Abstract
Pulmonary varix is a rare entity that presents as a focal aneurysmal dilatation of the pulmonary vein and is frequently mistaken for a pulmonary arteriovenous malformation (PAVM). It is important to distinguish between pulmonary varix and PAVM because the former does not usually require treatment. We present the findings of non–contrast-enhanced magnetic resonance angiography with the time-spatial labeling inversion pulse technique in case of pulmonary varix and PAVM and the utility of this method for differentiating between these diseases.
Keywords: Pulmonary varix, Pulmonary arteriovenous malformation, Time-spatial labeling inversion pulse, Non–contrast-enhanced magnetic resonance angiography
Introduction
Pulmonary varix is a rare entity that presents as a focal aneurysmal dilatation of the pulmonary vein [1]. It may exist either as an isolated malformation or in association with pulmonary venous hypertension and is frequently mistaken for a pulmonary arteriovenous malformation (PAVM) [2]. It is important to distinguish between pulmonary varix and PAVM because pulmonary varix does not usually require treatment in contrast with PAVM, which may be a risk factor for serious complications, such as stroke, cerebral abscess, and fatal hemoptysis [3], [4]. In recent years, multidetector computed tomography (CT) with contrast medium has become an important modality for evaluating these conditions owing to its noninvasiveness [5], although digital subtraction angiography (DSA) is the gold standard. However, both DSA and CT necessitate exposure to ionizing radiation and are associated with the risk of nephrotoxicity from iodinated contrast agents. Non–contrast-enhanced magnetic resonance angiography with the time-spatial labeling inversion pulse technique (time-SLIP MRA) is a relatively new non–contrast-enhanced MRA that facilitates the selective visualization of blood flow in a region of interest using an arterial spin labeling technique [6]. The utility of time-SLIP MRA for the evaluation of PAVM has been recently reported [7]. Here, we present the findings of time-SLIP MRA in case of pulmonary varix and PAVM and the utility of this method for differentiating between these diseases.
Case reports
Patients
The patients included a 47-year-old female with pulmonary varix accompanied with anomalous unilateral single pulmonary vein and a 43-year-old female with PAVM. Both the patients were referred to our hospital to abnormal chest radiographs during a routine medical check-up. They were asymptomatic with an unremarkable history. Examinations and other investigations, including electrocardiography (ECG), pulmonary function test, blood count, and arterial blood gas analysis, were unremarkable. Both patients underwent contrast-enhanced multidetector CT and time-SLIP MRA for evaluation of pulmonary varix and PAVM. A definitive diagnosis was made by DSA within 1 or 2 days after image acquisition by time-SLIP MRA.
Time-SLIP MRA procedure
Time-SLIP MRA was performed using a 3-tesla magnetic resonance imaging (MRI) system (Vantage Titan 3T; Toshiba Medical Systems, Tochigi, Japan) using 4- and 8-element-phased array body surface coil and receiver channels combined with parallel imaging capability (SPEEDER; Toshiba Medical Systems). Details of the procedures of this time-SLIP technique are described elsewhere [6], [7]. Briefly, after acquisition of axial, coronal, and sagittal field echo images to localize the heart for time-SLIP pulse (tag) placement and to determine the acquisition area, combined respiratory- and ECG-triggered 3-dimensional (3D) single-shot half Fourier fast advanced spin echo (FASE; Toshiba Medical Systems) images with fat saturation were acquired in the axial plane using time-SLIP with an alternate tag-on/off subtraction technique. 3D FASE images with and without time-SLIP pulse (tag) were acquired alternately in the same slice plane, thereby, generating 2 separate images. When a tag is used as the labeling slab, the signal of blood from the tagged region is decreased. These images were individually reconstructed and then subtracted to yield a final angiogram. Consequently, this technique allows depiction of only signals from the tagged region, which is referred to as a time-SLIP image, by cancellation of the background signal via subtraction. The image acquisition and reconstruction procedures are shown in Figure 1. The 3D FASE scan was conducted with the following parameters: 1 respiration interval/1; flip angle, 90°; echo train spacing, 30 ms; slice thickness, 2.0 mm; inversion time, 180 ms for fat suppression; slice section, 60 for tag-on and tag-off images, respectively; field of view, 370 × 370 mm; matrix size, 256 × 256; reconstructed matrix size, 512 × 512; number of acquisitions, 1; and a parallel imaging factor of 2 in the phase direction. The final images were reconstructed into apparent spatial resolutions of 1 × 0.6 × 0.6 mm. The total scanning times were approximately 20 minutes. Respiratory triggering was conducted at the beginning of expiration. For ECG gating, the trigger time of both time-SLIP pulse and 3D FASE image acquisition was set at diastole. The interval between time-SLIP pulse application and 3D FASE image acquisition was within 1 cardiac cycle. A time-SLIP pulse (tag) was placed on the range that covered the right atrium and ventricle, and the superior and inferior vena cava in the sagittal direction. The delay after the time-SLIP pulse, which is referred to as the black blood inversion time, was 1000 ms.
Fig. 1.
Three-dimensional fast advanced spin echo (FASE) with and without time-SLIP (tag) pulse were acquired alternately in the same slice plane. The tag-off image shows the signals of pulmonary vascular structures, including the pulmonary artery (PA), pulmonary vein (PV), and background (BG). Time-SLIP pulse was applied to the right-sided blood flow. Because the signal of the blood from the tagged region was decreased, the signal of the PA was suppressed on the tag-on image. Consequently, these images were subtracted from the time-SLIP image, which resulted in only signals of the PA. ECG, electrocardiography trigger; Resp, respiratory trigger.
Image findings
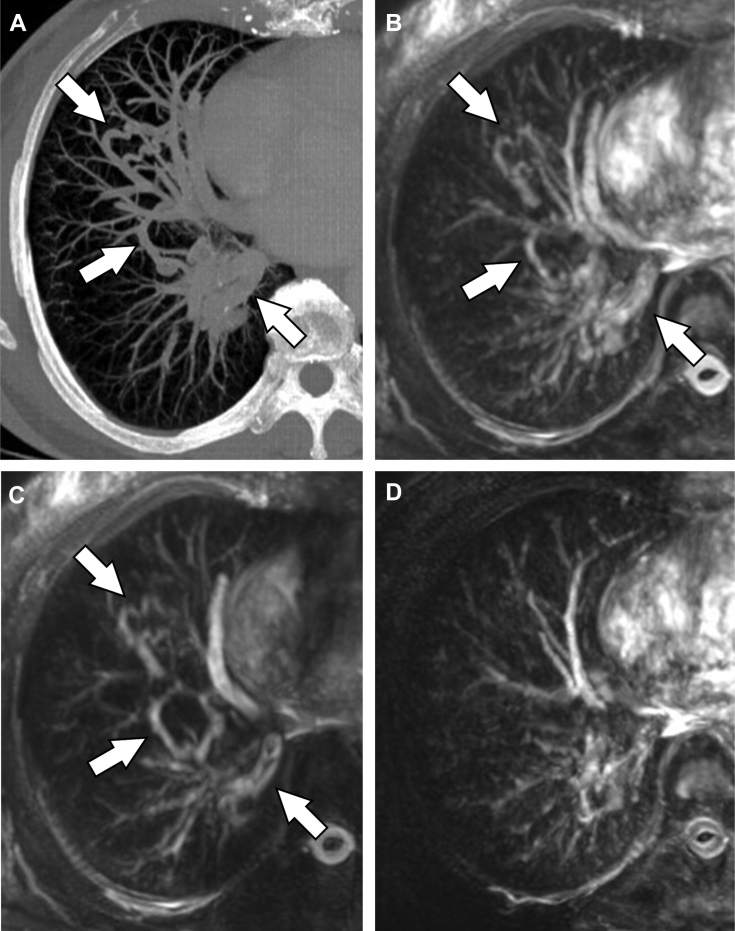
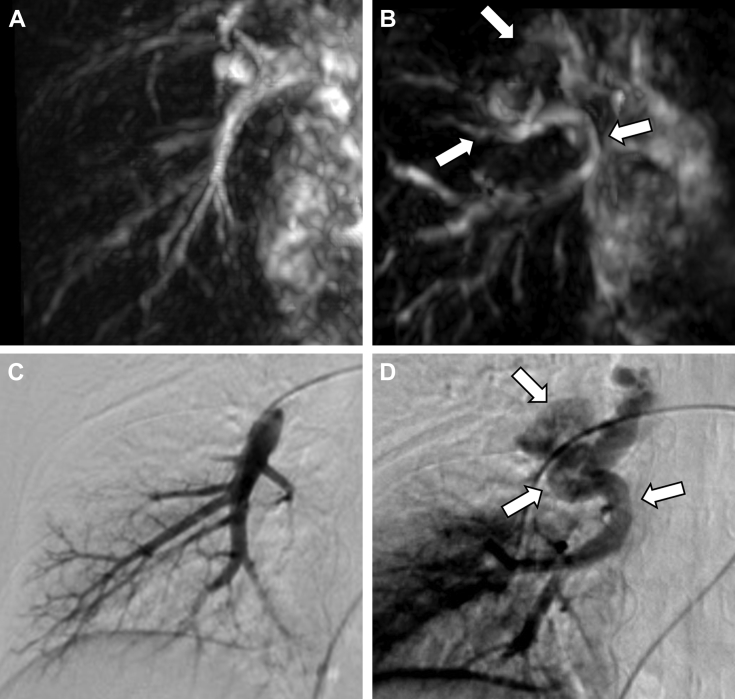
In both cases, the patients underwent time-SLIP MRA without any difficulty, and the signals from uninvolved pulmonary arteries and veins were separately described on time-SLIP images and tag-on images, respectively. In the case of pulmonary varix, the tortuous dilated vascular structures and the background pulmonary veins were visualized on tag-on images but not on the time-SLIP images, indicating a diagnosis of pulmonary varix (Fig. 2). These findings were well correlated with those of DSA (Fig. 3). A final diagnosis of pulmonary varix accompanied with anomalous unilateral single pulmonary vein was made. Since this patient was asymptomatic, no treatment was initiated. In the case of PAVM, the aneurysmal dilated sac that continued from the lower branch of pulmonary artery was visualized on a time-SLIP image (Fig. 4). The diameters of feeding arteries and aneurysmal sac were 5.4, 1.9, and 8.9 mm, respectively. The tag-on image revealed part of an aneurysmal sac continuous with an enlarged single-draining vein. These findings were quite similar to those by DSA (Fig. 5). After arriving at a final diagnosis, the PAVM was successfully treated by transcatheter coil embolization.
Fig. 2.
Time-SLIP MRA in the case of pulmonary varix. Contrast-enhanced CT with partial maximum intensity projection (MIP) showed tortuous dilated vascular structures (arrows) in the right lung field (A). In the time-SLIP MRA with partial-MIP, the abnormal vascular structures and background pulmonary veins were visualized on the tag-off (B) and tag-on images (C), but not on the time-SLIP image (D). Note that the signals of the uninvolved pulmonary arteries and veins were visualized on the time-SLIP image and tag-on image, respectively. Time-SLIP MRA, non–contrast-enhanced magnetic resonance angiography with the time-spatial labeling inversion pulse technique.
Fig. 3.
Time-SLIP image of time-SLIP MRA in the right oblique coronal view only showed the uninvolved pulmonary artery (A), whereas the tag-on image revealed the tortuous dilated vascular structures (arrows) connected to the right superior pulmonary vein (B). The arterial phase of DSA showed no abnormal vascular structures (C). On the venous phase image (D), the site of the atretic segment of the right inferior pulmonary vein is well-visualized with a subsequent large intrapulmonary collateral superior communication (arrows).
Fig. 4.
Time-SLIP MRA of a pulmonary arteriovenous malformation. Contrast-enhanced CT with partial-MIP showed a tortuous dilated vascular structure in the left lower lung field (A) (arrows). On time-SLIP MRA with partial-MIP, this abnormal vascular structure was visualized on tag-off (B) and time-SLIP images (D). (C) Tag-on image.
Fig. 5.
A left anterior oblique view of a time-SLIP image (A) showed a continuity between the feeding arteries (arrows) and aneurysmal sac (arrowhead). The tag-on image (B) visualized part of an aneurysmal sac and the draining vein. These findings were well correlated with the arterial and venous phases by DSA (C and D).
Discussion
Although DSA remains the gold standard for identifying pulmonary varix and PAVM, in recent years, the diagnosis of these diseases has been generally established with contrast-enhanced CT and MRA because of their noninvasiveness [5], [8], [9], [10], [11]. However, the necessity of exposure to ionizing radiation and administration of considerable volumes of potentially nephrotoxic iodinated contrast agents are considerable disadvantages of contrast-enhanced CT, particularly in young patients, women of child-bearing age, and patients with renal dysfunction. Furthermore, the potential neurotoxic concerns associated with gadolinium-based MRI contrast materials [12], particularly noncyclic agents, and the concerns for rare, but severe adverse effects of these agents including nephrogenic systemic fibrosis [13] and anaphylaxis, remain a drawback to contrast-enhanced MRA. Hence, to establish the non–contrast-enhanced MRA technique for assessment of pulmonary vascular malformations would be beneficial for the patient. In present report, time-SLIP MRA provided selective visualization of the pulmonary varix and background pulmonary veins on tag-on (pulmonary vein) images but not on the time-SLIP (pulmonary artery) images, whereas the aneurysmal sac of PAVM and pulmonary artery were visualized on the time-SLIP images but not on tag-on images. These findings were quite similar to those of DSA, indicating that our method not only definitively identifies a pulmonary varix, but can also differentiate the malformation from a PAVM. To the best of the authors’ knowledge, this is the first clinical report to demonstrate the utility of non–contrast-enhanced MRA for assessment of a pulmonary varix.
The major advantages of the time-SLIP MRA compared with other modalities are that it can provide not only morphologic, but also hemodynamic, information similar with DSA without the use of ionizing radiation or contrast media. This property is particularly beneficial in patients with renal insufficiency, in whom iodine- and gadolinium-based contrast agents are contraindicated. A review of the literature reveals that other non–contrast-enhanced MRA techniques with gradient-recalled echo cine and 2-dimensional phase contrast sequence can be used to diagnose PAVM [14]. However, these techniques have not been accepted in clinical routine as a substitute for DSA or contrast-enhanced CT and MRA because they are unable to provide simultaneous hemodynamic and morphologic information as can be done with our technique. Moreover, time-SLIP MRA has additional advantages compared with contrast-enhanced MRA. First, image acquisition can occur as many times as desired in the time-SLIP MRA; however, image data retrieval must be performed within a brief window during the first pass of the contrast agent in the contrast-enhanced MRA [15]. Second, our approach facilitates data acquisition for increased spatial resolution even in patients with reduced breath-hold capability because of the use of the respiratory-gating method. Finally, a relatively brief acquisition window in the late expiration phase enables high temporal resolution imaging with further reduction of motion artifacts [16]. Based on these findings, time-SLIP MRA is less invasive to patients than DSA or contrast-enhanced CT and MRA, and this technique may have the potential to replace the current diagnostic modalities used in identifying pulmonary varix and PAVM as well as distinguishing between these diseases.
Despite these advantages, there are some drawbacks to time-SLIP MRA in comparison with contrast-enhanced MRA and CT. First, the acquisition time of the sequence described in this study was approximately 8-12 minutes, which is much longer than that of contrast-enhanced MRA and CT. Second, the number of acquisitions in a single session is limited in time-SLIP MRA, and therefore, it can be difficult to cover the entire lung field with high spatial resolution during a single session. However, because there is no time constraint with time-SLIP MRA, an image of the entire lung field can be acquired by planning multiple sessions as time allows.
In conclusion, although this report included a small number of cases, these findings indicate that time-SLIP MRA is a useful method for diagnosis of pulmonary varix and distinguishing this malformation from PAVM.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Ben-menachem Y., Kuroda K., Kyger E.R., Brest A.N., Copeland O.P., Coan J.D. The various forms of pulmonary varices. Am J Roentgenol Radium Ther Nucl Med. 1975;125:881–889. doi: 10.2214/ajr.125.4.881. [DOI] [PubMed] [Google Scholar]
- 2.Gill S.S., Roddie M.E., Shovlin C.L., Jackson J.E. Pulmonary arteriovenous malformations and their mimics. Clin Radiol. 2015;70:96–110. doi: 10.1016/j.crad.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Gossage J.R., Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998;158:643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 4.Shovlin C.L., Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax. 1999;54:714–729. doi: 10.1136/thx.54.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanherreweghe E., Rigauts H., Bogaerts Y., Meeus L. Pulmonary vein varix: diagnosis with multi-slice helical CT. Eur Radiol. 2000;10:1315–1317. doi: 10.1007/s003300000381. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki M., Akahane M. Non-contrast enhanced MR angiography: established techniques. J Magn Reson Imaging. 2012;35:1–19. doi: 10.1002/jmri.22789. [DOI] [PubMed] [Google Scholar]
- 7.Hamamoto K., Matsuura K., Chiba E., Okochi T., Tanno K., Tanaka O. Feasibility of non-contrast-enhanced MR angiography using the time-SLIP technique for the assessment of pulmonary arteriovenous malformation. Magn Reson Med Sci. 2016;15:253–265. doi: 10.2463/mrms.mp.2015-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno Y., Hatabu H., Takenaka D., Adachi S., Hirota S., Sugimura K. Contrast-enhanced MR perfusion imaging and MR angiography: utility for management of pulmonary arteriovenous malformations for embolotherapy. Eur J Radiol. 2002;41:136–146. doi: 10.1016/s0720-048x(01)00419-3. [DOI] [PubMed] [Google Scholar]
- 9.Schneider G., Uder M., Koehler M., Kirchin M.A., Massmann A., Buecker A. MR angiography for detection of pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. AJR Am J Roentgenol. 2008;190:892–901. doi: 10.2214/AJR.07.2966. [DOI] [PubMed] [Google Scholar]
- 10.Boussel L., Cernicanu A., Geerts L., Gamondes D., Khouatra C., Cottin V. 4D time-resolved magnetic resonance angiography for noninvasive assessment of pulmonary arteriovenous malformations patency. J Magn Reson Imaging. 2010;32:1110–1116. doi: 10.1002/jmri.22384. [DOI] [PubMed] [Google Scholar]
- 11.Berecova Z., Neuschl V., Boruta P., Masura J., Ghersin E. A complex pulmonary vein varix - diagnosis with ECG gated MDCT, MRI and invasive pulmonary angiography. J Radiol Case Rep. 2012;6:9–16. doi: 10.3941/jrcr.v6i12.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muldoon L.L., Neuwelt E.A. Dose-dependent neurotoxicity (seizures) due to deposition of gadolinium-based contrast agents in the central nervous system. Radiology. 2015;277:925–926. doi: 10.1148/radiol.2015151028. [DOI] [PubMed] [Google Scholar]
- 13.Marckmann P., Skov L., Rossen K., Dupont A., Damholt M.B., Heaf J.G. Nephro-genic systemic fibrosis: suspected etiological role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 14.Silverman J.M., Julien P.J., Herfkens R.J., Pelc N.J. Magnetic resonance imaging evaluation of pulmonary vascular malformations. Chest. 1994;106:1333–1338. doi: 10.1378/chest.106.5.1333. [DOI] [PubMed] [Google Scholar]
- 15.Grist T.M. Magnetic resonance angiography of renal arterial stenosis. Coron Artery Dis. 1999;10:151–156. doi: 10.1097/00019501-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Vasbinder G.B., Maki J.H., Nijenhuis R.J., Leiner T., Wilson G.J., Kessels A.G. Motion of the distal renal artery during three-dimensional contrast-enhanced breath-hold MRA. J Magn Reson Imaging. 2002;16:685–696. doi: 10.1002/jmri.10214. [DOI] [PubMed] [Google Scholar]