Abstract
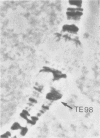
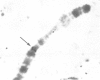
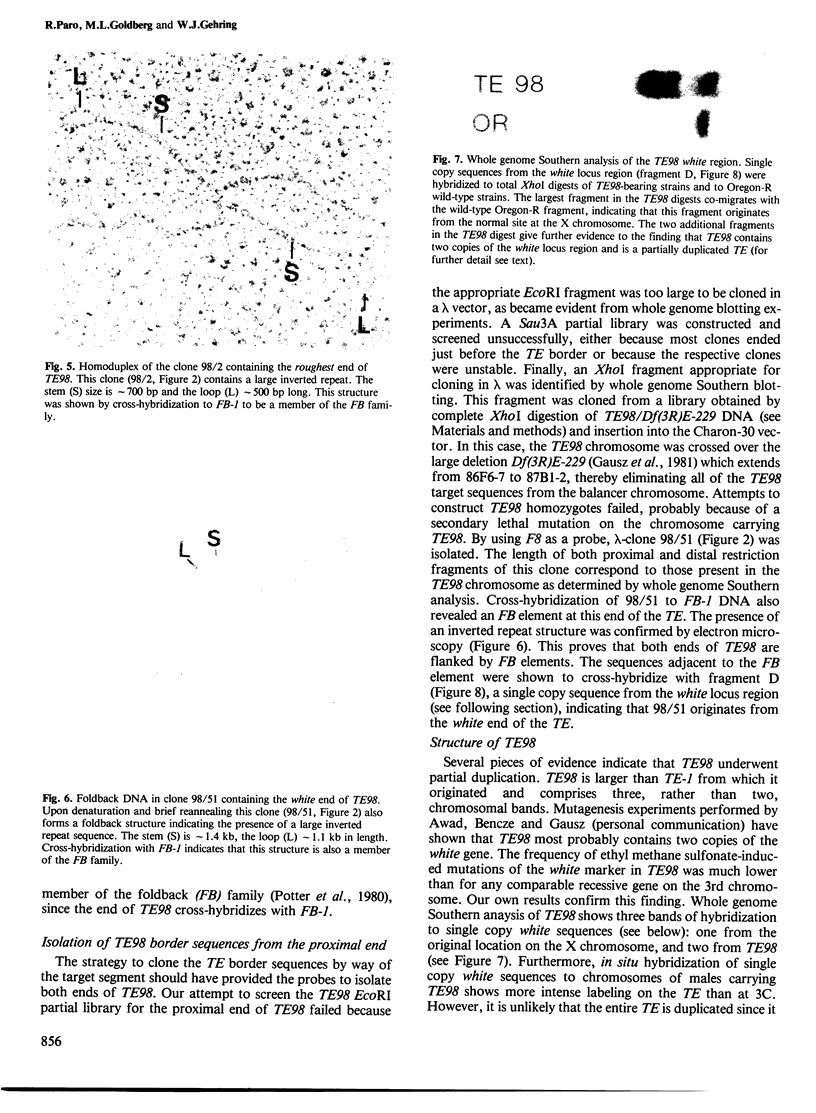
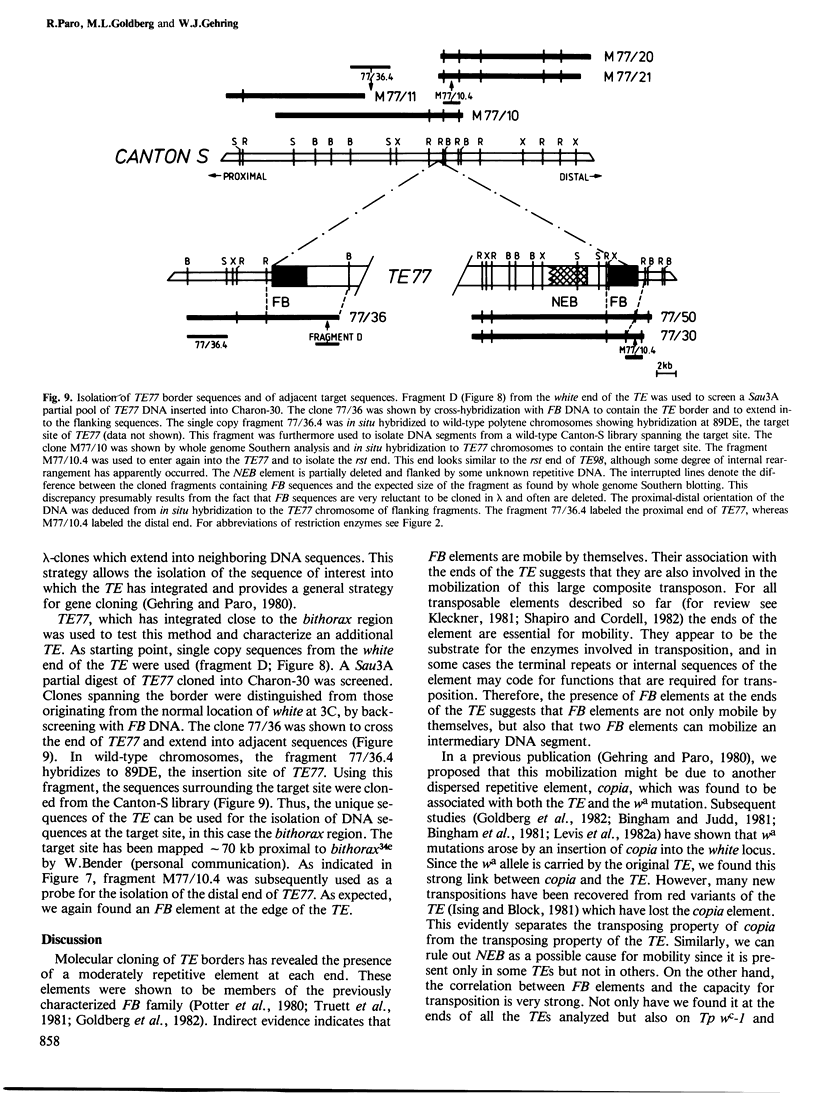
A large transposable element (TE) comprising the white-apricot and roughest genes has been found to transpose to well over a hundred sites scattered over the Drosophila genome. We report the cloning of the essential parts of several TEs. TE98 and TE28 sequences were cloned by `walking' along the chromosome from the previously cloned heatshock genes. The ends of the TEs are characterized by dispersed repetitive elements belonging to the foldback (FB) family. FB elements are also associated with two independently isolated transposable elements originating from the white locus, Tp wc-1 and Tp w+IV. The strong correlation between FB elements and large composite transposons suggests that a pair of these elements can mobilize large intermediary DNA segments. One particular FB family member, FB-NOF, is associated with TE28, the white-crimson (wc) mutant, the wc-derived Tp wc-1 and probably also with Tp w+IV. A unique sequence located close to the white end of TE28 was used to clone the borders of TE77 and the surrounding sequences in the bithorax region, indicating that the TE can be used as a probe for gene isolation. Some evolutionary implications of the large composite transposons are discussed.
Keywords: Drosophila, white locus, transposable elements
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham P. M., Judd B. H. A copy of the copia transposable element is very tightly linked to the Wa allele at the white locus of D. melanogaster. Cell. 1981 Sep;25(3):705–711. doi: 10.1016/0092-8674(81)90177-x. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Bingham P. M. The Regulation of White Locus Expression: A Dominant Mutant Allele at the White Locus of DROSOPHILA MELANOGASTER. Genetics. 1980 Jun;95(2):341–353. doi: 10.1093/genetics/95.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M., Rubin G. M. Structure of the Drosophila mutable allele, white-crimson, and its white-ivory and wild-type derivatives. Cell. 1982 Aug;30(1):71–79. doi: 10.1016/0092-8674(82)90013-7. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Ish-Horowicz D. Extrachromosomal circular copies of the eukaryotic transposable element copia in cultured Drosophila cells. Nature. 1981 Aug 13;292(5824):591–595. doi: 10.1038/292591a0. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Gausz J., Gyurkovics H., Bencze G., Awad A. A., Holden J. J., Ish-Horowicz D. Genetic characterization of the region between 86F1,2 and 87B15 on chromosome 3 of Drosophila melanogaster. Genetics. 1981 Aug;98(4):775–789. doi: 10.1093/genetics/98.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J., Paro R. Isolation of a hybrid plasmid with homologous sequences to a transposing element of Drosophila melanogaster. Cell. 1980 Apr;19(4):897–904. doi: 10.1016/0092-8674(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Paro R., Gehring W. J. Molecular cloning of the white locus region of Drosophila melanogaster using a large transposable element. EMBO J. 1982;1(1):93–98. doi: 10.1002/j.1460-2075.1982.tb01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Two genes for the major heat-shock protein of Drosophila melanogaster arranged as an inverted repeat. Nucleic Acids Res. 1980 Jan 25;8(2):235–252. doi: 10.1093/nar/8.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. Controlling element mediated transpositions of the white gene in Drosophila melanogaster. Genetics. 1969 Feb;61(2):429–441. doi: 10.1093/genetics/61.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. Mapping a Drosophila melanogaster "controlling element" by interallelic crossing over. Genetics. 1969 Feb;61(2):423–428. doi: 10.1093/genetics/61.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. The genetics of a mutable gene at the white locus of Drosophila melanogaster. Genetics. 1967 Jul;56(3):467–482. doi: 10.1093/genetics/56.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M. Genomic organization of the 87A7 and 87Cl heat-induced loci of Drosophila melanogaster. J Mol Biol. 1980 Sep 15;142(2):231–245. doi: 10.1016/0022-2836(80)90047-9. [DOI] [PubMed] [Google Scholar]
- Ising G., Block K. Derivation-dependent distribution of insertion sites for a Drosophila transposon. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):527–544. doi: 10.1101/sqb.1981.045.01.069. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Levis R., Bingham P. M., Rubin G. M. Physical map of the white locus of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Jan;79(2):564–568. doi: 10.1073/pnas.79.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Collins M., Rubin G. M. FB elements are the common basis for the instability of the wDZL and wC Drosophila mutations. Cell. 1982 Sep;30(2):551–565. doi: 10.1016/0092-8674(82)90252-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Potter S. S. DNA sequence analysis of a Drosophila foldback transposable element rearrangement. Mol Gen Genet. 1982;188(1):107–110. doi: 10.1007/BF00333002. [DOI] [PubMed] [Google Scholar]
- Potter S. S. DNA sequence of a foldback transposable element in Drosophila. Nature. 1982 May 20;297(5863):201–204. doi: 10.1038/297201a0. [DOI] [PubMed] [Google Scholar]
- Potter S., Truett M., Phillips M., Maher A. Eucaryotic transposable genetic elements with inverted terminal repeats. Cell. 1980 Jul;20(3):639–647. doi: 10.1016/0092-8674(80)90310-4. [DOI] [PubMed] [Google Scholar]
- Rasmuson B., Green M. M. Genetic instability in Drosophila melanogaster. A mutable tandem duplication. Mol Gen Genet. 1974;133(3):249–260. doi: 10.1007/BF00267674. [DOI] [PubMed] [Google Scholar]
- Rasmuson B., Montell I., Rasmuson A., Svahlin H., Westerberg B. M. Genetic instability in Drosophila melanogaster: evidence for regulation, excision and transposition at the white locus. Mol Gen Genet. 1980;177(4):567–570. doi: 10.1007/BF00272664. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Schedl P., Artavanis-Tsakonas S., Steward R., Gehring W. J., Mirault M. E., Goldschmidt-Clermont M., Moran L., Tissières A. Two hybrid plasmids with D. melanogaster DNA sequences complementary to mRNA coding for the major heat shock protein. Cell. 1978 Aug;14(4):921–929. doi: 10.1016/0092-8674(78)90346-x. [DOI] [PubMed] [Google Scholar]
- Shiba T., Saigo K. Retrovirus-like particles containing RNA homologous to the transposable element copia in Drosophila melanogaster. Nature. 1983 Mar 10;302(5904):119–124. doi: 10.1038/302119a0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Drosophila genome organization: conserved and dynamic aspects. Annu Rev Genet. 1981;15:219–264. doi: 10.1146/annurev.ge.15.120181.001251. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Tobin S. L., Zulauf E., Sánchez F., Craig E. A., McCarthy B. J. Multiple actin-related sequences in the Drosophila melanogaster genome. Cell. 1980 Jan;19(1):121–131. doi: 10.1016/0092-8674(80)90393-1. [DOI] [PubMed] [Google Scholar]
- Truett M. A., Jones R. S., Potter S. S. Unusual structure of the FB family of transposable elements in Drosophila. Cell. 1981 Jun;24(3):753–763. doi: 10.1016/0092-8674(81)90101-x. [DOI] [PubMed] [Google Scholar]