol (22). T-cell phenotype was assessed at various time-points through the priming process by staining with anti-CD3-APC, CD4-vioblue, CD8-PerCPCy5, CTLA4-APC and TIM-3-PE antibodies.
For TCR Vβ spectratyping analysis, SMX-NO-primed naïve T-cells were restimulated with fresh mature DCs (DC:T-cell ratio, 1:25) and SMX-NO (50 µM; 24 well plate) for 72 h before being subject to a CD45RO+ magnetic bead isolation as previously described. While some of the obtained memory T-cells were used for TCR Vβ FACS analysis, 2 x 106 CD45RO+ T-cells were also immediately frozen down in RNAlater. Naïve T-cells primed to the β-lactam antibiotic piperacillin (2mM) were also subjected to TCR Vβ spectratyping analysis and compared to the SMX-NO data.
Generation and characterisation of T-cell clones
T-cell clones were generated from both memory and naïve T-cell priming cultures using previously described methods of serial dilution and subsequent mitogen-driven expansion (23, 24). For testing antigen-specific responses in T-cell clones, autologous EBV-transformed B-cells were generated as previously described (25) and utilised as functional antigen presenting cells. To validate antigen-specificity, T-cell clones (5 x 104/well; 96 well plate; total volume, 200 µl) were cultured in duplicate per experimental condition with irradiated EBV-transformed B-cells (1 x 104/well) ± SMX-NO (40 µM). After incubation for 48 h (37°C/5% CO2), [3H] thymidine was added and cultures were incubated for a further 16 h before measurement of cellular proliferation by scintillation counting. Clones deemed antigen specific, by obtaining a stimulation index (mean cpm drug-treated wells / mean cpm of control wells) of > 2, were repetitively stimulated with allogeneic PBMCs (5x104/well; 96 well plate; total volume, 200 µl) in R9 medium supplemented with PHA (5 µg/ml) and IL-2. T-cell clone phenotype was assessed using flow cytometry by staining with anti-CTLA4-APC or TIM-3-PE. Further assays to determine the SMX-NO-dependent proliferation of T-cell clones or the secretion of IFN-γ, IL-13, and granzyme B ± PD-L1, CTLA4, or TIM-3 blocking antibodies were assessed by [3H] thymidine incorporation and ELISpot, respectively.
Flow cytometry
A FACS Canto II flow cytometer was used to acquire cells based upon forward/side scatter characteristics. A minimum sample size of 50,000 cells was acquired and data was analysed using associated FACS DIVA or Cyflogic software.
T-cell receptor Vβ analysis by flow cytometry
TCR Vβ protein expression was determined using the IOTest Beta Mark TCR Vβ repertoire kit (Immunotech, Beckman Coulter, UK). Aliquots of T-cells were stained with 24 different fluorescence-conjugated TCR Vβ antibodies for 20 min in the dark, with further inclusion of CD3-APC enabling the gating of T-cells. T-cells were washed with PBS and resuspended in 300 μl 10% FBS HBSS. Data was analysed using Cyflogic software (CyFlo Ltd., Finland).
RNA extraction and reverse transcription
Total RNA was extracted from naïve and SMX-NO-primed memory T-cells (2 x 106) using the RNeasy mini kit (Qiagen, UK), following the manufacturer’s protocol. After RNA elution from the spin column using RNase-free water, the eluate was passed over the column a second time in order to increase the RNA concentration. Total RNA was quantified using the NanoDrop N-8000 spectrophotometer (Thermo Scientific, UK), and RNA integrity assessed using the Agilent Bioanalyser (Agilent, UK). Total RNA (0.4 μg) was reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad, UK) as recommended by the manufacturer.
PCR amplification of cDNA
As described previously by Pannetier and colleagues, analysis of the CDR3 sizes within the 24 TCR Vβ chains was performed in a two-step polymerase chain reaction (PCR) (26). CDR3 spectratyping was performed. For each sample, aliquots of cDNA (20 ng) were amplified with 1 of 24 Vβ forward primers (10 μM; metabion, Germany) and a Cβ reverse primer (10 μM) on the thermal cycler Bio-Rad iCycler (Bio-Rad, Germany) using the HotStarTaq Master Mix kit (Qiagen, Germany). The final reaction mix contained 12.5 μl master mix, 1.3 μl Vβ forward primer, 1.3 μl reverse primer, 1 μl cDNA and 8.9 μl RNase-free water. The thermal cycling conditions were as follows: heat activation of Taq polymerase at 95 °C for 5 min, followed by 40 cycles of denaturation at 95°C for 45s, annealing at 60°C for 45 s, extension at 72 °C for 1 min, and a final extension step at 72 °C for 10 min.
Run-off PCR
PCR products from the first PCR were submitted to three cycles of an elongation run-off reaction with a FAM-labelled Cβ primer (10 μM; metabion, Germany). The final reaction mix contained 0.1 μl run-off primer, 0.1 μl Pfu polymerase (Promega, Germany), 1 μl dNTPs (30 μM; Promega, Germany), 1 μl 10 x Pfu buffer (Promega, Germany), 1 μl PCR product, and 6.8 μl RNase-free water. The PCR was performed on the thermal cycler Bio-Rad iCycler (Bio-Rad, Germany) under the following conditions: 94 °C for 2 min, 5 cycles of 94 °C for 25 s, 60 °C for 45 s, and 72 °C for 45 s, followed by a final elongation step of 72 °C for 10 min.
CDR3 spectratyping by GeneScan
Run-off PCR product (2 μl) was added to a mix of Hi-Di formamide (10 μl; Applied Biosystems, Germany) and 500 LIZ size standard (1 μl; Applied Biosystems, Germany) in a Thermo-Fast 96 PCR Detection plate (Applied Biosystems, Germany). The plate was sealed with a 96 well Plate Septa (Applied Biosystems, Germany) and the samples denaturated at 94 °C for 2 min. CDR3 size was determined on the ABI PRISM 3300 Genetic Analyzer, and data analysed using GeneMapper 4.0 software (Applied Biosystems, USA).
Statistics
Proliferation data from the in vitro priming of naïve and memory T-cells was performed in triplicate cultures and on 3 or more separate donors. Proliferative responses and the secretion of cytokines/cytolytic molecules from SMX-NO-responsive T-cell clones were also conducted in triplicate. Data is presented as the mean of the triplicate values with associated standard deviation. A paired t test was implemented for statistical analysis (Sigmaplot 13 software).
Results
TCR Vβ usage of SMX-NO-responsive T-cells
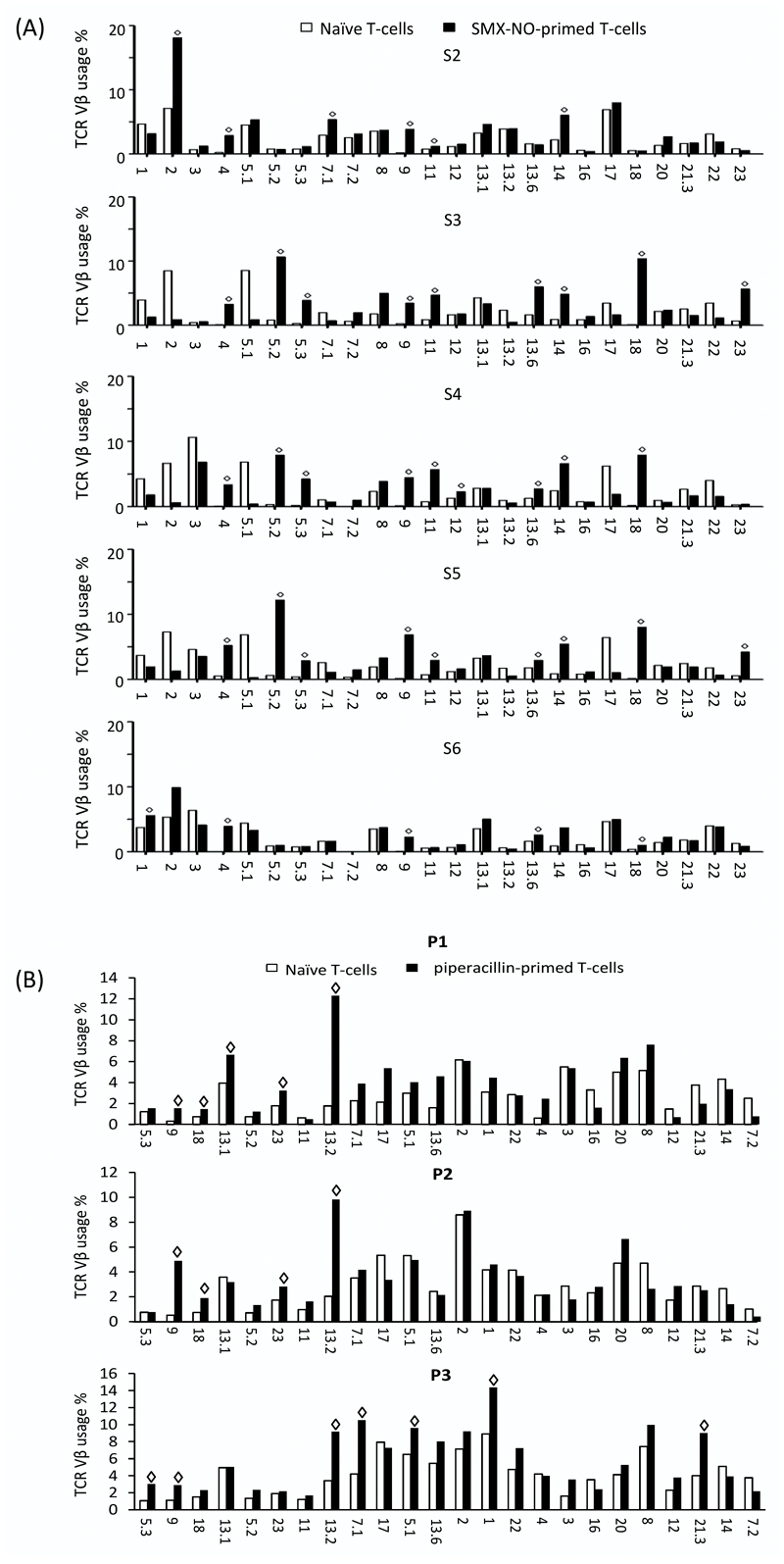
Flow cytometry was initially used to explore the comparative TCR Vβ expression between naïve T-cells and the corresponding SMX-NO-primed memory population. An expansion of the TCR Vβ repertoire was observed in T-cells from five donors following in vitro priming to SMX-NO, indicating that clonal expansion of drug-specific T-cells had occurred (Table 1).
Table 1. Flow cytometric analysis of TCR Vβ usage during SMX-NO-specific naïve T-cell priming.
| TCR Vβ coverage (%) 1 | S1 | S2 | S3 | S4 | S5 | S6 | ||
|---|---|---|---|---|---|---|---|---|
| Naïve | 61 | 56 | 52 | 57 | 53 | 50 | ||
| Memory | 65 | 83 | 78 | 71 | 76 | 65 | ||
| TCR Vβ 2 | 4 | 5.2 | 5.3 | 9 | 11 | 13.6 | 14 | 18 |
| S2 | ● | ● | ● | ● | ||||
| S3 | ● | ● | ● | ● | ● | ● | ● | ● |
| S4 | ● | ● | ● | ● | ● | ● | ● | ● |
| S5 | ● | ● | ● | ● | ● | ● | ● | ● |
| S6 | ● | ● | ● | ● | ||||
Coverage of the TCR Vβ repertoire in T-cells before (naïve) and after (memory) priming to SMX-NO.
Summary of common skewed TCR Vβ usage in SMX-NO-responsive T-cells from five healthy donors.
All 24 TCR Vβs could be detected from naïve T-cells, with mean expression levels ranging from 0.18 % for Vβ 9 up to 6.98 % for Vβ 2 (supplementary table 1). After in vitro priming, an increase in expression levels of up to 16/24 TCR Vβs was observed (figure 1a). Skewing of individual TCR Vβ subtypes was examined as described by Hashizume et al. (2002) who defined skewed TCR Vβ usage as the percentage of a particular TCR Vβ which is above the mean percentage + 3 SD of the same TCR Vβ in normal cells (in this study, naïve T-cells) (27). Skewed usage of TCR Vβ 4 and 9 was subsequently detected in all five individuals, while usage of TCR Vβ 11, 13.6, 14 and 18 was skewed in four donors, and usage of TCR Vβ 5.2 and 5.3 in three donors (Table 1).
Figure 1. Clonogram of naïve and (a) SMX-NO- and (b) piperacillin-primed memory T-cell TCR Vβ usage from healthy donors.
TCR Vβ subtype expression in naïve (white bars) and drug-primed memory (black bars). Data represent mean percentages of T-cells expressing the individual TCR Vβ subtypes. (◊ = skewed TCR Vβ usage, defined as percentage above mean value +3SD of TCR Vβ in naïve T-cells).
The same analysis was conducted on T-cells primed against an alternative drug hapten, the β-lactam antibiotic piperacillin. After priming, an increase in expression of 10 TCR Vβs was observed (figure 1b). Skewed usage of TCR Vβ 9 and 13.2 was detected in all 3 individuals, while TCR Vβ 18 and 23 was skewed in 2 donors and usage of TCR Vβ 5.3, 13.1, 7.1, 5.1, 1 and 21.3 was seen in one donor.
CDR3 spectratyping analysis of TCR Vβ diversity
The TCR Vβ repertoire of naïve and SMX-NO-responsive memory T-cells was further analysed by CDR3 spectratyping. Total RNA from naïve and memory T-cells was reverse transcribed to cDNA, and TCR Vβ chain transcripts amplified prior to analysis on an automated sequencer to determine the CDR3 size pattern of each TCR Vβ subtype. In the absence of antigenic stimulation, CDR3 profiles of individual TCR Vβs show a Gaussian-like distribution. A distortion of the CDR3 profiles, characterised by the emergence of single or multiple dominant peaks, signifies antigen specific T-cell stimulation leading to clonal expansion. Representative CDR3 spectratyping profiles of skewed TCR Vβs are shown in supplementary figure 1a and b. No spectratyping profile could be established for a small number of TCR Vβ’s as the genetic analyser failed to detect the size standard.
19/24 TCR Vβ subtypes were detected in naïve T-cells from all five healthy volunteers. As expected, the majority of TCR Vβ spectratyping profiles of naïve T-cells displayed a polyclonal distribution of CDR3 lengths (defined as quasi-Gaussian profile with ≥ 5 peaks). Transcripts of TCR Vβ 2, 4, 11, 20 and 21 were present in some donors, but absent in others. In SMX-NO-primed memory T-cells, 19/24 TCR Vβ chains were present in all five donors. TCR Vβ 2, 3, 4, 20 and 21 could only be detected in some of the donors. After SMX-NO priming, up to 80 % of TCR Vβ subtypes showed an oligoclonal distribution of CDR3 lengths (skewed Gaussian profile with at least one dominant peak). Skewing of CDR3 profiles from poly- to oligoclonal distribution could be observed for between six and fifteen TCR Vβs, indicating the expansion of a clonal T-cell population reactive to SMX-NO. SMX-NO specific memory T-cells from all five volunteers showed oligoclonal expression of TCR Vβ 18 (Table 2). Skewed usage of TCR Vβ 13B was observed in four donors, and TCR Vβ 1, 5, 9, 13A, and 14 displayed a skewed CDR3 pattern in three individuals.
Table 2. Spectratyping analysis of TCR Vβ subtypes with oligoclonal distribution of CDR3 sizes following SMX-NO priming.
| TCR Vβ | 1 | 2 | 3 | 4 | 5 | 6A | 6BC | 7 | 8 | 9 | 11 | 12 | 13A | 13B | 14 | 15 | 16 | 17 | 18 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | ●1 | X2 | X | ● | ● | ● | ● | ● | ||||||||||||||||
| S3 | X | S3 | S | S | ● | ● | ● | ● | ● | ● | S | |||||||||||||
| S4 | X | ● | ● | ● | ● | ● | ● | ● | ● | X | X | |||||||||||||
| S5 | ● | X | S | ● | ● | S | S | ● | ● | ● | S | ● | X | S | ||||||||||
| S6 | ● | X | ● | X | ● | ● | ● | ● | X | ● | ● | ● | ● | ● | ● | X | ● | ● | ● |
● = oligoclonal expansion (oligoclonal distribution of CDR3 lengths defined as skewed Gaussian profile with at least one dominant peak)
x = not detected
S = no sizing data
The comparative effect of immune checkpoint receptor blockade between drug antigen-induced naïve and memory T-cell activation
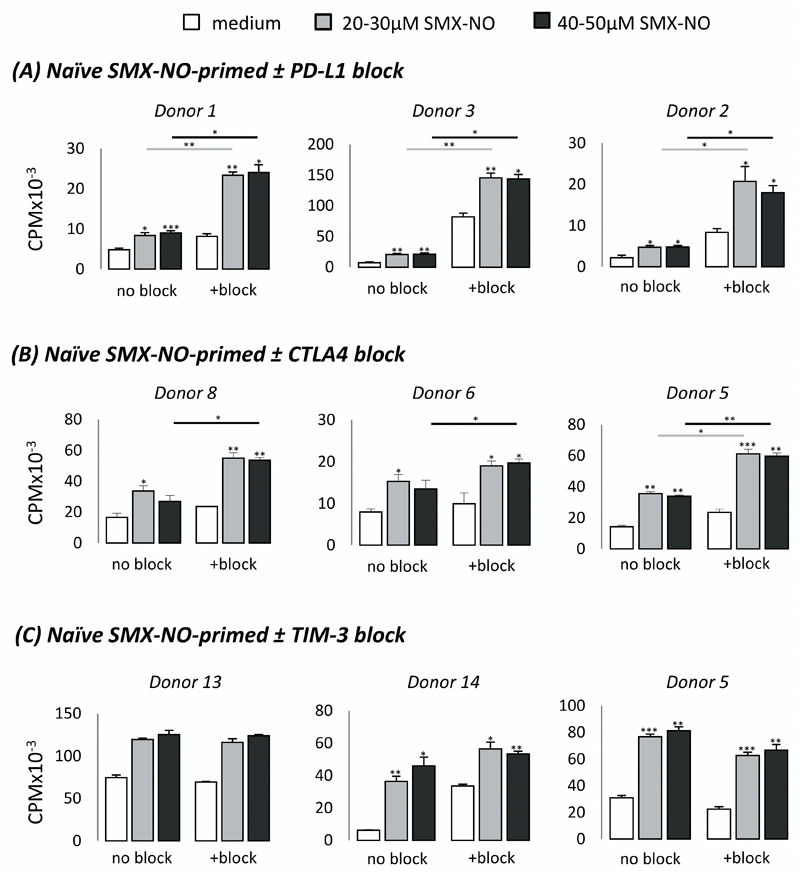
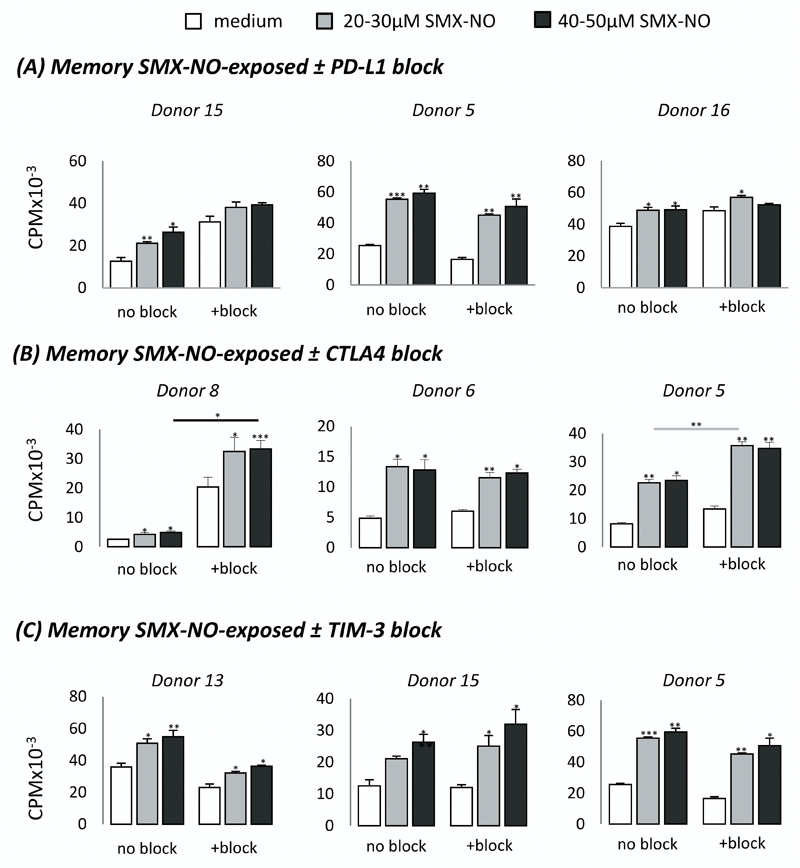
Following the TCR Vβ analysis, we examined the propensity for co-inhibitory pathways to modulate the activation thresholds of these SMX-NO-responsive and therefore TCR Vβ-restricted T-cell populations. Mature DCs generated from drug-naïve donors were co-cultured with autologous naïve or memory CD3+ T-cells in the presence of SMX-NO ± PD-L1/CTLA4/TIM-3 block for 8 days. DCs expressed a characteristic phenotype (CD1anegative CD11ahigh CD11chigh CD14negative CD40high CD83low MHC class Ihigh MHC class IIhigh). Importantly, mature DCs also expressed PD-L1high (PD-1 ligand), CD80mid and CD86high (CTLA4 ligands), and galectin-9high (TIM-3 ligand, supplementary figure c-e). Upon restimulation, primed naïve and memory T-cells displayed a dose-dependent proliferative response to SMX-NO (figures 2 and 3; *p < 0.05; SMX-NO, 20-50 µM). For naïve T-cell priming, blocking PD-L1 and CTLA4 markedly enhanced the proliferative response (figure 2) in 4/5 and 6/9 SMX-NO-responsive donors, respectively (Supplementary Table 2; *p < 0.05). The blockade of CTLA4 resulted in a lesser fold increase in proliferative response (average 2.5-fold increase) than the comparative effect after inclusion of PD-L1 block (average 4.4-fold increase). In contrast to naïve T-cell priming, blocking PD-L1 had no effect on the proliferation of SMX-NO-exposed memory T-cells, whereas inclusion of CTLA4 blockade markedly enhanced the proliferative response in 3/6 donors (figure 3, Supplementary Table 3; *p < 0.05). Blocking TIM-3 did not enhance the proliferative response of SMX-NO-primed naïve or memory T-cells from any donor (figures 2 and 3, Supplementary Tables 2 and 3, respectively).
Figure 2. Proliferation of SMX-NO-primed naïve T-cells ± co-inhibitory receptor blocking antibodies.
Naive T-cells from healthy donors were cultured with mature autologous monocyte-derived DCs in the presence of SMX-NO (50 μM) for one 1 week, ± (A) PD-L1 (5µg/ml), (B) CTLA4 (5µg/ml), or (C) TIM-3 (7.5μg/ml) blocking antibodies. T-cells were harvested and re-stimulated with fresh mature DCs and SMX-NO (20-50 μM) for 48 hrs. [3H]-thymidine was added and incubated for a further 16 hrs. Data from three representative donors shows the mean ± SD of triplicate cultures. Statistical significance denotes a significant increase in proliferative response compared to ‘medium only’ treated wells within that condition (i.e. TIM-3 blocked cells only). Statistical significance between conditions denotes a significant increase in proliferative response compared to ‘no block’ treated wells at a particular drug concentration after normalisation of all data values to account for differing basal stimulation (*p ≤ 0.05; **p ≤ 0.005; ***p < 0.001).
Figure 3. Proliferation of SMX-NO-exposed memory T-cells ± co-inhibitory receptor blocking antibodies.
Memory T-cells from healthy donors were cultured with mature autologous monocyte-derived DCs in the presence of SMX-NO (50 μM) for one 1 week, ± (A) PD-L1 (5µg/ml), (B) CTLA4 (5µg/ml), or (C) TIM-3 (7.5μg/ml) blocking antibodies. T-cells were harvested and re-stimulated with fresh mature DCs and SMX-NO (20-50 μM) for 48 hrs. [3H]-thymidine was added and incubated for a further 16 hrs. Data from three representative donors shows the mean ± SD of triplicate cultures. Statistical significance denotes a significant increase in proliferative response compared to ‘medium only’ treated wells within that condition (i.e. TIM-3 blocked cells only). Statistical significance between conditions denotes a significant increase in proliferative response compared to ‘no block’ treated wells at a particular drug concentration after normalisation of all data values to account for differing basal stimulation (*p ≤ 0.05; **p ≤ 0.005; ***p < 0.001).
PD-1, CTLA4, and TIM-3 display discrete expression profiles during drug antigen-induced T-cell activation
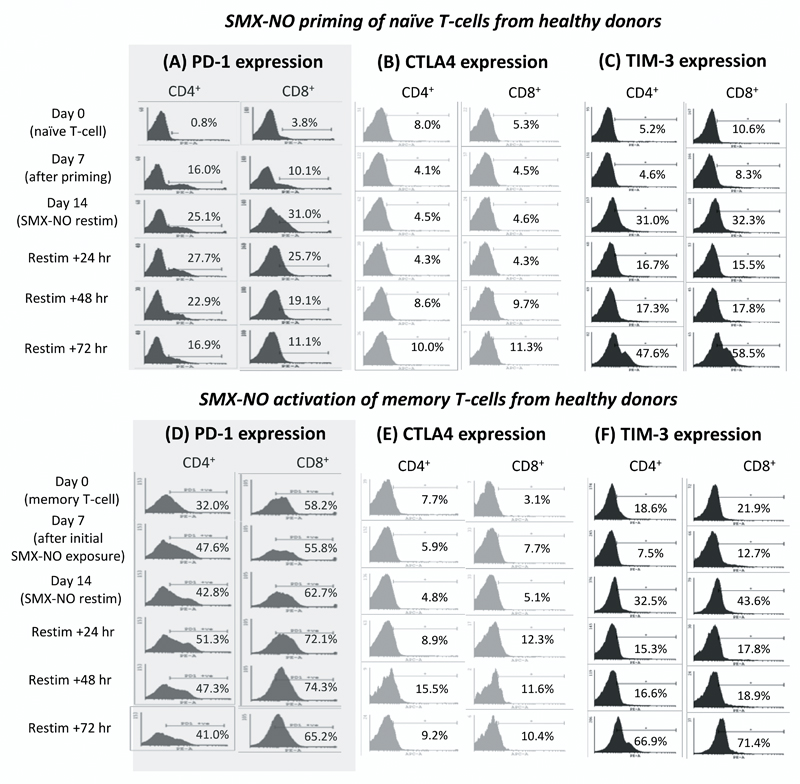
We previously measured the expression of PD-1 before and during the priming of naïve T-cells to SMX-NO. PD-1 expression on CD4+ and CD8+ T-cells increased rapidly after priming with SMX-NO. Greater than 20 % of T-cells stained positive for 14 days and for 48 h after restimulation of the primed T-cells (figure 4a). During this investigation we completed the profiling of PD-1 expression on drug-antigen stimulated memory T-cells, and similarly assessed the expression kinetics of CTLA4 and TIM-3 on naïve and memory T-cells.
Figure 4. Co-inhibitory receptor expression during the SMX-NO-specific activation of CD4+ and CD8+ naive and memory T-cells from healthy donors.
The expression of co-inhibitory receptors on naïve [(A) PD-1 (B) CTLA4 (C) TIM-3] or memory T-cells [(D) PD-1 (E) CTLA4 (F) TIM-3] from healthy donors during stimulation, and re-stimulation with SMX-NO (50 μM) using mature autologous DCs. Samples of T-cells were harvested at various time-points and labelled with CD4, CD8, and PD-1/CTLA4/TIM-3 fluorochrome-bound antibodies. Percentages indicate the proportion of cells which stain positive for each co-inhibitory receptor.
While 5-8 % of naïve T-cells expressed CTLA4, the percentage of naïve-derived CTLA4+ T-cells decreased directly after initial exposure to antigen, before rising above pre-stimulation levels by 48 h post re-stimulation (figure 4b). Although SMX-NO-specific activation of naïve T-cells increased the percentage of CTLA4+ CD4+ T-cells by 2 % (8-10 %), 6 % more (5.3-11.3 %) CD8+ T-cells expressed CTLA4 after restimulation. TIM-3 expression was also detected on naïve T-cells, but in contrast to CTLA4, the percentage of TIM-3+ T-cells increased during SMX-NO priming (figure 4c). After re-stimulation, the number of TIM-3+ cells fluctuated before again rising by 72 h post re-stimulation so that > 50 % of naïve-derived T-cells expressed TIM-3. The maximal increase in TIM-3 expression was similar for both CD4+ and CD8+ naïve T-cells, with a 42.4 %, and 47.9 % rise in comparison to unstimulated naïve CD4+ and CD8+ T-cells, respectively.
Similar to naïve T-cells, CTLA4 was expressed on fewer than 10 % of resting memory T-cells (figure 4e). In contrast, PD-1 and TIM-3 were expressed on a much higher proportion of quiescent memory T-cells (figure 4d and 4f, respectively) than naïve T-cells. The expression kinetics of each receptor during memory T-cell stimulation followed those previously observed on naive T-cells. Although PD-1 expression increased, peak expression remained < 10 % greater than basal memory T-cell expression. In contrast, peak TIM-3 expression was > 45 % greater than on quiescent memory T-cells. For both PD-1 and TIM-3, the percentage increase in receptor expression was similar between CD4+ and CD8+ T-cells (PD-1: CD4+, 9 % [32.0-41.0 %]; CD8+, 7 % [58.2-65.2 %]) (TIM-3: CD4+, 48.3 % [18.6-66.9 %]; CD8+, 49.5 % [21.9-71.4 %]). In contrast, as with naïve T-cell expression, CTLA4 expression on memory T-cells was more greatly enhanced on CD8+ T-cells (7.3 % [3.1-10.4 %]) than CD4+ T-cells (1.5 % [7.7-9.2 %]) (figure 4e).
The expression of CTLA4 and TIM-3 is enhanced on drug antigen-primed dividing T-cells
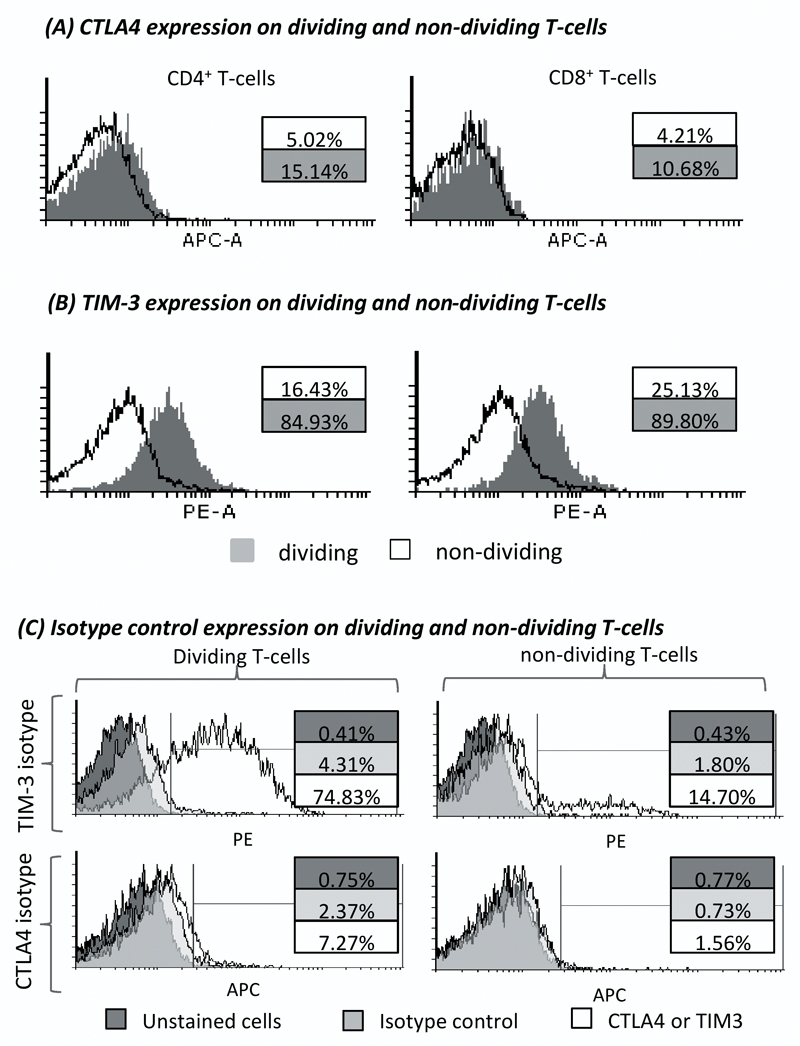
The high expression of co-inhibitory receptors has previously been associated with T-cell exhaustion. In our previous investigation we identified that PD-1 was expressed on dividing T-cells and was therefore not individually a marker of exhaustion. We again questioned this association for CTLA4 and TIM-3 by performing a detailed analysis of SMX-NO-primed naïve T-cell proliferation by co-staining cells with CFSE. CTLA4 was expressed on a significant proportion of CD4+ (15.1 %) and CD8+ (10.7 %) dividing T-cells, both of which represented greater proportions than CTLA4 expression on the corresponding non-dividing T-cell populations (figure 5a). Similarly, TIM-3 expression was found on 5- and 3-fold more dividing CD4+ and CD8+ T-cells than those in a quiescent state, respectively (figure 5b).
Figure 5. Expression of CTLA4 and TIM-3 on dividing and non-dividing T-cells derived from priming healthy donor naïve T-cells to SMX-NO.
Naïve T-cells from healthy donors were cultured with mature autologous DCs in the presence of SMX-NO (50 μM) for one 1 week. T-cells were stained with CFSE and then re-stimulated with fresh mature DCs and SMX-NO (50 μM) for 72 hrs. T-cells were incubated with CTLA4-APC* or TIM-3-PE, and CD4- and CD8-specific fluorochromes. Histograms detail the expression of (A) CTLA4 and (B) TIM-3 expression on CD4+ or CD8+ T-cell populations separately (grey histogram, expression on dividing T-cells; black line, expression on non-dividing T-cells). Percentages indicate the percentage of each population gated positive for each co-inhibitory receptor.
The expression level of individual co-inhibitory receptors has no effect on the response of drug antigen-responsive T-cell clones
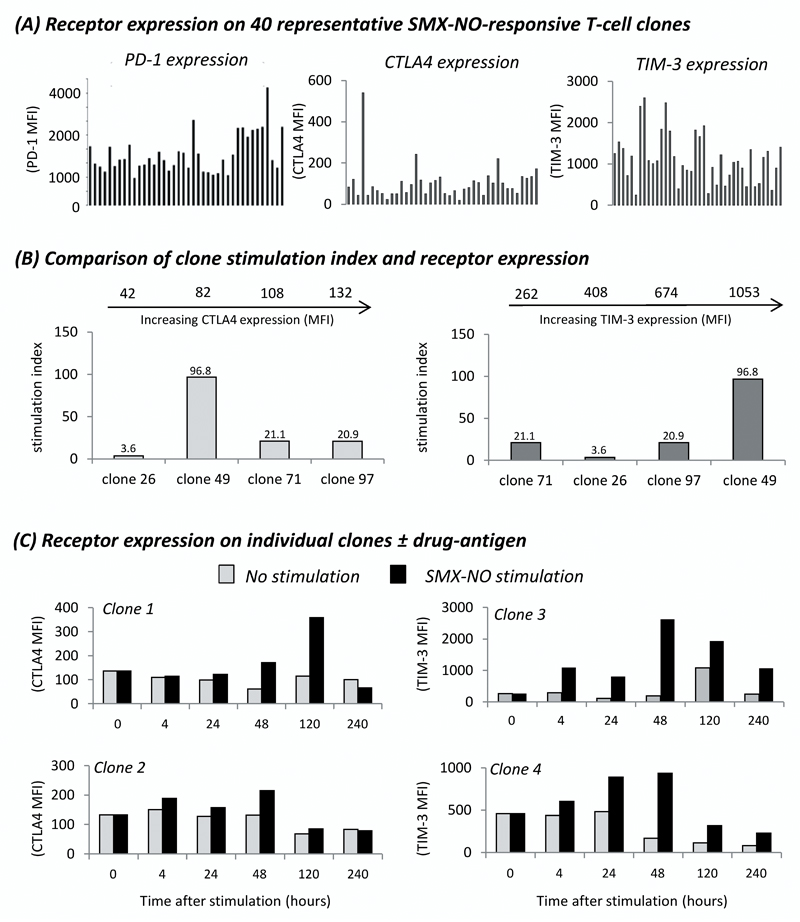
In comparison to the modest 4-fold difference in PD-1 expression previously reported between T-cell clones, flow cytometric analysis on 40 representative SMX-NO-specific T-cell clones revealed > 27-fold difference in CTLA4 expression, and a > 10-fold difference in TIM-3 surface expression (figure 6a). We previously reported that the degree of PD-1 expression had no effect on T-cell clone activity. Despite a much greater variation in expression of CTLA4 and TIM-3 between T-cell clones, the level of expression of these co-inhibitory receptors did not affect the proliferative response of each clone (figure 6b). While the clone with the highest TIM-3 expression (clone 49) was also the clone with the highest stimulation index, the previous 3 clones showed no correlation with TIM-3 expression. For example, clone 71 had a similar SI to that of clone 97 but only one third of the TIM-3 surface expression.
Figure 6. Expression-activity relationship of CTLA4 and TIM-3 on SMX-NO-responsive T-cell clones.
(A) Expression of PD-1, CTLA4 and TIM-3 on 40 representative T-cell clones derived from priming healthy donor naïve T-cells to SMX-NO. (B) The proliferative response of T-cell clones expressing varied levels of CTLA4 or TIM-3 in response to SMX-NO stimulation, as measured by [3H] thymidine incorporation. The mean fluorescence intensity (MFI) analysed by flow cytometry indicating the relative expression of CTLA4 or TIM-3 is indicated for each clone at the top of the graphs (C) The expression of CTLA4 and TIM-3 on representative T-cell clones over 240hrs ± SMX-NO stimulation. Grey bars indicate no stimulation, black bars indicate SMX-NO stimulation (40µM).
We then measured the surface expression of CTLA4 and TIM-3 on clones over a period of 10 days, with and without SMX-NO stimulation. While staining over a similar time-period had shown relatively stable PD-1 expression, the expression profile for CTLA4 and TIM-3 was more varied (figure 6c). In the absence of antigen, the expression of both receptors remained relatively constant over a period of 240 h. In the presence of SMX-NO, the expression of CTLA4 and TIM-3 increased above that of basal levels in clone 1 (CTLA4), and clones 3 and 4 (TIM-3). For clone 1, maximal CTLA4 expression was observed at 120 h, at which point expression on SMX-NO-stimulated wells was > 2.5 fold higher than prior to SMX-NO exposure (0 hr MFI: 136, 120 hr MFI: 358), and > 3-fold greater than the expression at a similar time-point on antigen untreated cells (untreated cells 120 h MFI: 115). Both clones analysed for TIM-3 expression showed maximal expression at 48 h post SMX-NO exposure. At this time-point, expression of TIM-3 on SMX-NO stimulated cells was > 10-fold and > 2-fold higher than on cells prior to SMX-NO exposure for clone 3 and 4, respectively (clone 3: 0 h MFI, 262; 48 h MFI, 2626; clone 4: 0 h MFI, 458; 48 h MFI, 936), and > 13.5-fold and > 5.5-fold greater than the expression at a similar time-point of antigen untreated wells (untreated cells 120 h MFI: clone 3, 192; clone 4, 168).
PD-L1, CTLA4, and TIM-3 have no effect on the effector response of SMX-NO-responsive T-cell clones
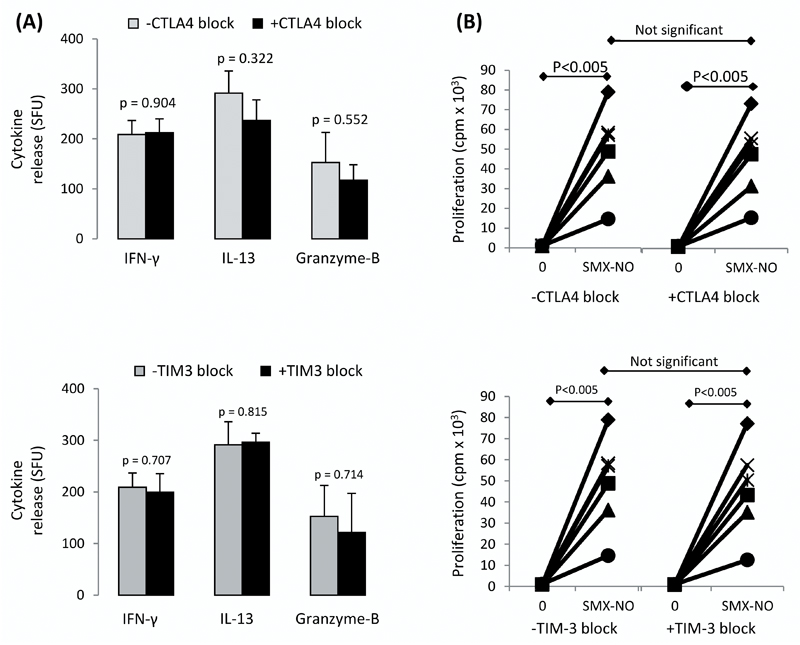
To further assess the role of immune checkpoint receptors in the regulation of secondary T-cell responses, we explored the role of CTLA4 and TIM-3 on SMX-NO-specific T-cell clones generated from healthy donor primed naïve T-cells as previous work had shown that PD-L1 block led to a modest increase in cytokine secretion from T-cell clones. Both the proliferative capacity and ability to secrete cytokines and cytolytic molecules in the presence of SMX-NO was assessed on 6 representative T-cell clones, with and without CTLA4 or TIM-3 blockade (figure 7a and 7b, respectively). All clones significantly proliferated in the presence of SMX-NO (*p < 0.005), and the addition of CTLA4- or TIM-3-blocking antibodies did not significantly increase the proliferative response or the secretion of IFN-γ, IL-13 or granzyme B in response to SMX-NO for any of the six T-cell clones. The inability of CTLA4 and TIM-3 to regulate the responses of T-cell clones reflects the reduced capacity of such pathways to control secondary (memory) responses, especially where every cell has specificity for the drug, in comparison to the initial priming response.
Figure 7. The activation of SMX-NO-responsive T-cell clones ± co-inhibitory receptor blockade.
The (A) secretion of IFN-γ, IL-13 and granzyme B, and (B) the proliferative response of T-cell clones in response to SMX-NO stimulation, ± anti-CTLA4 (10µg/ml) or anti-TIM-3 (7.5µg/ml) treatment. Cytokine secretion was measured by ELISpot and proliferative analysis by [3H] thymidine incorporation. Statistical significance between conditions denotes a significant increase/decrease in proliferative response or cytokine release.
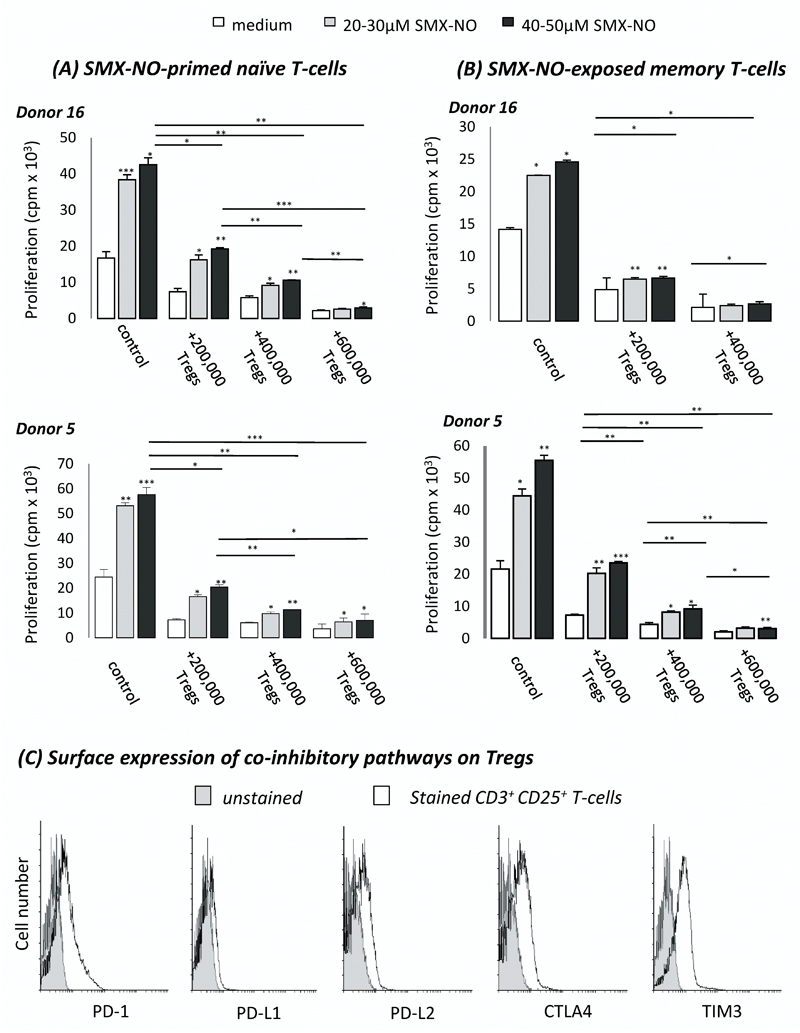
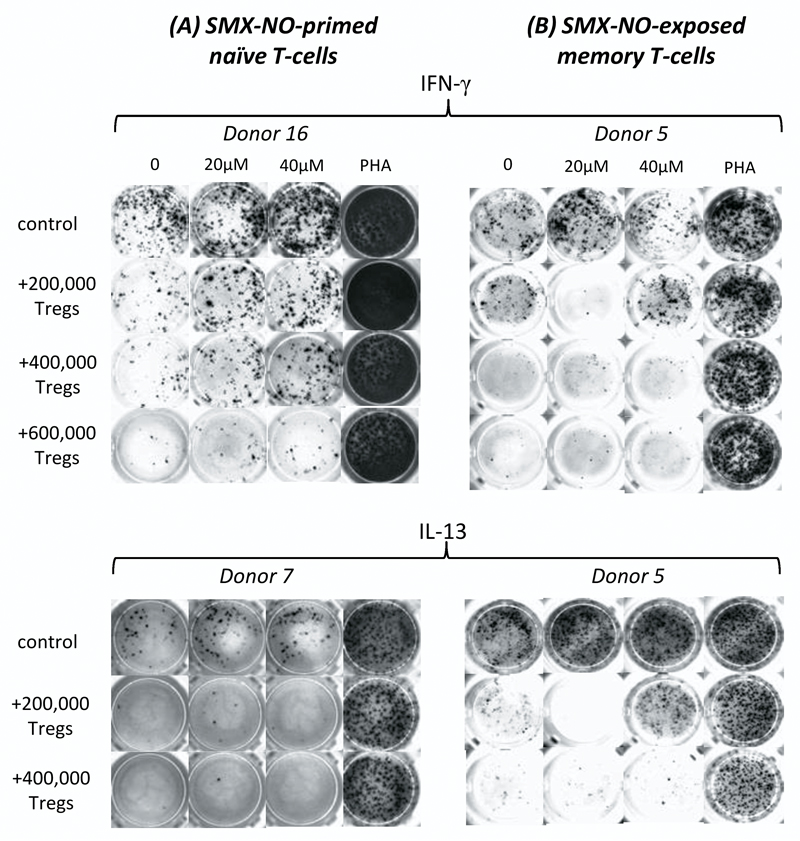
CD25+ T-cells (Tregs) effectively dampen SMX-NO-induced naïve and memory T-cell activation
The Treg population was selectively removed from the T cell population using CD25+ as a marker for these cells. To determine the scale of regulation imposed by CD25+ Tregs on drug antigen-induced immune activation, we seeded autologous CD25+ T-cells into the co-culture. The number of conditions performed for each donor varied depending on the number of T-cells obtained. The addition of 2 x 105 CD25+ T-cells led to a significant reduction in the drug-antigen induced proliferation of both naïve- and memory-derived T-cells from all donors (figure 8a and 8b, respectively). A similar trend was observed upon analysis of IFN-γ and IL-13 secretion from both naïve- and memory-derived cells (figure 9a and 9b, respectively). Increasing the number of CD25+ T-cells further reduced the SMX-NO-specific response. These functionally suppressive Tregs expressed co-inhibitory receptors and ligands as determined by flow cytometry (figure 8c).
Figure 8. The suppressive effect of autologous CD25+ Tregs on the SMX-NO-induced activation of naïve and memory T-cells from healthy donors.
(A) Naïve or (B) memory T-cells were cultured with mature DCs and SMX-NO (50µM) for 1 week, ± autologous CD25+ Tregs (2-6 x 105/well). T-cells were harvested and restimulated with fresh mature DCs and SMX-NO (20-50 µM) for 48 hrs before measurement of proliferative capacity through the incorporation of [3H] thymidine. Statistical significance between conditions denotes a significant increase/decrease in proliferative response (*p ≤ 0.05; **p ≤ 0.005; ***p < 0.001) (C) The expression of PD-1, PD-L1, PD-L2, CTLA4, and TIM3 on autologous Tregs was analysed by flow cytometry to identify the propensity for co-inhibitory pathways to aid Treg-mediated suppression of SMX-NO-induced T-cell responses. Grey histograms represent unstained cells, white histograms represent co-inhibitory marker-stained cells.
Figure 9. The quantitative effect of CD25+ Treg-mediated inhibition of cytokine secretion on drug antigen-mediated T-cell activation.
The secretion of IFN-γ and IL-13 after SMX-NO-induced activation of 2.5 x 106 (A) naïve or (B) memory T-cells from heathy donors ± 2-6 x 105 CD25+ Tregs/well. Autologous Tregs were removed from the control naïve and memory T-cell populations. Tregs were then supplemented back into the assays prior to addition of the drug antigen.
Discussion
The association of specific HLA alleles with drug hypersensitivity attracted the scientific community to reinvestigate the role of T cells in this form of iatrogenic disease. However, for the vast majority of drugs, HLA allele associations have not been identified or do not solely account for the onset of a reaction (28). It is therefore apparent that predisposition to hypersensitivity is seldom simplistic and or mediated by singular genetic traits, but is more frequently facilitated by a combination of susceptibility factors.
Three key areas have the potential to influence drug antigen-induced T-cell activation. Firstly, Ko et al demonstrated the importance of investigating both sides of the MHC-TCR interaction as they found that the use of specific TCR Vβ’s alongside HLA-B*15:02 is required for hypersensitivity to the antiepileptic drug carbamazepine (7). Secondly, multiple studies have identified that dysregulated co-inhibitory receptors involved in T-cell activation can propagate autoimmunity (29–35), and that blocking these pathways in cancer models can significantly enhance functional T-cell responses (14, 36, 37). Thus, it is conceivable that dysregulated regulatory pathways may contribute to the inadvertent activation of T-cells to drug-antigens. Thirdly, it has been reported that Tregs can prevent TEN-associated epidermal injury in model systems, highlighting that susceptibility in patients may be linked to a defect in normal Treg function (16, 17). We are interested in examining these factors within a single model system. Using the drug antigen SMX-NO, for which HLA associations have not been identified (38), we have investigated the role of specific TCR Vβ subtypes, immune checkpoint signalling, and Tregs during drug antigen-induced T-cell activation.
To assess the role of individual TCR subtypes during drug antigen-specific T-cell responses, we explored the TCR Vβ repertoire of T-cells before and after SMX-NO-priming. The successful priming of naïve T-cells from all donors was confirmed by proliferation analysis in response to SMX-NO. To profile TCR Vβ usage, we analysed (a) protein expression by flow cytometry, and (b) mRNA expression using molecular CDR3 spectratyping as both techniques have been widely used across different disease settings (39–42). SMX-NO-primed T-cells displayed broad TCR Vβ usage, with increased expression levels in up to 16 TCR Vβs. Skewing was observed for nine different Vβ subtypes across the five donors indicating that multiple TCRs may be involved in drug antigen recognition. Given that SMX-NO is known to modify a range of cellular proteins and that protein adducts stimulate T-cells from hypersensitive patients (43–46), the observation that multiple TCRs are activated was anticipated. Furthermore, common skewing of particular TCR Vβ subtypes (Vβ 4 and Vβ 9) in all drug-responsive individuals indicates that a public TCR repertoire may be involved in recognition of SMX-NO-derived antigens. Similar observations were made herein with the β-lactam antibiotic piperacillin, which forms protein adducts through the covalent modification of lysine residues, and elsewhere with abacavir, in which case the extensive TCR usage is thought to be responsible for the recognition of diverse novel self-peptides presented on HLA-B*57:01 in the presence of abacavir (8).
Detailed mRNA analysis using CDR3 spectratyping showed that eleven TCR Vβ subtypes were present in both naïve and SMX-NO-specific T-cells from all volunteers, whereas six TCR Vβs (2, 3, 4, 11, 20, and 21) were only detected in some donors. In naive T-cells most TCRs displayed polyclonal CDR3 patterns, whereas CDR3 profiles of SMX-NO specific T-cells showed oligoclonal expansions in up to 16 different Vβ subtypes. CDR3 spectra only partially match the results obtained by flow cytometry, which is likely due to the enhanced sensitivity of CDR3 spectratyping, failure to amplify specific TCR Vβ transcripts due to inadequate binding specificity of the corresponding Vβ primer. Despite this, common skewing of particular TCR Vβ subtypes was observed between the two techniques. For spectratyping analysis, skewing was observed for TCR Vβ 18 in 5/5 donors, TCR Vβ 13B in 4/5 donors, and TCR Vβ 1, 5, 9, 13A, and 14 in 3/5 volunteers, respectively. These findings strengthen the proposal that T-cell responses to SMX-NO-derived antigens may be controlled by public TCRs, which are present in all responsive individuals, alongside private TCR repertoires specific to each individual.
While the TCR Vβ analysis builds towards a full characterisation for the role of signal 1 during SMX-NO-induced T-cell activation, the role of signal 2 on the resultant antigen-specific and TCR Vβ-restricted T-cells remains largely undefined. An in vitro T-cell assay was subsequently used to block individual immune checkpoints in order to ‘peel away’ and reveal the influence of individual layers of regulation, and assess the kinetics of co-inhibitory receptor expression during the drug antigen-specific activation of naïve or memory T-cells. This work builds upon our previously published work exploring the effects of PD-1 on SMX-NO-induced T-cell activation (47), and expands further to similarly detail the function of the CTLA4 and TIM-3 receptor pathways. We identified an enhanced proliferative T-cell response after priming naïve T-cells to SMX-NO in the presence of PD-L1 and CTLA4 blocking antibodies. PD-L1 block had a greater regulatory role during primary T-cell responses to SMX-NO with an average 4.4-fold increase in proliferative response, compared to a 2.5-fold increase for CTLA4 block. These data concur with the work of Parry et al who studied T-cell activation-induced gene transcripts, and found the generation of just 67 % of transcripts was hindered by CTLA4 ligation, in comparison to 90 % upon PD-1 activation (48). In contrast, we detected no significant increase with the inclusion of TIM-3 block. The apparent lack of regulation by TIM-3 may be partly related to its more restricted expression profile compared to CTLA4 and PD-1 (expressed on all T-cells) (49, 50). As the characterisation of TIM-3 has largely been performed in mice, it may be that there are functional discrepancies between human and mouse TIM-3.
Lucas et al recently described the ability of drug-derived antigens to stimulate memory T-cells from drug-naïve donors via heterologous immunity (9). Thus, when considering the onset of hypersensitivity, it is necessary to evaluate the role of immune checkpoints during both primary and secondary T-cell responses. Using our in vitro assay, and in complete contrast to that of naïve T-cell priming, memory T-cell responses to SMX-NO were not enhanced by the blockade of PD-L1. Similarly, blocking TIM-3 did not increase the memory T-cell response. In contrast, blocking CTLA4 enhanced memory drug antigen-specific responses; however, it did so in a smaller proportion of donors (3/6) than for primary T-cell responses. As expected, our data describe a greater role for co-inhibitory regulation during primary, rather than secondary T-cell responses, in line with the more stringent requirements and higher activation thresholds for the activation of naïve T-cells. The requirement for additional signalling minimises the risk of a naïve T-cell response to non-harmful or self-antigen, a requirement which is unnecessary for memory T-cells due to their pre-defined antigen-specificity.
We further examined the dynamics of co-inhibitory receptor expression on naïve and memory T-cells during T-cell activation. The percentage of PD-1+ memory cells greatly increased after initial antigen stimulation, peaking shortly after restimulation in a similar manner to that previously described on naïve T-cells (47). CTLA4 expression on naïve T-cells has been shown to be low and inducible upon activation (51), while the TIM-3 profiles of CD4+ and CD8+ T-cells are less well-defined. Hastings et al found that TIM-3 expression was generally restricted to CD4+ T-cells, which were only found in human lymph nodes, with peripheral CD4+ T-cells showing little expression until induced post-stimulation (52). Although we found that exposure of naïve T-cells to SMX-NO did not initially enhance CTLA4 expression, in line with previous reports citing an increase after 2-3 days (53), we observed a slight increase in the percentage of naïve T-cells expressing CTLA4 between 48-72 h after re-stimulation with SMX-NO. CTLA4 expression on memory T-cells was enhanced similarly after restimulation. The proportion of naïve and memory TIM-3+ cells reduced after both initial antigen exposure and re-stimulation, but increased towards the end of both culture periods. This pattern is likely imposed to promote a strong initial response by reducing expression, before increasing at later time-points to prevent a long-lasting, potentially damaging response. This timeline is supported by two previous observations regarding the TIM-3 ligand, galectin-9. Firstly, galectin-9 expression is downregulated in the lymph nodes after immune activation which is thought to allow the initial generation of a Th1 response (49). Secondly, the subsequent expansion of Th1 T-cells results in IFN-γ secretion, which in turn upregulates galectin-9 in a negative feedback loop to prevent a runaway immune response (54). In both naïve and memory cultures the percentage of TIM-3+ cells increased by a similar amount upon antigen stimulation for CD4+ and CD8+ T-cell populations indicating that TIM-3 equally regulates all responsive T-cells during a drug-derived antigen-specific response. The expression profiles of PD-1, CTLA4, and TIM-3 paint a complex picture of regulation, with different receptors regulating different phases of a response. Specifically, these data show that PD-1 expression is increased soon after T-cell activation and so likely has a more prominent role in regulation during the initial encounter of T-cells with antigen. In contrast, TIM-3 and CTLA4 may play a more prominent role during late stage T-cell responses.
As co-inhibitory pathways can function to prevent T-cell stimulation, multiple studies have linked the high expression of specific co-inhibitory receptors to an exhausted T-cell phenotype; a quiescent state characterised by an inability to proliferate and produce cytokines (55). Previously, we reported that a high proportion of dividing SMX-NO-specific T-cells were PD-1+ and thus PD-1 does not represent a lone marker for exhaustion. However, similar investigations have not been performed for CTLA4 and TIM-3. Indeed, assessment of the functionality of PD-1+ TIM-3+ tumour-infiltrating T-cells found that these cells represented an exhausted phenotype, while other studies report that TIM-3 expression can identify exhausted T-cells in patients with HIV or HCV infection (56, 57). Upon analysis of both CD4+ and CD8+ T-cells, we found a significant proportion of actively proliferating cells expressed either CTLA4 or TIM-3, as quantified by CFSE-incorporation. In all instances, the proportion of dividing T-cells expressing either receptor was greater than the comparative proportion of non-dividing T-cells. As up to 7 different co-inhibitory receptors are expressed on functionally exhausted T-cells during chronic infection (58), our data highlight the importance of not singling out individual receptors as markers of T-cell exhaustion. Moreover, using T-cell clones we demonstrate that the level of CTLA4 or TIM-3 expression can vary widely among autologous, antigen-responsive cells. Importantly, the level of expression did not strongly correlate with the strength of the SMX-NO-specific T-cell responses.
Finally, we explored the inhibitory effect of Tregs that expressed PD-L1, PD-L2, CTLA-4 and Tim-3 by reintroducing autologous CD25+ T-cells into the priming culture at graded quantities. The stimulation of both naïve and memory T-cell responses were dampened by the addition of Tregs, and that suppression was increased with the addition of more Tregs. Further work should investigate whether Treg-mediated suppression of drug-specific T-cells is regulated by co-inhibitory receptor signalling and/or the secretion of suppressive cytokines.
In summary, we have successfully utilised an in vitro model to highlight the propensity for various non-HLA risk factors to enhance the likelihood of drug antigen-induced T-cell activation. We show that T-cell responses to SMX-NO are dependent on the availability of specific TCR Vβ repertoires and that these antigen-responsive T-cells are highly regulated by co-inhibitory mechanisms that can be enforced by resident Tregs. Our report demonstrates that an individual may be predisposed to develop hypersensitivity by (a) distinct TCR Vβ subtypes that form both private and public repertoires, (b) dysregulation of the PD-1 or CTLA4 pathways which predominantly control naïve and memory T-cell responses, respectively, or (c) a lack of functional Tregs as autologous CD25+ Tregs can effectively supress effector naïve and memory T-cell responses at a 1:5 ratio. Until recently, many of these factors have been overlooked by the field’s predominant interest in the role of HLA interactions. While these associations remain important, it is highly likely that the exploration of other risk factors will be required to fully define individual predisposition to drug hypersensitivity.
Supplementary Material
Acknowledgements
The authors would like to thank the volunteers for their generous blood donations.
This work received funding from the MRC Centre for Drug Safety Science (Grant Number G0700654) and also the MIP-DILI project (supported by the European Community under the Innovative Medicines Initiative Programme through Grant Agreement number 115336).
References
- 1.Holt M, Ju C. Mechanisms of drug-induced liver injury. AAPS J. 2006;8:E48–E54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng X, Jenkins RE, Berry NG, Maggs JL, Farrell J, Lane CS, Stachulski AV, French NS, Naisbitt DJ, Pirmohamed M, Park BK. Direct evidence for the formation of diastereoisomeric benzylpenicilloyl haptens from benzylpenicillin and benzylpenicillenic acid in patients. J Pharmacol Exp Ther. 2011;338:841–849. doi: 10.1124/jpet.111.183871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nhim C, Delluc S, Halgand F, de Chaisemartin L, Weaver RJ, Claude N, Joseph D, Maillere B, Pallardy M. Identification and frequency of circulating CD4(+) T lymphocytes specific to Benzylpenicillin in healthy donors. Allergy. 2013;68:899–905. doi: 10.1111/all.12173. [DOI] [PubMed] [Google Scholar]
- 4.Chung W-H, Hung S-I, Hong H-S, Hsih M-S, Yang L-C, Ho H-C, Wu J-Y, Chen Y-T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature. 2004;428:486–486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 5.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. The Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 6.Martin MA, Klein TE, Dong BJ, Pirmohamed M, Haas DW, Kroetz DL, C. Clinical Pharmacogenetics Implementation Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Ther. 2012;91:734–738. doi: 10.1038/clpt.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko T-M, Chung W-H, Wei C-Y, Shih H-Y, Chen J-K, Lin C-H, Chen Y-T, Hung S-I. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128:1266–1276. doi: 10.1016/j.jaci.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, Miles JJ, Kjer-Nielsen L, Gras S, Williamson NA, Burrows SR, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 9.Lucas A, Lucas M, Strhyn A, Keane NM, McKinnon E, Pavlos R, Moran EM, Meyer-Pannwitt V, Gaudieri S, D'Orsogna L, Kalams S, et al. Abacavir-reactive memory T cells are present in drug naïve individuals. PloS one. 2015;10 doi: 10.1371/journal.pone.0117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative Disorders with Early Lethality in Mice Deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 12.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 13.Teft WA, Kirchhof MG, Madrenas J. A MOLECULAR PERSPECTIVE OF CTLA-4 FUNCTION. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 14.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavani A. T regulatory cells in contact hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:294–298. doi: 10.1097/ACI.0b013e3283079ea4. [DOI] [PubMed] [Google Scholar]
- 16.Azukizawa H, Sano S, Kosaka H, Sumikawa Y, Itami S. Prevention of toxic epidermal necrolysis by regulatory T cells. Eur J Immunol. 2005;35:1722–1730. doi: 10.1002/eji.200425773. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective Regulatory T Cells In Patients with Severe Drug Eruptions: Timing of the Dysfunction Is Associated with the Pathological Phenotype and Outcome. J Immunol. 2009;182:8071–8079. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 18.Grattan M, Mi Q-S, Meagher C, Delovitch TL. Congenic Mapping of the Diabetogenic Locus Idd4 to a 5.2-cM Region of Chromosome 11 in NOD Mice: Identification of Two Potential Candidate Subloci. Diabetes. 2002;51:215–223. doi: 10.2337/diabetes.51.1.215. [DOI] [PubMed] [Google Scholar]
- 19.Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 21.Niwa H, Satoh T, Matsushima Y, Hosoya K, Saeki K, Niki T, Hirashima M, Yokozeki H. Stable form of galectin-9, a Tim-3 ligand, inhibits contact hypersensitivity and psoriatic reactions: A potent therapeutic tool for Th1- and/or Th17-mediated skin inflammation. Clin Immunol. 2009;132:184–194. doi: 10.1016/j.clim.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Faulkner L, Martinsson K, Santoyo-Castelazo A, Cederbrant K, Schuppe-Koistinen I, Powell H, Tugwood J, Naisbitt DJ, Park BK. The Development of In Vitro Culture Methods to Characterize Primary T-Cell Responses to Drugs. Toxicol Sci. 2012;127:150–158. doi: 10.1093/toxsci/kfs080. [DOI] [PubMed] [Google Scholar]
- 23.Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J Immunol. 1995;155:462–472. [PubMed] [Google Scholar]
- 24.Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of Sulfamethoxazole and Its Reactive Metabolites by Drug-Specific CD4+ T Cells from Allergic Individuals. J Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- 25.Naisbitt DJ, Britschgi M, Wong G, Farrell J, Depta JPH, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Hypersensitivity Reactions to Carbamazepine: Characterization of the Specificity, Phenotype, and Cytokine Profile of Drug-Specific T Cell Clones. Mol Pharmacol. 2003;63:732–741. doi: 10.1124/mol.63.3.732. [DOI] [PubMed] [Google Scholar]
- 26.Pannetier C, Levraud J, Lim A, Even J, Kourilsky P. The immunoscope approach for the analysis of T cell repertoires. The antigen T cell receptor: selected protocols and applications. 1997:287. [Google Scholar]
- 27.Hashizume H, Takigawa M, Tokura Y. Characterization of Drug-Specific T Cells in Phenobarbital-Induced Eruption. J Immunol. 2002;168:5359–5368. doi: 10.4049/jimmunol.168.10.5359. [DOI] [PubMed] [Google Scholar]
- 28.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR, Graham J, et al. HLA-B[ast]5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 29.Chae S-C, Park Y-R, Shim S-C, Yoon K-S, Chung H-T. The polymorphisms of Th1 cell surface gene Tim-3 are associated in a Korean population with rheumatoid arthritis. Immunol Lett. 2004;95:91–95. doi: 10.1016/j.imlet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka KV, Mäurer M, Wiendl H. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58:50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 31.Chuang WY, Scröbel P, Gold R, Nix W, Schalke B, Kiefer R, Opitz A, Klinker E, Müller-Hermelink HK, Marx A. A CTLA4high genotype is associated with myasthenia gravis in thymoma patients. Ann Neurol. 2005;58:644–648. doi: 10.1002/ana.20577. [DOI] [PubMed] [Google Scholar]
- 32.Donner H, Braun J, Seidl C, Rau H, Finke R, Ventz M, Walfish PG, Usadel KH, Badenhoop K. Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinal Metab. 1997;82:4130–4132. doi: 10.1210/jcem.82.12.4406. [DOI] [PubMed] [Google Scholar]
- 33.Donner H, Rau H, Walfish PG, Braun J, Siegmund T, Finke R, Herwig J, Usadel KH, Badenhoop K. CTLA4 alanine-17 confers genetic susceptibility to Graves' disease and to type 1 diabetes mellitus. J Clin Endocrinal Metab. 1997;82:143–146. doi: 10.1210/jcem.82.1.3699. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Escribano MF, Rodriguez R, Valenzuela A, Garcia A, Garcia-Lozano JR, Nunez-Roldan A. CTLA4 polymorphisms in Spanish patients with rheumatoid arthritis. Tissue Antigens. 1999;53:296–300. doi: 10.1034/j.1399-0039.1999.530311.x. [DOI] [PubMed] [Google Scholar]
- 35.Kouki T, Sawai Y, Gardine CA, Fisfalen M-E, Alegre M-L, DeGroot LJ. CTLA-4 Gene Polymorphism at Position 49 in Exon 1 Reduces the Inhibitory Function of CTLA-4 and Contributes to the Pathogenesis of Graves’ Disease. J Immunol. 2000;165:6606–6611. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 36.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, et al. Ipilimumab (Anti-CTLA4 Antibody) Causes Regression of Metastatic Renal Cell Cancer Associated With Enteritis and Hypophysitis. J immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alfirevic A, Vilar FJ, Alsbou M, Jawaid A, Thomson W, Ollier WER, Bowman CE, Delrieu O, Park BK, Pirmohamed M. TNF, LTA, HSPA1L and HLA-DR gene polymorphisms in HIV-positive patients with hypersensitivity to cotrimoxazole. Pharmacogenomics. 2009;10:531–540. doi: 10.2217/pgs.09.6. [DOI] [PubMed] [Google Scholar]
- 39.Okajima M, Wada T, Nishida M, Yokoyama T, Nakayama Y, Hashida Y, Shibata F, Tone Y, Ishizaki A, Shimizu M, Saito T, et al. Analysis of T cell receptor Vbeta diversity in peripheral CD4 and CD8 T lymphocytes in patients with autoimmune thyroid diseases. Clin Exp Immunol. 2009;155:166–172. doi: 10.1111/j.1365-2249.2008.03842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh YC, Chang ST, Huang WT, Kuo SY, Chiang TA, Chuang SS. A comparative study of flow cytometric T cell receptor Vbeta repertoire and T cell receptor gene rearrangement in the diagnosis of large granular lymphocytic lymphoproliferation. Int J Lab Hematol. 2013;35:501–509. doi: 10.1111/ijlh.12041. [DOI] [PubMed] [Google Scholar]
- 41.Tzifi F, Kanariou M, Tzanoudaki M, Mihas C, Paschali E, Chrousos G, Kanaka-Gantenbein C. Flow cytometric analysis of the CD4+ TCR Vbeta repertoire in the peripheral blood of children with type 1 diabetes mellitus, systemic lupus erythematosus and age-matched healthy controls. BMC Immunol. 2013;14:33. doi: 10.1186/1471-2172-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortonne N, Huet D, Gaudez C, Marie-Cardine A, Schiavon V, Bagot M, Musette P, Bensussan A. Significance of circulating T-cell clones in Sezary syndrome. Blood. 2006;107:4030–4038. doi: 10.1182/blood-2005-10-4239. [DOI] [PubMed] [Google Scholar]
- 43.Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- 44.Farrell J, Naisbitt DJ, Drummond NS, Depta JP, Vilar FJ, Pirmohamed M, Park BK. Characterization of sulfamethoxazole and sulfamethoxazole metabolite-specific T-cell responses in animals and humans. J Pharmacol Exp Ther. 2003;306:229–237. doi: 10.1124/jpet.103.050112. [DOI] [PubMed] [Google Scholar]
- 45.Callan HE, Jenkins RE, Maggs JL, Lavergne SN, Clarke SE, Naisbitt DJ, Park BK. Multiple Adduction Reactions of Nitroso Sulfamethoxazole with Cysteinyl Residues of Peptides and Proteins: Implications for Hapten Formation. Chem Res Toxicol. 2009;22:937–948. doi: 10.1021/tx900034r. [DOI] [PubMed] [Google Scholar]
- 46.Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, Naisbitt DJ. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:411–418 e414. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Gibson A, Ogese M, Sullivan A, Wang E, Saide K, Whitaker P, Peckham D, Faulkner L, Park BK, Naisbitt DJ. Negative Regulation by PD-L1 during Drug-Specific Priming of IL-22–Secreting T Cells and the Influence of PD-1 on Effector T Cell Function. J Immunol. 2014;192:2611–2621. doi: 10.4049/jimmunol.1302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 50.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic Self-Tolerance Maintained by Cd25+Cd4+Regulatory T Cells Constitutively Expressing Cytotoxic T Lymphocyte–Associated Antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Xin XZ, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 54.Imaizumi T, Kumagai M, Sasaki N, Kurotaki H, Mori F, Seki M, Nishi N, Fujimoto K, Tanji K, Shibata T, Tamo W, et al. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72:486–491. [PubMed] [Google Scholar]
- 55.Wherry EJ, Ahmed R. Memory CD8 T-Cell Differentiation during Viral Infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative Immune Regulator Tim-3 Is Overexpressed on T Cells in Hepatitis C Virus Infection and Its Blockade Rescues Dysfunctional CD4+ and CD8+ T Cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.