Abstract
Objectives
Distinguishing intestinal tuberculosis (ITB) from Crohn's disease (CD) is difficult, although studies have reported clinical, endoscopic, imaging, and laboratory findings that help to differentiate these two diseases. We aimed to produce estimates of the predictive power of these findings and construct a comprehensive model to predict the probability of ITB vs. CD.
Methods
A systematic literature search for studies differentiating ITB from CD was conducted in MEDLINE, PUBMED, and EMBASE from inception until September 2015. Fifty-five distinct meta-analyses were performed to estimate the odds ratio of each predictive finding. Estimates with a significant difference between CD and ITB and low to moderate heterogeneity (I2 <50%) were incorporated into a Bayesian prediction model incorporating the local pretest probability.
Results
Thirty-eight studies comprising 2,117 CD and 1,589 ITB patients were included in the analyses. Findings in the model that significantly favored CD included male gender, hematochezia, perianal disease, intestinal obstruction, and extraintestinal manifestations; endoscopic findings of longitudinal ulcers, cobblestone appearance, luminal stricture, mucosal bridge, and rectal involvement; pathological findings of focally enhanced colitis; and computed tomographic enterography (CTE) findings of asymmetrical wall thickening, intestinal wall stratification, comb sign, and fibrofatty proliferation. Findings that significantly favored ITB included fever, night sweats, lung involvement, and ascites; endoscopic findings of transverse ulcers, patulous ileocecal valve, and cecal involvement; pathological findings of confluent or submucosal granulomas, lymphocyte cuffing, and ulcers lined by histiocytes; a CTE finding of short segmental involvement; and a positive interferon-γ release assay. The model was validated by gender, clinical manifestations, endoscopic, and pathological findings in 49 patients (27 CD, 22 ITB). The sensitivity, specificity, and accuracy for diagnosis of ITB were 90.9%, 92.6%, and 91.8%, respectively.
Conclusions
A Bayesian model based on the meta-analytic results is presented to estimate the probability of ITB and CD calibrated to local prevalence. This model can be applied to patients using a publicly available web application.
Introduction
Differentiating intestinal tuberculosis (ITB) from Crohn's disease (CD) remains a challenging clinical problem in regions where ITB is prevalent and CD incidence is increasing. This differentiation is also increasingly a problem in countries where ITB is not common, but rapidly growing immigrant populations from areas of high ITB prevalence make ITB an important diagnostic possibility. A definite diagnosis of ITB depends on methods that have unsatisfactorily low sensitivities, including 5.3–37.5% for acid-fast bacilli tissue staining (1–3), 23–46% for mycobacterial culture (4,5), and 36.4–67.9% for PCR (3,4,6–8). Therefore, ITB still cannot be confidently excluded even when all the above results are negative, thus the current Asia-Pacific guidelines recommend 8–12 weeks of empirical antituberculosis treatment for patients with diagnostic uncertainty, owing to the potentially fatal complications if immunosuppressive agents are wrongly prescribed to ITB patients (9). However, antituberculosis treatment can cause many side effects and facilitate the development of Mycobacterium tuberculosis drug resistance. Additionally, 8–12 weeks of empiric antituberculosis treatment can delay proper CD treatment and lead to severe flares and complications. Therefore, many studies have been undertaken to identify features that can differentiate between these two diseases, and have found that individual clinical, endoscopic, imaging, and serologic laboratory findings help to guide physicians in selecting empirical treatment (1,4,6,10–17). However, the results of these studies are heterogeneous with inconsistent findings and recommendations. Thus, physicians need a better model to differentiate ITB from CD. Here we report the results of 55 meta-analyses of factors predictive of ITB or CD based on published data, and we present a Bayesian model for discriminating ITB from CD using these factors and the local pretest probability in a publicly available web application.
Methods
Search strategy and study selection
To obtain all studies related to differentiating between CD and ITB, we searched with the term “intestinal tuberculosis” AND “Crohn's disease” for studies published in PubMed, Medline, and Embase from the origination of each database up to September 2015. We included studies that used any of the following to differentiate CD from ITB: clinical manifestations, inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), colonoscopic findings, pathologic findings, computed tomography (CT) findings, serological tests including anti-Saccharomyces cerevisiae antibody and interferon-γ release assay (IGRA), or a PCR-based assay performed on either tissue biopsies or stool. We included all studies without language restriction. In cases where there was a suspicion of overlapping study populations, the larger study population was selected for inclusion. Duplicate articles were manually deleted. We excluded (i) studies that did not report the number of patients (e.g., reported only the number of mucosal biopsies), (ii) studies that included only complicated cases requiring surgery, and (iii) studies published before the year 2000 that used imaging, owing to the potential for relatively poor imaging quality.
Data extraction and quality assessment
Eligible articles were reviewed independently by two investigators (J.L. and A.B.S.). Disagreements were resolved by consensus and, as necessary, involvement of a third reviewer (P.D.R.H.). All variables to distinguish CD from ITB were recorded. We selected the variables that were included in at least three studies in our analyses. In total, we included age, gender, 14 clinical manifestations, 3 inflammatory markers, 18 colonoscopic findings (10 for lesion characteristics, 8 for location of involvement), 11 pathologic findings, 5 CT findings, and 2 serological findings (anti-Saccharomyces cerevisiae antibody and IGRA). These 55 variables included the 52 dichotomous variables shown in Table 1, and five continuous variables including age, duration of symptoms, ESR, CRP, and albumin; ESR and CRP were reported in both dichotomous and continuous variables. The definition criteria of all variables are provided in Supplementary Table S1 online in the Supplementary Documents. A separate random-effects meta-analysis was performed for each predictor variable.
Table 1. ORs and corresponding 95% confidence intervals (CI), I2, positive and negative likelihood ratios of all variables.
| Findings | N of studies | (+) in CD (%) | (+) in TB (%) | OR for CD | 95% CI | I2 (%) | +LR for CD | −LR for CD | +LR for ITB | −LR for ITB |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic data | ||||||||||
| Male gender (1–3,6,8, 10–18, 28-30, 32–36, 44–16) | 25 | 874/1,390 (62.9) | 560/1,124 (49.8) | 1.63 | 1.28–2.08 | 48.1 | 1.26 | 0.74 | 0.79 | 1.35 |
| Clinical manifestations | ||||||||||
| Abdominal pain (1–3,6,8, 11–15,29,30,32–35,46,47) | 18 | 900/1,068 (84.3) | 692/821 (84.3) | 0.97 | 0.67–1.41 | 43.2 | ||||
| Diarrhea (1–3, 6, 8, 11–15, 28, 30, 32-35, 37, 46, 47) | 19 | 680/1,078 (63.1) | 376/846 (44.4) | 2.09 | 1.44–3.05 | 69.9 | 1.42 | 0.66 | 0.70 | 1.50 |
| Hematochezia (1-3, 6, 8, 11, 12, 15, 28, 30, 33–35, 46) | 14 | 304/885 (34.4) | 99/673 (14.7) | 2.13 | 1.52–2.98 | 57.3 | 2.33 | 0.77 | 0.43 | 1.30 |
| Perianal disease (1, 6, 8, 11, 12, 15,28-30,32,34,37,38,45,47) | 15 | 209/897 (23.3) | 23/702 (3.3) | 6.11 | 3.92–9.51 | 0 | 7.11 | 0.79 | 0.14 | 1.26 |
| Obstruction (2, 3, 6, 11, 12, 14, 15, 29, 30, 33, 34, 47) | 12 | 212/882 (24.0) | 113/600 (18.8) | 1.59 | 0.91–2.78 | 69.4 | ||||
| Abdominal mass (1–3, 12, 28–30, 32–34, 46) | 11 | 93/580 (16.0) | 76/489 (15.5) | 1.10 | 0.75–1.62 | 0 | ||||
| Fever (1-3, 6, 8, 11–15, 29, 30, 32–35, 37, 46, 47) | 19 | 326/1,107 (29.4) | 415/840 (49.4) | 0.44 | 0.33–0.58 | 41.0 | 0.59 | 1.39 | 1.67 | 0.71 |
| Weight loss (1, 3, 6, 11–15, 30, 32–35, 37, 46) | 15 | 602/895 (67.3) | 546/739 (73.9) | 0.67 | 0.42–1.07 | 73.4 | ||||
| Night sweats (2, 8, 11, 12, 15, 30, 34, 35) | 8 | 74/603 (12.3) | 155/393 (39.4) | 0.20 | 0.12–0.33 | 53.7 | 0.31 | 1.44 | 3.21 | 0.69 |
| Anemia (3, 11,29,30,33,37) | 6 | 192/405 (47.4) | 162/327 (49.5) | 0.81 | 0.42–1.54 | 70.2 | ||||
| Lung involvement (2, 3, 11, 12, 15, 29, 30, 33, 34, 37, 45) | 11 | 50/785 (6.4) | 205/515 (39.8) | 0.11 | 0.06–0.18 | 36.2 | 0.16 | 1.56 | 6.25 | 0.64 |
| Ascites (2, 3, 8, 11, 12, 15, 30, 32, 33, 44, 47) | 11 | 41/720 (5.7) | 138/483 (28.6) | 0.17 | 0.10–0.27 | 15.2 | 0.20 | 1.32 | 5.02 | 0.76 |
| Extraintestinal manifestations (1, 3,11, 15, 33, 34, 45, 48) | 8 | 222/865 (25.6) | 51/603 (8.5) | 4.10 | 2.92–5.75 | 0 | 3.0 | 0.81 | 0.33 | 1.23 |
| Inflammatory markers | ||||||||||
| Erythrocyte sedimentation rate (11, 15, 17, 34) | 4 | 263/403 (65.3) | 188/278 (67.6) | 1.18 | 0.40–3.48 | 88.3 | ||||
| C-reactive protein (11, 15, 34) | 3 | 269/378 (71.2) | 128/238 (53.8) | 2.05 | 1.43–2.92 | 0 | 1.32 | 0.62 | 0.76 | 1.60 |
| Endoscopic findings | ||||||||||
| Longitudinal ulcers (1, 2, 6, 8, 10–12, 14, 16, 30, 33, 34, 47) | 13 | 340/744 (45.7) | 45/558 (8.1) | 9.30 | 6.53–13.24 | 0 | 5.67 | 0.59 | 0.18 | 1.69 |
| Transverse ulcers (1, 2, 8, 10–12, 14, 16, 30, 34, 35, 47) | 12 | 97/698 (13.9) | 239/513 (46.6) | 0.15 | 0.11–0.20 | 0 | 0.30 | 1.61 | 3.35 | 0.62 |
| Aphthous ulcers (1, 6, 8, 10–14, 16, 30, 33–35) | 13 | 242/705 (34.3) | 112/592 (18.9) | 3.05 | 1.81–5.13 | 60.6 | 1.81 | 0.81 | 0.55 | 1.23 |
| Cobblestone appearance (1,2, 6, 8, 10–12, 14, 16,30,33–35,46) | 14 | 193/763 (25.3) | 32/610 (5.2) | 5.03 | 3.02–8.38 | 26.2 | 4.82 | 0.79 | 0.21 | 1.27 |
| Patulous ileocecal valve (1, 10–12, 16, 30, 34) | 7 | 48/462 (10.4) | 129/362 (35.6) | 0.22 | 0.11–0.41 | 48.0 | 0.29 | 1.39 | 3.43 | 0.72 |
| Pseudopolyps (1, 2, 8, 10, 12, 14, 16, 32, 33, 46, 47) | 11 | 175/469 (37.3) | 134/374 (35.8) | 1.45 | 0.82–2.55 | 62.1 | ||||
| Stricture (1, 2, 8, 10, 11, 14, 30, 32, 33, 35, 37, 47) | 12 | 184/557 (33.0) | 111/469 (23.7) | 1.45 | 1.08–1.94 | 0 | 1.40 | 0.88 | 0.72 | 1.14 |
| Mucosal bridge (10, 34,46) | 3 | 16/181 (8.8) | 2/143 (1.4) | 5.02 | 1.38–18.33 | 0 | 6.32 | 0.92 | 0.16 | 1.08 |
| Skip lesions (1,6, 12,33) | 4 | 87/162 (53.7) | 38/146 (26.0) | 4.00 | 1.48–10.79 | 64.2 | 2.06 | 0.63 | 0.48 | 1.60 |
| Nodularity (6, 13, 14) | 3 | 26/144 (18.0) | 56/133 (42.1) | 0.25 | 0.06–1.09 | 82.3 | ||||
| Location of involvement defined by colonoscopy | ||||||||||
| Rectum (6, 11, 12, 14,30,33,34,47) | 8 | 151/502 (30.1) | 56/404 (13.9) | 3.11 | 1.81–5.34 | 45.7 | 2.17 | 0.81 | 0.46 | 1.23 |
| Sigmoid colon (6, 11, 12, 14, 30) | 5 | 146/325 (44.9) | 65/297 (21.9) | 2.92 | 1.09–7.87 | 84.7 | 2.05 | 0.71 | 0.49 | 1.42 |
| Descending colon (6, 11, 12, 14,30) | 5 | 123/323 (38.1) | 64/297 (21.5) | 2.47 | 0.91–6.70 | 84.3 | ||||
| Transverse colon (6, 11, 12, 14, 30, 47) | 6 | 128/363 (35.3) | 85/308 (27.6) | 1.47 | 0.52–4.16 | 85.4 | ||||
| Ascending colon (6,11,12,14, 30, 47) | 6 | 151/357 (42.3) | 155/305 (50.8) | 0.80 | 0.43–1.50 | 67.0 | ||||
| Cecum (11, 12, 14) | 3 | 96/223 (43.0) | 117/205 (57.1) | 0.56 | 0.38–0.83 | 0 | 0.75 | 1.33 | 1.33 | 0.75 |
| Ileocecal valve (11, 12, 14, 30, 33) | 5 | 164/281 (58.4) | 183/256 (71.5) | 0.53 | 0.36–0.77 | 0 | 0.82 | 1.46 | 1.22 | 0.68 |
| Ileum (6, 11, 12, 14,30,33) | 6 | 191/326 (58.6) | 162/295 (54.9) | 1.08 | 0.65–1.79 | 43.3 | ||||
| Pathologic findings | ||||||||||
| Granuloma (6–8, 10, 12, 13, 17, 31–33, 45–47, 49) | 14 | 177/467 (37.9) | 308/480 (64.2) | 0.26 | 0.15–0.44 | 57.0 | 0.59 | 1.73 | 1.69 | 0.58 |
| Confluent granuloma (1, 7, 12, 31, 33, 45, 49) | 7 | 4/211 (1.9) | 86/206 (41.7) | 0.04 | 0.02–0.10 | 0 | 0.05 | 1.68 | 22.0 | 0.59 |
| Large granuloma (6, 7, 12,31,45,49) | 6 | 26/208 (12.5) | 127/209 (60.8) | 0.07 | 0.02–0.17 | 59.8 | 0.21 | 2.23 | 4.86 | 0.45 |
| Multiple granulomas/section (6, 12, 31, 45) | 4 | 8/135 (5.9) | 32/121 (26.4) | 0.18 | 0.07–0.49 | 11.6 | 0.22 | 1.28 | 4.55 | 0.78 |
| Mucosal granuloma (6, 31, 45, 49) | 4 | 62/129 (48.1) | 65/124 (52.4) | 0.86 | 0.52–1.42 | 0 | ||||
| Submucosal granuloma (6, 31, 45, 49) | 4 | 10/129 (7.8) | 41/124 (33.1) | 0.17 | 0.08–0.37 | 0 | 0.23 | 1.38 | 4.27 | 0.73 |
| Microgranuloma (1,6,31, 49) | 4 | 37/134 (27.6) | 23/130 (17.7) | 3.56 | 0.82–15.49 | 51.4 | ||||
| Cuffing lymphocyte (6, 7, 49) | 3 | 17/126 (13.5) | 66/141 (46.8) | 0.15 | 0.08–0.29 | 0 | 0.29 | 1.63 | 3.47 | 0.61 |
| Ulcer lined by histiocytes (6, 7, 31, 45, 49) | 5 | 10/171 (5.8) | 69/179 (38.5) | 0.11 | 0.05–0.27 | 29.2 | 0.15 | 1.53 | 6.59 | 0.65 |
| Disproportionate submucosal inflammation (6, 12, 31, 45) | 4 | 29/117 (24.8) | 57/110 (51.8) | 0.22 | 0.04–1.24 | 81.7 | ||||
| Focally enhanced colitis (6, 31, 49) | 3 | 53/104 (60.0) | 26/106 (24.5) | 3.65 | 1.94–6.85 | 0 | 2.08 | 0.65 | 0.48 | 1.54 |
| Computed tomographic enterography findings | ||||||||||
| Short segmental involvement (14, 16, 44) | 3 | 52/175 (29.7) | 68/99 (68.7) | 0.11 | 0.05–0.26 | 30.0 | 0.43 | 2.24 | 2.31 | 0.45 |
| Wall stratification (14, 16, 44) | 3 | 79/175 (45.1) | 22/99 (22.2) | 2.32 | 1.04–5.17 | 42.6 | 2.03 | 0.70 | 0.49 | 1.42 |
| Asymmetrical wall thickening (14–16, 44) | 4 | 135/316 (42.7) | 8/146 (5.5) | 9.24 | 0.90–94.27 | 82.3 | ||||
| Comb sign (14–16, 44) | 4 | 252/316 (79.7) | 28/146 (19.2) | 19.77 | 4.87–80.38 | 81.1 | 4.16 | 0.25 | 0.24 | 3.99 |
| Fibrofatty proliferation (14–16, 44) | 4 | 128/316 (40.5) | 18/146 (12.3) | 4.05 | 1.87–8.79 | 35.7 | 3.29 | 0.68 | 0.30 | 1.47 |
| Serology | ||||||||||
| Positive IGRA (2, 3, 17, 38, 50–54) | 9 | 69/483 (14.3) | 266/317 (83.9) | 0.02 | 0.01–0.04 | 35.3 | 0.17 | 5.33 | 5.87 | 0.19 |
| Positive ASCA (1–3, 33, 38, 55, 56) | 7 | 116/333 (34.8) | 55/247 (22.3) | 1.94 | 0.97–3.86 | 53.4 | ||||
ASCA, anti-Saccharomyces cerevsiae antibody; CD, Crohn's disease; CI, confidence interval; IGRA, interferon-γ-releasing assay; ITB, intestinal tuberculosis; +LR, positive likelihood ratio; −LR, negative likelihood ratio; OR, odds ratio.
Bold font indicates significant OR and low heterogeneity (/2<50%).
We assessed for bias in the studies using the QUADAS-2 tool. The tool comprises of four domains: patient selection, index test, reference standard, and flow and timing. The risk of bias was judged as “low,” “high,” or “unclear.” The “unclear” category was used only when insufficient data were reported to permit a judgment. A sensitivity analyses including only low-bias studies was performed.
Statistical analyses and development of a Bayesian model to predict the probabilities of ITBs
Meta-analysis of each finding was carried out using a random-effects model to combine estimates from each study. For the predictor variables with dichotomous results, the diagnostic odds ratio (OR) and corresponding 95% confidence interval (CI) for CD were calculated to determine the findings that significantly favored CD vs. ITB. Heterogeneity was determined by the I2. Positive and negative likelihood ratios (LRs) for both ITB and CD were calculated to determine the effect size of each finding. For example, to calculate the positive LR for ITB for a particular finding, the percentage of ITB patients with the positive findings was divided by the percentage of CD patients with that positive findings. For the continuous variables, the results were reported in mean difference and 95% CI.
The significant variables with dichotomous results were selected to build the Bayesian model, which calculated the probability of ITB based on the relative prevalence of ITB vs. CD (the pretest probability of ITB) and LR for ITB of each predictor variable in the model. The pretest probability is converted into odds, then multiplied by each of the LRs, and then converted back into a probability. The formula used is
where P0 is the pretest probability of ITB, which is the relative prevalence of ITB, and P′ is the post-test probability, which is the probability of diagnosis of ITB. For any findings without available results, the model defaults to an LR of 1 for that finding.
For variable selection, because of uncertain cutoff values of heterogeneity and effect size to determine which variables should be included to obtain the best model, three models with different cutoff s of the variables' heterogeneity and LR were generated. The first model comprises of all variablesthat significantly favored either CD or ITB with low to moderate heterogeneity (I2<50%) and strong effect size (LR≥2). The second model included all significant variables with low to moderate heterogeneity, regardless of LR. The third model included all significant variables regardless of heterogeneity and LR. The model with best performance for differentiating ITB from CD was selected to be the final model.
To assess the performance of the models, we did a retrospective cohort review of all newly diagnosed CD and ITB patients in Siriraj Hospital, Bangkok, Thailand from January 2012 to December 2015. The criteria for diagnosis of ITB was any of the following: (i) presence of acid-fast bacilli or caseating granuloma in pathological specimens, (ii) tissue culture growing mycobacterial tuberculosis, (iii) presence of proven tuberculosis elsewhere in the body, and (iv) clinical and endoscopic response to antituberculosis treatment without subsequent recurrence. CD was diagnosed based on clinical, endoscopic, and pathological findings with clinical response to CD treatment with at least 6 months follow-up period. The clinical manifestations and laboratory data were manually reviewed in medical records (CH.L. and R.B.). The endoscopic findings and pathological findings were reviewed by a gastroenterologist (J.L.) and a gastrointestinal pathologist (A.P.), respectively, who were blinded from final diagnosis and any other predictive data. The data from this cohort was applied to all models. The performance of the model was determined by area under the curve of receiver operating characteristic curves, sensitivity, specificity, positive predicted value (PPV), and negative predicted value (NPV) to differentiate ITB from CD. This protocol was approved by an independent ethics committee according to local requirements in Bangkok.
All analyses were performed in R version 3.2.2. The package metafor was used for meta-analysis. The package OptimalCutpoints was used to create ROC curves and define the cut-point providing the best performance of the model. The Bayesian model web application was built in the Shiny web application framework (shiny.rstudio.com).
Results
Studies selected for inclusion
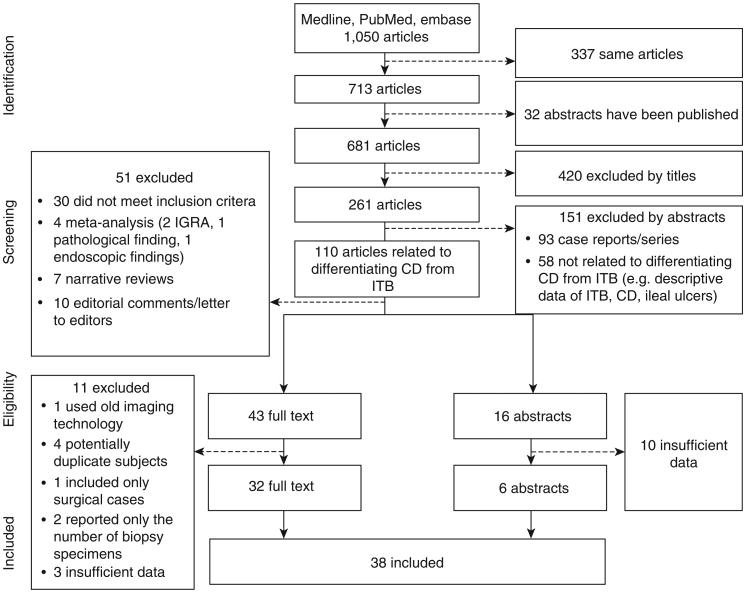
One thousand and fifty articles were found in the systematic literature search. Of these, 59 articles (43 full text, 16 abstracts) met the inclusion criteria. Eleven full-text articles were excluded; four of these contained potentially duplicated subjects (18–21), two reported only the number of biopsy specimens (22,23), three did not provide the desired data (5,24,25), one relied on outdated CT techniques (26), and one included only complicated surgical cases (27). However, we decided to include two other studies with only surgical cases: one where the indication for surgery was for diagnosis in some cases, and the other one was from 1981 when the efficacy of medical treatment was poor and early surgery may have been indicated (28,29). The severity of disease in these two studies appeared comparable to the other studies included. Ten abstracts were excluded because they did not report sufficient detailed data. Finally, 38 articles (32 full-text articles and 6 abstracts) comprising 2,117 CD and 1,589 ITB patients were included in the analyses (Figure 1). Among these studies, most were published in English. There were two studies published in Chinese and one study published in Korean, and these were translated for this analysis. A summary of all of the 38 analyzed studies is available in Supplementary Table S2 online in the Supplementary Documents.
Figure 1.
Flow diagram of study selection. CD, Crohn's disease; IGRA, interferon-γ-releasing assay; ITB, intestinal tuberculosis.
Significant predictor variables for differentiating ITBs from CD selected to build the Bayesian model
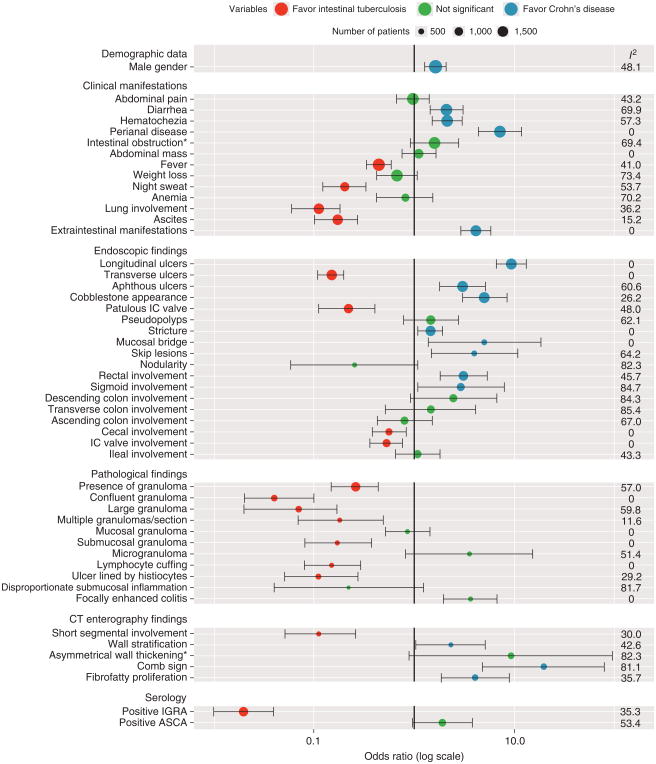
The diagnostic OR for CD and corresponding 95% CI, the I2 measure of heterogeneity, and the positive and negative LR of each predictor variable are shown in Figure 2 and Table 1. The variables with an OR >1 and the lower bound of the 95% CI >1 significantly favored CD, whereas the variables with an OR <1 and the upper bound of the 95% CI <1 significantly favored ITB. The forest plots for each of the 55 predictors are included in Supplementary Figures online.
Figure 2.
Forest plot presenting odds ratios, 95% confidence intervals, and I2 of all predictor variables. “Clinical manifestation of intestinal obstruction” and “CT enterography finding of asymmetrical wall thickening” became significant in sensitivity analyses using only the studies with low bias. ASCA, anti-Saccharomyces cerevisiae antibody; CT, computed tomography; IC, ileocecal valve; IGRA, interferon-γ-releasing assay.
Demographic data
The proportion of males was significantly higher in CD patients (62.9% in CD vs. 49.8% in ITB, OR=1.63, 95% CI=1.28–2.08). Gender was selected to build the model. CD patients trended younger than ITB patients, but the difference was not statistically significant. The mean age difference was –1.71 (–3.96 to 0.55) years when CD was compared with ITB (Supplementary Figures online).
Clinical manifestations
The duration of disease was longer in CD (Table 2). Seven studies reported the median and seven studies reported the mean duration of symptoms. The median duration in CD (6–53.3 months, range 0.3–300 months) was significantly greater than that in ITB (3–23.4 months, range 0–120 months) in five of the studies (1,6,12–14,16,30). In the other studies, the reported mean duration of symptoms in meta-analysis was 26.5 months (95% CI=15.7–37.3) longer for CD compared with that for ITB (2,11,15,31–34). Although the studies reporting means had a very high heterogeneity with I2 of 97.7%, all of the studies showed a longer duration in CD (Supplementary Figures online). However, our Bayesian model based on LRs requires significant dichotomous differences. Therefore, duration of disease could not be included in the model.
Table 2. Summary of duration of symptoms before diagnosis of Crohn's disease or intestinal tuberculosis in each study.
| Author | Year | N | Duration of symptoms (months) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| CD | ITB | CD | ITB | |||||
| Median | Range | Median | Range | |||||
| Makharia et al. (6) | 2010 | 53 | 53 | 53.3 | 9–300 | 23.4 | 1–120 | <0.001 |
| Dutta et al. (1) | 2011 | 30 | 30 | 24 | 6–180 | 3 | 1–24 | <0.001 |
| Yu et al. (12) | 2012 | 53 | 43 | 28.9 | 0.3–132 | 12.5 | 0.5–96 | 0.009 |
| Liu et al. (30) | 2014 | 38 | 30 | 19.5 | NA | 3 | NA | 0.001 |
| Larsson et al. (13) | 2014 | 37 | 38 | 6 | 1–78 | 6 | 0–60 | 0.58 |
| Kedia et al. (14) | 2015 | 54 | 50 | 27 | 16.5–63 | 12 | 4–24 | <0.001 |
| Mao et al. (16 | 2015 | 67 | 38 | 13 | 1–252 | 8.5 | 1–96 | 0.147 |
| Mean | S.d. | Mean | S.d. | |||||
| Pulimood et al. (31) | 1999 | 20 | 20 | 31.7 | 21 | 16.4 | 21 | NA |
| Amarapurkar et al. (32) | 2004 | 20 | 60 | 42 | 7.3 | 7 | 2.5 | NA |
| Amarapurkar et al. (33) | 2008 | 26 | 26 | 58.1 | 9.8 | 7.2 | 3.4 | <0.05 |
| Li et al. (11) | 2011 | 130 | 122 | 30.9 | 17.6 | 15.9 | 11.2 | 0.011 |
| Li et al. (2) | 2012 | 65 | 19 | 46.5 | 51.5 | 10.2 | 8.8 | 0.003 |
| Cheng et al. (34) | 2013 | 107 | 69 | 30.8 | 16.8 | 15.8 | 11 | 0.032 |
| Zhao et al. (15) | 2014 | 141 | 47 | 35.7 | 12.4 | 17.6 | 6.9 | 0.001 |
CD, Crohn's disease; ITB, intestinal tuberculosis; NA, no data available. Bold font indicates significant values.
Diarrhea, hematochezia, presence of perianal disease, and extraintestinal manifestations significantly favored CD, whereas fever, night sweats, lung involvement, and ascites significantly favored ITB. All of them were selected to build the model. Abdominal pain, abdominal mass, presence of intestinal obstruction, weight loss, and anemia were not significantly different between CD and ITB.
Inflammatory markers
ESR was reported in continuous values in six studies (four for mean and two for median) (1,11,12,15,17,34) and in dichotomous results (normal vs. abnormal value) in four studies (2,33,35,36). Meta-analyses were performed separately. There was no difference between the proportion of patients with high ESR among the studies reporting dichotomous results. Patients with ITB had higher ESR level than patients with CD among the studies reporting mean, but the mean difference was quite small, at 4.09 (2.22–5.96) mm/h (Supplementary Figures online). Therefore, ESR was not included in the model.
CRP was reported in continuous value in seven studies (five for mean and two for median) (2,12,13,35–38) and in dichotomous results in three studies (11,15,34). The proportion of patients with high CRP was significantly higher in CD in the studies reporting dichotomous results; however, CRP level tended to be higher but not statistically significant in ITB among the studies reporting CRP in mean (Supplementary Figures online). Owing to these conflicting results, CRP was not included in the model.
Albumin level was reported in six studies (1,11,12,15,34,35). There was no significant difference in the albumin level between CD and ITB, and it was not included in the model.
Endoscopic findings
The following endoscopic findings significantly favored CD: longitudinal ulcers, aphthous ulcers, cobblestone appearance, luminal stricture, mucosal bridge, and skip lesions. Transverse ulcers and a patulous ileocecal (IC) valve significantly favored ITB. All of them were selected to build the model. Pseudopolyps did not distinguish the two diseases. Mucosal nodularity was found more of en in ITB, but this was not statistically significant.
For the site of involvement, rectal and sigmoid colon involvement were significant predictors of CD, while involvement of the IC valve and cecum significantly favored ITB. Involvement of rectum, sigmoid colon, and cecum were selected to build the model. Involvement of IC valve may be correlated with patulous IC valve. To avoid the possibility of including potentially interdependent variables in the model, patulous IC valve that has been reported as a significant predictor in many studies was selected. Involvement of ileum, ascending colon, transverse colon, and descending colon was not significantly different between CD and ITB.
Pathologic findings
Granulomas were found more frequently in ITB. Some characteristics of the granulomas were significantly associated with ITB such as confluent granuloma, large granuloma, multiple granulomas per section, submucosal granuloma, and granuloma with surrounding cuffing lymphocytes. An ulcer lined by histiocytes also significantly favored ITB, while focally enhanced colitis significantly favored CD. All of these findings had low heterogeneity and were initially selected to build the model. However, the presence of a confluent granuloma may be correlated with the presence of large or multiple granulomas/section. Only conf uent granuloma, which had the least heterogeneity and highest positive LR was selected for the model. Microgranulomas and disproportionate submucosal inflammation were observed more commonly in CD and ITB, respectively, but the differences were not significant. They were not included in the model.
Imaging findings
Four studies using CT were included; all used the CT enterography technique. The presence of wall stratification, comb sign, and f brofatty proliferation significantly favored CD, while short segmental involvement significantly supported the diagnosis of ITB. These predictors were selected to build the model. Asymmetrical wall thickening was found more commonly in CD, but the difference was not statistically significant. The presence of necrotic intra-abdominal lymph nodes was found only in ITB. Because we considered this as a diagnostic finding for ITB, we did not include it as a predictive variable.
Serological testing results
IGRA has a pooled sensitivity of 84% and a specificity of 86% for diagnosis of ITB. Its positive result strongly favored ITB (I2 =35.3%, positive LR for ITB=5.87), and it was selected to build the model. A positive anti-Saccharomyces cerevisiae antibody favored CD, but it was not statistically significant, and was not included in the model.
Sensitivity analysis by QUADAS-2 bias level
The evaluation of bias with the QUADAS-2 tool is shown in Supplementary Table S3 online. Sensitivity analyses using only the studies with low bias were carried out and are summarized in Supplementary Table S4 online in the Supplementary Documents. Sensitivity analyses were not performed for some predictor variables because very few studies qualified after the exclusion of studies with high or unclear bias. The results of the sensitivity analyses largely confirmed the results of the initial meta-analyses with the following exceptions: the clinical manifestation of intestinal obstruction and the presence of asymmetrical bowel wall thickening on computed tomographic enterograph became significant in favoring CD with low heterogeneity, and these predictors were added to the model development. The heterogeneity was reduced for the presence of night sweats and the presence of comb sign on computed tomographic enterograph.
Development of the Bayesian model for predicting the probability of ITBs
The predictor variables with significant OR were selected to build the model. Three models using different criteria for the heterogeneity and LR of predictor variables as we described in the Methods section were shown in Table 3. In the models, we retained the LR from the original analysis for most predictor variables. We used the LR from the low bias sensitivity analysis for the predictor variables that became significant or had reduced heterogeneity in the low bias sensitivity analysis. The model uses local relative prevalence of ITB vs. CD for the pretest probability, and calculates the probability of ITB based on the measured predictor variables. Predictor findings that are not assessed are assigned an LR of 1.
Table 3. The models with different criteria for the cutoff values of variables' heterogeneity and likelihood ratio.
| Parameters | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Demographic data and clinical manifestations | |||
| Variables with I2 <50% and LR≥2 | Yes | Yes | Yes |
| Variables favoring Crohn's disease | |||
| Perianal disease | |||
| Intestinal obstruction | |||
| Extraintestinal manifestations | |||
| Variables favoring Intestinal tuberculosis | |||
| Night sweats | |||
| Pulmonary involvement | |||
| Ascites | |||
| Variables with I2 <50% and LR<2 | Yes | Yes | |
| Variables favoring Crohn's disease | |||
| Male gender | |||
| Hematochezia | |||
| Variables favoring intestinal tuberculosis | |||
| Fever | |||
| Variables with I2 ≥50%, any LR | Yes | ||
| Variables favoring Crohn's disease | |||
| Diarrhea | |||
| Endoscopic findings | |||
| Variables with I2 <50% and LR≥2 | Yes | Yes | Yes |
| Variables favoring Crohn's disease | |||
| Longitudinal ulcers | |||
| Cobblestone appearance | |||
| Mucosal bridge | |||
| Rectal involvement | |||
| Variables favoring intestinal tuberculosis | |||
| Transverse ulcers | |||
| Patulous ileocecal valve | |||
| Variables with I2 <50% and LR<2 | Yes | Yes | |
| Variables favoring Crohn's disease | |||
| Luminal stricture | |||
| Variables favoring intestinal tuberculosis | |||
| Cecal involvement | |||
| Variables with I2 ≥50%, any LR | Yes | ||
| Variables favoring Crohn's disease | |||
| Aphthous ulcers | |||
| Skip lesion | |||
| Sigmoid colon involvement | |||
| Pathological findings | |||
| Variables with I2 <50% and LR≥2 | Yes | Yes | Yes |
| Variables favoring Crohn's disease | |||
| Focally enhanced colitis | |||
| Variables favoring intestinal tuberculosis | |||
| Confluent granuloma | |||
| Submucosal granuloma | |||
| Lymphocyte cuffing around granuloma | |||
| Ulcers lined by histiocytes | |||
| Computed tomography enterography findings | |||
| Variables with I2 <50% and LR≥2 | Yes | Yes | Yes |
| Variables favoring Crohn's disease | |||
| Asymmetrical wall thickening | |||
| Intestinal wall stratification | |||
| Comb sign | |||
| Fibrofatty proliferation | |||
| Variables favoring intestinal tuberculosis | |||
| Short segmental involvement | |||
| Serological tests | |||
| Variables with I2 <50% and LR≥2 | Yes | Yes | Yes |
| Variables favoring intestinal tuberculosis | |||
| Interferon-γ release assay | |||
LR, likelihood ratio.
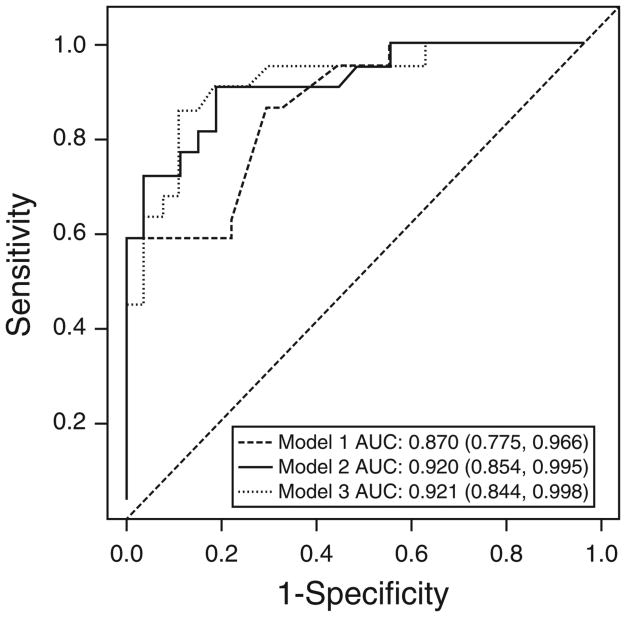
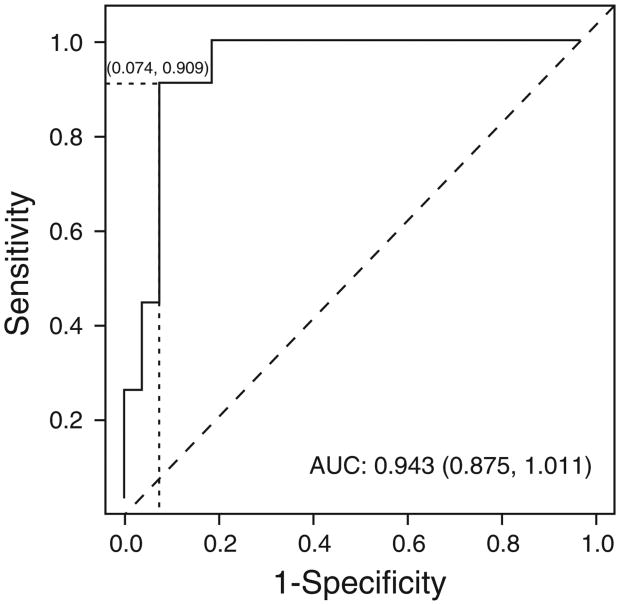
To compare the performance of each of the three models, a validation cohort was identified in Bangkok, Thailand. The three models were applied to the data of 27 CD patients and 22 ITB patients. Because the differences between each model were found only in the gender, clinical manifestations, and endoscopic findings parameters (only these had predictors with low LRs or high heterogeneity) (Table 3), the calculated probability of ITB based on these parameters was selected for the three model comparison. The ROC curves of all three models are shown in Figure 3. The sensitivity, specificity, PPV, NPV, false positive (misdiagnosis of ITB in CD patients), and false negative (misdiagnosis of CD in ITB patients) for the cut-point to obtain the best model performance and the cut-point to obtain the NPV of 100% for diagnosis of ITB (avoiding a potentially fatal outcome from wrongly prescribing immunosuppressive agents to patients with ITB) of each model are shown in Table 4. The area under the curve of receiver operating characteristic curves of Model 1, Model 2, and Model 3 were 0.870, 0.920, and 0.921, respectively. Both Model 2 and Model 3 appear to be quite accurate. When we evaluated the best cut-point to obtain an NPV of 100% for diagnosis of ITB, the number of false positives were 15, 15, and 17 for Model 1, Model 2, and Model 3, respectively. Model 2 was selected as the final model because it had an area under the curve of receiver operating characteristic curve comparable to Model 3, but had fewer false positives using a cut-point to obtain an NPV of 100%. When the pathological data was added to this model, the area under the curve of receiver operating characteristic curve of Model 2 increased to 0.943 (0.875–1.011) (Figure 4). At the cut-point of 85.83% of the probability of ITB, the sensitivity, specificity, PPV, NPV, and accuracy for differentiating ITB from CD were 90.9%, 92.6%, 90.9%, 92.6%, and 91.8%, respectively. Moreover, when the cut-point of 49.04% was used to obtain a NPV of 100% for diagnosis of ITB, the number of patients with CD who would be misdiagnosed with ITB decreased to 5/27 (18.5%). The data for predictors of CT enterography and IGRA was available in only 16 patients and one patient, respectively, in our cohort. Therefore, the full model performance could not be assessed in this validation cohort.
Figure 3.
Receiver operating characteristic curve for three models for differentiating intestinal tuberculosis from Crohn's disease based on gender, clinical manifestations, and endoscopic findings. Model 1 includes the significant parameters with low heterogeneity (I2<50) and likelihood ratio (LR) ≥2, Model 2 includes the significant parameters with low heterogeneity (I2<50), and Model 3 includes the significant parameters, regardless of heterogeneity and LR. AUC, area under the curve.
Table 4. The performance of three models based on gender, clinical manifestations, and endoscopic findings to differentiate intestinal tuberculosis from Crohn's disease.
| Cut-point to obtain the best performance of the models | |||||||
|---|---|---|---|---|---|---|---|
| Cut-point (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | ||
| Model 1 | 75.12 | 59.1 | 100 | 100 | 75.0 | 81.6 | |
| Model 2 | 41.27 | 90.9 | 81.5 | 80.0 | 91.7 | 85.7 | |
| Model 3 | 74.52 | 86.4 | 88.9 | 86.4 | 88.9 | 87.8 | |
| Cut-point to obtain the NPV of 100% for diagnosis of intestinal tuberculosis | |||||||
| Cut-point (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | FP | FN | |
| Model 1 | 17.29 | 100 | 44.4 | 59.5 | 100 | 15/27 | 0 |
| Model 2 | 20.62 | 100 | 44.4 | 59.5 | 100 | 15/27 | 0 |
| Model 3 | 7.27 | 100 | 37.0 | 56.4 | 100 | 17/27 | 0 |
FP, false-positive (misdiagnosis of intestinal tuberculosis in patients with Crohn's disease); FN, false-negative (misdiagnosis of Crohn's disease in patient with intestinal tuberculosis); NPV, negative predicted value; PPV, positive predicted value.
Figure 4.
Receiver operating characteristic curve of the final Bayesian model for differentiating intestinal tuberculosis from Crohn's disease based on gender, clinical manifestation, endoscopic, and pathological findings.
Model access
Readers with estimates of the local prevalence of ITB vs. CD can access and use the model in an individual patient with the Shiny web application at https://www.pathology.med.umich.edu/shiny/tbcrohns/orbit.ly/ITBvsCD. Users with an estimate of the pretest probability of ITB can choose for each predictor whether the finding is present, absent, or not assessed, and estimate the probability of ITB and CD in their patient.
Discussion
T is meta-analysis and Bayesian model summarizes the reported factors helpful in differentiating CD and ITBs. We selected predictor variables with a significant OR and low heterogeneity, and built a Bayesian model incorporating pretest probability and diagnostic LRs to produce estimates of the probability of ITB and CD that are calibrated to local prevalence. The model was validated and demonstrated a high accuracy in differentiating ITB from CD in our validation cohort from Bangkok, Thailand.
Regarding the differentiation of ITB from CD, different diagnostic scoring systems have been developed, but unfortunately there is little agreement on which factors to include. Recently, Jung et al. (39) proposed a model with good performance based on the results of a large retrospective cohort in seven centers in Korea, but the model has not been validated outside of Korea. There have been three recent published meta-analyses carried out to determine the utility of pathologic findings and single serological tests such as anti-Saccharomyces cerevisiae antibody and IGRA (40–42). This meta-analysis integrates data from all published studies from inception to September 2015 on 55 distinct predictors and summarizes the findings that have proven useful in differentiating these two conditions.
This study confirms that common clinical manifestations infrequently help to make a diagnosis between ITB and CD. Abdominal pain, weight loss, or anemia was not significantly different between the two diseases. Some other common manifestations were found to be significantly different, but their diagnostic value was limited because of their small effect size, such as fever for ITB. The significant manifestations with high positive LR that are strong predictors, such as the presence of perianal disease, extraintestinal manifestations for CD, night sweats, lung involvement, and ascites for TB, are uncommon findings; all were present in less than half of patients. Furthermore, some endoscopic findings that are strong predictors, such as longitudinal ulcers, cobblestone appearance, and rectal involvement for CD, and transverse ulcers and patulous IC valve for ITB, are also uncommon findings; none of these findings were reported in more than half of the patients. Moreover, while the pathologic finding of granulomas, whether confluent, large, or present in high numbers, strongly favors the diagnosis of ITB, granulomas were found in only 38% of CD and 64% of ITB in this meta-analysis. All of these findings indicate that conventional tools are useful, but that integration of multiple parameters is necessary to produce a strong positive or negative diagnostic probability. Our study results showed that integration of significant clinical manifestations, endoscopic, and pathological findings could accurately diagnose ITB and CD in 91.8% of patients in a validation cohort. These results need more validation in larger studies.
Owing to the limited value of clinical, endoscopic, and pathologic findings in distinguishing these two diseases, new tools have been developed for this purpose. The IGRA has performed well in differentiating CD and ITB in previous studies. Its pooled sensitivity and specificity were 84% and 86%, respectively, in this meta-analysis. These data are consistent with another recent meta-analysis (41). However, this test is not available in all centers, and can be positive in either active or latent tuberculosis (43), which can lead to a misdiagnosis of ITB in CD patients who have been exposed to tuberculosis. Imaging with CTE has shown some promising findings. Demonstration of peritoneal thickening or ascites or short segmental involvement support the diagnosis of ITB. The comb sign, f brofatty proliferation, asymmetrical wall thickening, and wall stratification support the diagnosis of CD. However, there is risk of kidney injury from contrast and additional risks of radiation exposure, thus selecting the patients who will obtain the most benefit based on pretest probability is warranted.
This study developed a Bayesian model that comprised of the significant parameters based on meta-analytic results to calculate the estimated probability of either ITB or CD in an individual patient. This model considers the effect of each variable independent of whether the results are positive or negative. Furthermore, physicians are able to use the model by selecting only the variables available in their center or in each particular case (unmeasured variables have an LR=1). In addition, this model may help to decrease unnecessary additional testing. For example, physicians can calculate the probability of ITB or CD based on clinical, endoscopic, and pathologic predictors. With this estimate in hand, they can then calculate whether adding CT enterography or IGRA results will change the probability enough to change their treatment decision.
The main strength of this study is that it is the largest series of meta-analyses evaluating predictor variables that have been reported to help differentiate CD from ITB. We collected all published studies without language restriction, most of which have been performed in Asia; therefore, the results should be generaliz-able to populations in which ITB prevalence is fairly high. Sensitivity analyses were performed in different criteria of variable selection, and the model with best performance was selected. The model demonstrated a high accuracy in differentiating ITB from CD in our validation cohort. However, the results were based on a small number of patients, and we have not validated the model when the CT enterography findings and IGRA are included. We would hope that additional predictor data would enhance the performance of the model, but that remains to be proven in a larger data set. Future studies with larger and different populations, and with the availability of all parameters are needed.
This study has several limitations. We included studies with large heterogeneities. Across studies, there is significant heterogeneity in sample populations by ethnicity, race, and disease severity. There may be local variation in subjectively evaluated findings such as endoscopic lesions, pathologic findings, and imaging characteristics. We tried to reduce this variance by excluding some studies that have a high potential for bias. For instance, we excluded the studies with a high potential for bias in subject selection by excluding studies that recruited only complicated cases requiring surgery. We also excluded studies using older generation imaging techniques as these results may not be comparable to those using current imaging techniques. Furthermore, we performed sensitivity analyses including only the studies with low bias that confirmed the significance of the parameters we selected to build the model.
Another limitation is that the structure of our Bayesian model is based on LRs, thus we could only incorporate dichotomized variables, such as the presence or absence of a finding. Continuous variables were not incorporated. Furthermore, our model is based on the assumption that each variable is independent of the other variables in the model, as many of these variables have been reported as independent predictors for differentiating ITB from CD in previous studies (6,11,33). We also did deliberately exclude three potentially interdependent variables from the model when they were, by definition, likely to be highly correlated with other predictors. For example, we selected confluent granuloma and excluded large granulomas and high numbers of granulomas, and we selected patulous IC valve and excluded the finding of IC valve involvement. Next, the model can be applied only for the differentiation of CD and ITB in patients where other potential diagnoses have been already excluded. Moreover, we did not include abstracts that did not provide adequate information, thus there may be other data that could affect our results. Last, this model relies on the availability of an estimate of the local pretest probability of ITB to calculate the probabilities, thus it needs external validation in various populations. We invite other groups to validate the model in their local population.
This study addresses a common clinical problem by summarizing the findings of 55 meta-analyses to identify estimates for the predictors that differentiate between CD and ITB and by providing a Bayesian model to help physicians calculate the estimated probability of CD or ITB to make treatment decisions with greater confidence in cases of diagnostic uncertainty.
Supplementary Material
Study Highlights.
What is Current Knowledge
✓ Multiple clinical manifestations, endoscopic findings, pathological findings, computed tomography findings, and the interferon-γ -releasing assay are helpful in differentiating intestinal tuberculosis (ITB) and Crohn's disease (CD).
✓ The significant findings vary widely across individual studies.
What is New Here
✓ Meta-analyses including all significant findings of clinical manifestations, endoscopic, pathological, and computed tomography (CT) enterography findings, and interferon-γ-releasing assay to differentiate ITBs from CD.
✓ A Bayesian model that integrates these significant findings and can calculate the probability of the diagnosis of ITBs and CD.
✓ The model is publicly available at https://www.pathology.med.umich.edu/shiny/tbcrohns/orbit.ly/ITBvsCD.
Acknowledgments
We thank Dr Kim and Dr Lu for their assistance in Korean and Chinese language translation, respectively.
Footnotes
Conflict of Interest: Guarantor of the article: Peter D.R. Higgins, MD, PhD, MSc.
Specific author contributions: Study concept and design: JL, N P, SP, SM, PDRH; acquisition of data for meta-analysis: JL and ABS; acquisition of patient data for model validation: CL, RB, JL, AP; analysis and interpretation of data: JL and PDRH; drafting of the manuscript: JL and ABS; critical revision of the manuscript for important intellectual content: PDRH; statistical analysis: JL and PDRH; study supervision: PDRH.
Financial support: This work was supported by Ananda Mahidol Foundation, Bangkok, Thailand.
Potential competing interests: None.
Supplementary Material is linked to the online version of the paper at http://www.nature.com/ajg
References
- 1.Dutta AK, Sahu MK, Gangadharan SK, et al. Distinguishing Crohn's disease from intestinal tuberculosis—a prospective study. Trop Gastroenterol. 2011;32:204–9. [PubMed] [Google Scholar]
- 2.Li Y, Zhang LF, Liu XQ, et al. The role of in vitro interferongamma-release assay in differentiating intestinal tuberculosis from Crohn's disease in China. J Crohns Colitis. 2012;6:317–23. doi: 10.1016/j.crohns.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Lei Y, Yi FM, Zhao J, et al. Utility of in vitro interferon-gamma release assay in differential diagnosis between intestinal tuberculosis and Crohn's disease. J Dig Dis. 2013;14:68–75. doi: 10.1111/1751-2980.12017. [DOI] [PubMed] [Google Scholar]
- 4.Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–6. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramadass B, Chittaranjan S, Subramanian V, et al. Fecal polymerase chain reaction for Mycobacterium tuberculosis IS6110 to distinguish Crohn's disease from intestinal tuberculosis. Indian J Gastroenterol. 2010;29:152–6. doi: 10.1007/s12664-010-0022-3. [DOI] [PubMed] [Google Scholar]
- 6.Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105:642–51. doi: 10.1038/ajg.2009.585. [DOI] [PubMed] [Google Scholar]
- 7.Jin XJ, Kim JM, Kim HK, et al. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn's disease. World J Gastroenterol. 2010;16:2496–503. doi: 10.3748/wjg.v16.i20.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fei BY, Lv HX, Zheng WH. Fluorescent quantitative PCR of Mycobacterium tuberculosis for differentiating intestinal tuberculosis from Crohn's disease. Braz J Med Biol Res. 2014;47:166–70. doi: 10.1590/1414-431X20133277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ooi CJ, Hilmi I, Makharia GK, et al. The Asia Pacific Consensus Statements on Crohn's Disease Part 1: definition, diagnosis and epidemiology. J Gastroenterol Hepatol. 2015;31:45–55. doi: 10.1111/jgh.12956. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Yang SK, Byeon JS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn's disease. Endoscopy. 2006;38:592–7. doi: 10.1055/s-2006-924996. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Liu X, Zou Y, et al. Predictors of clinical and endoscopic findings in differentiating Crohn's disease from intestinal tuberculosis. Dig Dis Sci. 2011;56:188–96. doi: 10.1007/s10620-010-1231-4. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Liu Y, Wang Y, et al. Clinical, endoscopic and histological differentiations between Crohn's disease and intestinal tuberculosis. Digestion. 2012;85:202–9. doi: 10.1159/000335431. [DOI] [PubMed] [Google Scholar]
- 13.Larsson G, Shenoy T, Ramasubramanian R, et al. Routine diagnosis of intestinal tuberculosis and Crohn's disease in Southern India. World J Gastroenterol. 2014;20:5017–24. doi: 10.3748/wjg.v20.i17.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedia S, Sharma R, Nagi B, et al. Computerized tomography-based predictive model for differentiation of Crohn's disease from intestinal tuberculosis. Indian J Gastroenterol. 2015;34:135–43. doi: 10.1007/s12664-015-0550-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhao XS, Wang ZT, Wu ZY, et al. Differentiation of Crohn's disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis. 2014;20:916–25. doi: 10.1097/MIB.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 16.Mao R, Liao WD, He Y, et al. Computed tomographic enterography adds value to colonoscopy in differentiating Crohn's disease from intestinal tuberculosis: a potential diagnostic algorithm. Endoscopy. 2015;47:322–9. doi: 10.1055/s-0034-1391230. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Liao WD, Yu C, et al. Differences in clinical features of Crohn's disease and intestinal tuberculosis. World J Gastroenterol. 2015;21:3650–6. doi: 10.3748/wjg.v21.i12.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BJ, Choi YS, Jang BI, et al. Prospective evaluation of the clinical utility of interferon-gamma assay in the differential diagnosis of intestinal tuberculosis and Crohn's disease. Inflamm Bowel Dis. 2011;17:1308–13. doi: 10.1002/ibd.21490. [DOI] [PubMed] [Google Scholar]
- 19.Liu XW, Li XF, Zou YY, et al. Screening of clinical and endoscopic parameters for differentiating Crohn's disease from intestinal tuberculosis by logistic regression analysis. World Chin J Digestol. 2010;18:621–7. [Google Scholar]
- 20.Li XF, Zhou MH, Lu FG, et al. Comparison of the pathologic characteristics of biopsy and operative specimens between Crohn's disease and intestinal tuberculosis: an analysis of 148 cases. World Chin J Digestol. 2010;18:409–12. [Google Scholar]
- 21.Huang S, Yi FM, Zhou R, et al. The utility of platelet, mean platelet volume, and red cell distribution width in the diagnosis of active Crohn's disease and intestinal tuberculosis. Saudi Med J. 2013;34:1161–6. [PubMed] [Google Scholar]
- 22.Gan H, Ouyang Q, Bu H, et al. Value of polymerase chain reaction assay in diagnosis of intestinal tuberculosis and differentiation from Crohn's disease. Chin Med J (Engl) 1995;108:215–20. [PubMed] [Google Scholar]
- 23.Gan HT, Chen YQ, Ouyang Q, et al. Differentiation between intestinal tuberculosis and Crohn's disease in endoscopic biopsy specimens by polymerase chain reaction. Am J Gastroenterol. 2002;97:1446–51. doi: 10.1111/j.1572-0241.2002.05686.x. [DOI] [PubMed] [Google Scholar]
- 24.Sekine K, Nagata N, Shindo T, et al. Combined identifying granuloma and biopsy culture is useful for diagnosing intestinal tuberculosis. Int J Colorectal Dis. 2015;30:939–45. doi: 10.1007/s00384-015-2208-8. [DOI] [PubMed] [Google Scholar]
- 25.Pulimood AB, Peter S, Rook GW, et al. In situ PCR for Mycobacterium tuberculosis in endoscopic mucosal biopsy specimens of intestinal tuberculosis and Crohn disease. Am J Clin Pathol. 2008;129:846–51. doi: 10.1309/DKKECWQWMG4J23E3. [DOI] [PubMed] [Google Scholar]
- 26.Makanjuola D. Is it Crohn's disease or intestinal tuberculosis? CT analysis Eur J Radiol. 1998;28:55–61. doi: 10.1016/s0720-048x(97)00097-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Kim JW, Jeong JB, et al. Diferential diagnosis of Crohn's disease and intestinal tuberculosis in patients with spontaneous small-bowel perforation. Dig Surg. 2014;31:151–6. doi: 10.1159/000363066. [DOI] [PubMed] [Google Scholar]
- 28.Liu TH, Pan GZ, Chen MZ. Crohn's disease. Clinicopathologic manifestations and differential diagnosis from enterocolonic tuberculosis Chin Med J (Engl) 1981;94:431–40. [PubMed] [Google Scholar]
- 29.Pulimood AB, Amarapurkar DN, Ghoshal U, et al. Differentiation of Crohn's disease from intestinal tuberculosis in India in 2010. World J Gastroenterol. 2011;17:433–43. doi: 10.3748/wjg.v17.i4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu YY, Chen MK, Cao Z, et al. Differential diagnosis of intestinal tuberculosis from Crohn's disease and primary intestinal lymphoma in China. Saudi J Gastroenterol. 2014;20:241–7. doi: 10.4103/1319-3767.136979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulimood AB, Ramakrishna BS, Kurian G, et al. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn's disease from tuberculosis. Gut. 1999;45:537–41. doi: 10.1136/gut.45.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amarapurkar DN, Patel ND, Amarapurkar AD, et al. Tissue polymerase chain reaction in diagnosis of intestinal tuberculosis and Crohn's disease. J Assoc Physicians India. 2004;52:863–7. [PubMed] [Google Scholar]
- 33.Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–6. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng L, Huang MF, Mei PF, et al. The clinical, endoscopic and pathologic features of Crohn's disease in the differentiation from intestinal tuberculosis. Zhonghua Nei Ke Za Zhi. 2013;52:940–4. [PubMed] [Google Scholar]
- 35.Gu Q, Ouyang Q, Zhang WY, et al. A comparison of clinical and pathologic characteristics between Crohn's disease and intestinal tuberculosis. Zhonghua Nei Ke Za Zhi. 2009;48:291–4. [PubMed] [Google Scholar]
- 36.Liu S, Ren J, Xia Q, et al. Preliminar y case–control study to evaluate diagnostic values of C-reactive protein and erythrocyte sedimentation rate in differentiating active Crohn's disease from intestinal lymphoma, intestinal tuberculosis and Behcet's syndrome. Am J Med Sci. 2013;346:467–72. doi: 10.1097/MAJ.0b013e3182959a18. [DOI] [PubMed] [Google Scholar]
- 37.Hatemi IEY, Kochan K, Aygun G, et al. Comparison of Crohn's disease and intestnal tuberculosis by clinical, laboratory, endoscopic, radiologic and histologic parameters. J Crohns Colitis. 2012;6:S76–7. [Google Scholar]
- 38.Kim YS, Kim YH, Kim WH, et al. Diagnostic utility of anti-Saccharomyces cerevisiae antibody (ASCA) and interferon-gamma assay in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Clin Chim Acta. 2011;412:1527–32. doi: 10.1016/j.cca.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 39.Jung Y, Hwangbo Y, Yoon SM, et al. Predictive factors for differentiating between Crohn's disease and intestinal tuberculosis in Koreans. Am J Gastroenterol. 2016;111:1156–64. doi: 10.1038/ajg.2016.212. [DOI] [PubMed] [Google Scholar]
- 40.Du J, Ma YY, Xiang H, et al. Confluent granulomas and ulcers lined by epithelioid histiocytes: new ideal method for differentiation of ITB and CD? A meta analysis PLoS One. 2014;9:e103303. doi: 10.1371/journal.pone.0103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng SC, Hirai HW, Tsoi KK, et al. Systematic review with meta-analysis: accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn's disease in Asians. J Gastroenterol Hepatol. 2014;29:1664–70. doi: 10.1111/jgh.12645. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Fan JH, Luo W, et al. Effectiveness of interferon-gamma release assays for differentiating intestinal tuberculosis from Crohn's disease: a meta-analysis. World J Gastroenterol. 2013;19:8133–40. doi: 10.3748/wjg.v19.i44.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darby J, Black J, Buising K. Interferon-gamma release assays and the diagnosis of tuberculosis: have they found their place? Intern Med J. 2014;44:624–32. doi: 10.1111/imj.12469. [DOI] [PubMed] [Google Scholar]
- 44.Park MJ, Lim JS. Computed tomography enterography for evaluation of inflammatory bowel disease. Clin Endosc. 2013;46:327–66. doi: 10.5946/ce.2013.46.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirsch R, Pentecost M, Hall Pde M, et al. Role of colonoscopic biopsy in distinguishing between Crohn's disease and intestinal tuberculosis. J Clin Pathol. 2006;59:840–4. doi: 10.1136/jcp.2005.032383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou ZY, Luo HS. Differential diagnosis between Crohn's disease and intestinal tuberculosis in China. Int J Clin Pract. 2006;60:212–4. doi: 10.1111/j.1742-1241.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee JN, Ryu DY, Park SH, et al. The usefulness of in vitro interferon-gamma assay for differential diagnosis between intestinal tuberculosis and Crohns disease. Korean J Gastroenterol. 2010;55:376–83. doi: 10.4166/kjg.2010.55.6.376. [DOI] [PubMed] [Google Scholar]
- 48.Singh B, Kedia S, Konijeti G, et al. Extraintestinal manifestations of inflammatory bowel disease and intestinal tuberculosis: frequency and relation with disease phenotype. Indian J Gastroenterol. 2015;34:43–50. doi: 10.1007/s12664-015-0538-7. [DOI] [PubMed] [Google Scholar]
- 49.Pulimood AB, Peter S, Ramakrishna B, et al. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn's disease. J Gastroenterol Hepatol. 2005;20:688–96. doi: 10.1111/j.1440-1746.2005.03814.x. [DOI] [PubMed] [Google Scholar]
- 50.Moon GLJ, Kwon HJ, Chung WJ, et al. QuantiFERON®-TB gold test in the differential diagnosis of intestinal tuberculosis and Crohn's disease. J Gastroenterol Hepatol. 2009;24:A48. [Google Scholar]
- 51.Baek DH, SG, Ryu DY, et al. The usefulness of in vitro interferon-g assay for differential diagnosis between intestinal tuberculosis and Crohn's disease. J Crohns Colitis. 2013;7:S63. [Google Scholar]
- 52.He YCY, Zhang Q, Cao Q, et al. The application of T-SPOT.TB in the differential diagnosis of Crohn's disease and intestinal tuberculosis. J Crohns Colitis. 2014;8:S115. [Google Scholar]
- 53.Kakkadasam Ramaswami P, Joshi H, Toke N, et al. Role of interferon gamma release assay (IGRA) in differentiating gastrointestinal tuberculosis from Crohn's disease. J Crohns Colitis. 2014;8:S123. [Google Scholar]
- 54.Kim SK, KY The clinical importance of in vitro interferon-gamma assay for differential diagnosis between intestinal tuberculosis and Crohn's disease. J Crohns Colitis. 2013;7:S63. [Google Scholar]
- 55.Ghoshal UC, Ghoshal U, Singh H, et al. Anti-Saccharomyces cerevisiae antibody is not useful to differentiate between Crohn's disease and intestinal tuberculosis in India. J Postgrad Med. 2007;53:166–70. doi: 10.4103/0022-3859.33857. [DOI] [PubMed] [Google Scholar]
- 56.Makharia GK, Sachdev V, Gupta R, et al. Anti-Saccharomyces cerevisiae antibody does not differentiate between Crohn's disease and intestinal tuberculosis. Dig Dis Sci. 2007;52:33–9. doi: 10.1007/s10620-006-9527-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.