Abstract
Objective
We aimed to clarify the correlation between bone mineral density (BMD) and the modified total Sharp score of the hand in Japanese patients with established rheumatoid arthritis (RA).
Methods
We examined the hands of 57 patients who had RA for more than 20 years. BMD for the whole hand was measured using dual-energy x-ray absorptiometry. Concurrently, the hands were analyzed using radiography to estimate the van der Heijde-modified total Sharp score (vdH-S).
Results
The patients were all women with a median age of 69.7 years and RA disease duration of 29.9 years. The correlation coefficients were −0.513 (P < 0.0001) for hand BMD and vdH-S of the hand, −0.576 (P < 0.0001) for hand BMD and the erosion score of the vdH-S, and −0.339 (P < 0.0001) for hand BMD and the joint narrowing score of the vdH-S.
Conclusions
Hand BMD is correlated with the vdH-S in long-established RA. The hand BMD is important for structural assessment of the hand. Additionally, we may be able to predict the vdH-S of the hand on the basis of the hand BMD in long-established RA.
Keywords: Bone mineral density, Hand, Modified total sharp score, Rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is associated with joint inflammation and destruction, which lead to pain, swelling, stiffness, and loss of function in joints throughout the body. The outcomes of treatment for RA have improved following recommendations and new criteria established by the European League against Rheumatism and American College of Rheumatology.1, 2 In the early stage of RA, involvement of the hand often causes pain and swelling. Approximately 90% of patients who experience early onset of RA demonstrate deformities of the metacarpophalangeal joint or wrist on magnetic resonance imaging.3 Additionally, more than 90% of the patients with RA experience at least one hand or wrist symptom for more than 8 years.4. It is important, at all stages of the disease, to prevent destruction of the hand. A study reported that the loss of bone mineral content in the hand, as assessed using dual-energy x-ray absorptiometry (DXA), over 5 years is significantly correlated with the responses on the Health Assessment Questionnaire (HAQ).5 Another previous study reported that the increase in the bone mineral density (BMD) of the femoral neck is correlated with improvements in the HAQ score. However, the BMD of the distal radius was not correlated with the HAQ score.6
Decreased hand and femoral BMD may cause functional disability. Chronic inflammation due to RA affects bone metabolism, disrupting the normal cycle of bone resorption and remodeling, thereby leading to generalized bone loss (osteoporosis) and local bone loss (joint destruction).7 Previous studies have suggested that generalized and local bone destruction are caused by osteoclast activation, and the mechanism of bone loss in RA is related to an imbalance in the ratio of receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin.7, 8, 9 Furthermore, the van der Heijde-modified total Sharp score (vdH-S) was found to be correlated with the bone marker urinary deoxypyridinoline in active RA.10 Body mass index, disease duration, and high serum cross-linked N-telopeptidases of type I collagen level are common risk factors of osteoporosis in postmenopausal women with RA.11 In RA, the changes in BMD are likely caused by accumulated inflammation.
The goal of RA treatment is clinical, structural, and functional remission. The outcomes of joint damage due to RA have been examined on the basis of the vdH-S.12 The vdH-S for the hand includes the whole hand as well as the proximal interphalangeal joint, metacarpophalangeal joint, and intercarpal joint. The joint damage is result of the combination of accumulated inflammation and changes in BMD.
We believe that bone loss in the whole hand may be related to joint damage, particularly in long-established RA. In daily practice, we treat several RA patients with postmenopausal and chronic diseases. The aim of this study was to evaluate the frequency of osteoporosis and the relationship of the BMD and vdH-S of the hand in established RA.
2. Materials and methods
This study investigated the clinical course and background variables of patients with RA that fulfilled the American College of Rheumatology classification criteria (1987).13 The disease duration of all patients with RA was more than 20 years. The control group comprised 50 women aged 20–39 years who were currently not receiving treatment for any diseases. A control group was necessary because there is no established baseline hand BMD for the Japanese population.
This study was approved by the ethics committee of Kamagaya General Hospital. All patients agreed to the terms of the study protocol.
Hand, lumbar spine (L2-L4 anteroposterior view), and total hip BMD were measured using DXA with the PRODIGY System (GE Healthcare, Madison, Wi, USA). In or der to measure the vdH-S, DXA and plain X-ray were performed simultaneously. The most affected hand for each patient was scanned. After excluding the hand operation history, the left hand and left hip were scanned. A flatbed scanner was used to capture radiographs as digital images. The hand BMD includes whole hand including the carpal bone (Fig. 1). Joint damage analysis for the hand was examined on the basis of the vdH-S. The vdH-S identifies 16 areas of erosion and 15 areas of joint space narrowing in hand.
Fig. 1.
The measurement range of the hand BMD by DXA.
BMD: bone mineral density, DXA: dual-energy x-ray absorptiometry
The data analysis for the hand, lumbar spine, and total hip BMD and total sharp score, erosion score and joint narrowing score of vdH-S in hand was performed using Spearman's rank correlation. Statistical significance was established at a P-value <0.05.
3. Results
In this study, 57 patients with RA (RA group) were enrolled. All of the enrolled patients were female and had established RA. The age and disease duration for the RA group (mean ± standard deviation) was 69.7 ± 8.1 years and 29.9 ± 8.7 years, respectively. Table 1 shows the demographic characteristics of the RA group and control group.
Table 1.
Demographic characteristics in RA group and control group.
| RA group (n = 57) | Control group (n = 50) | |
|---|---|---|
| Age, years, mean (SD) | 69.7 (8.1) | 30.6 (5.0) |
| Gender, female, n (%) | 57 (100) | 50 (100) |
| Body weight, kg, mean (SD) | 48.1 (6.6) | 53.5 (8.6) |
| Body mass index, mean (SD) | 21.3 (2.4) | 20.9 (3.4) |
| Disease duration, years (SD) | 29.9 (8.7) | – |
| PF positive, n (%) | 46 (80.7) | – |
| Anti-CCP positive, n (%) | 48 (84.2) | – |
| Biological DMARD use, n (%) | 31 (54.4) | – |
| MTX use, n (%) | 36 (63.2) | – |
| Corticosteroid use, n (%) | 24 (42.1) | – |
| Hand BMD, g/cm2, mean (SD) | 0.243 (0.055) | 0.382 (0.046) |
| Lumbar spine BMD, g/cm2, mean (SD) | 0.938 (0.209) | 1.208 (0.124) |
| Total hip BMD, g/cm2, mean (SD) | 0.666 (0.113) | 0.943 (0.146) |
RF: rheumatoid factor, Anti-CCP: anti-cyclic citrullinated peptide antibody, MTX: methotrexate, BMD: bone mineral density.
In comparison with the control group, the hand BMD was −2.5 SD below the mean for 70.2% of patients in the RA group. Similarly the lumbar spine BMD was −2.5 SD below the mean for 43.9% of patients and the hip BMD was −2.5 SD below the mean for 22.8% of patients in the RA group.
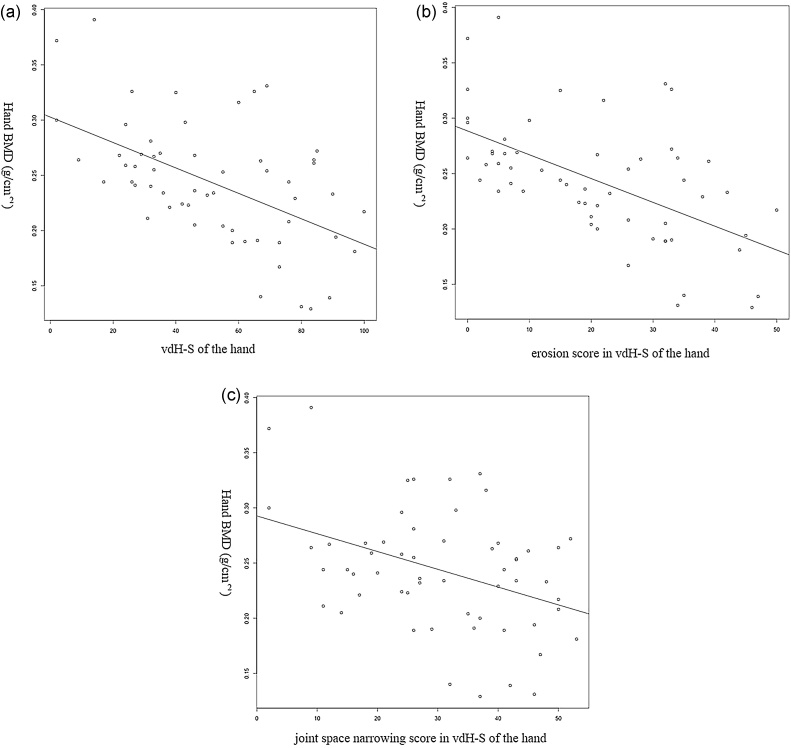
In all patients of RA group, the correlation coefficients of hand BMD and vdH-S were −0.513 (P < 0.001) for the total score, −0.576 (P < 0.001) for the erosion score, and −0.339 (P < 0.001) for the joint narrowing score (Fig. 2). The correlation coefficients of lumbar spine BMD and vdH-S of the hand were −0.161 (P = 0.232) for the total score, −0.301 (P = 0.023) for the erosion score, and 0.046 (P = 0.736) for the joint narrowing score. The correlation coefficients of hip BMD and vdH-S of the hand were −0.281 (P = 0.034) for the total score, −0.368 (P = 0.005) for the erosion score, and −0.135 (P = 0.316) for the joint narrowing score.
Fig. 2.
The correlation coefficients of hand BMD and vdH-S of hand in total score (a), erosion score (b), and joint space narrowing score (c) of all patients.
BMD: bone mineral density, vdH-S: van der Heijde-modified total sharp score
In the RA group treated with biological DMARDs, the correlation coefficients of hand BMD and vdH-S were −0.565 (P < 0.001) for the total score, −0.601 (P < 0.001) for the erosion score, and −0.466 (P < 0.001) for the joint narrowing score. The correlation coefficients of lumbar spine BMD and vdH-S of the hand were −0.156 (P = 0.402) for the total score, −0.296 (P = 0.106) for the erosion score, and 0.023 (P = 0.902) for the joint narrowing score. The correlation coefficients of hip BMD and vdH-S of the hand were −0.375 (P = 0.038) for the total score, −0.511 (P = 0.003) for the erosion score, and −0.203 (P = 0.273) for the joint narrowing score.
In the RA group treated with corticosteroids, the correlation coefficients of hand BMD and vdH-S of the hand were −0.368 (P = 0.077) for the total score, −0.434 (P = 0.039) for the erosion score, and −0.191 (P = 0.371) for the joint narrowing score. The correlation coefficients of lumbar spine BMD and vdH-S of the hand were 0.224 (P = 0.292) for the total score, 0.039 (P = 0.856) for the erosion score, and 0.467 (P = 0.021) for the joint narrowing score. The correlation coefficients of hip BMD and vdH-S of the hand were 0.102 (P = 0.636) for the total score, −0.006 (P = 0.979) for the erosion score, and 0.285 (P = 0.176) for the joint narrowing score.
The results of the correlation coefficients of the BMD and vdH-S of the hand are summarized in Table. 2.
Table 2.
The correlation coefficients of BMD and vdH-S of hand.
| Hand BMD |
Lumbar spine BMD |
Total hip BMD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TSS | EN | JSN | TSS | EN | JSN | TSS | EN | JSN | |
| All patients | −0.513** | −0.576** | −0.339** | −0.161 | −0.301* | 0.046 | −0.281* | −0.368* | −0.135 |
| Biological DMARDs treatment | −0.565** | −0.601** | −0.466** | −0.156 | −0.296 | 0.023 | −0.375* | −0.511* | −0.203 |
| Corticosteroid treatment | −0.368 | −0.434* | −0.191 | 0.224 | 0.039 | 0.467* | 0.102 | −0.006 | 0.285 |
BMD: bone mineral density, TSS: van der Heijde-modified total Sharp score of the hand, EN: erosion score in van der Heijde-modified total Sharp score of the hand, JSN: joint narrowing score in van der Heijde-modified total Sharp score of the hand, DMARDs: disease modified anti-rheumatic drugs; Spearman's rank correlation: * P < 0.05, **P < 0.01.
4. Discussion
Joint inflammation gradually exacerbates the osteoporosis and joint destruction during RA with hand involvement. This study showed that the frequency of osteoporosis of the hand was 70.2% in patients with RA, which was comparatively higher than the frequency of osteoporosis of the lumbar spine and hip in these patients. In the general Japanese population, the frequency of osteoporosis of the lumbar spine is approximately 10% in people aged 60–69 years and approximately 30% in those aged 70–79 years.14 In 50–70-year-old women with long-established (mean disease duration 17.3 years) RA, the reduction in hand BMD was more pronounced than those in lumbar spine BMD and hip BMD.15 The results of the vdH-S suggest that hand BMD is not affected by degenerative changes, and thus reflects disease specificity. Our results were in accordance with those from previous studies. In our study, the patients received sufficient treatment for an adequate period of time since the onset of RA. In Japan, methotrexate has been used for the treatment of RA since 1999, and biological DMARDs have been used since 2003. According to several reports, biological DMARDs, such as tumor necrosis factor-α inhibitors, increase the BMD of the lumbar spine and hip. However Güler-Yüksel et al. reported that one randomized trial of RA patients indicated that comparison of treatment with MTX alone, or in combination with infliximab, did not demonstrate any differences in BMD loss for the lumbar spine and hip.16 Vis et al. reported that in the RA patients treated with infliximab, spine and hip BMD loss is arrested, whereas metacarpal cortical hand bone loss is not attenuated.17 The patients treated with biological DMARDs showed reduced periarticular bone loss independently of the clinical response.18 We believe that the patients included in our study did not undergo aggressive treatments upon early onset of RA, and therefore, the loss of hand BMD was in progress after the initiation of aggressive treatment. Thus, the frequency of osteoporosis of the hand was high among the patients in our study.
A study reported that the loss of hand BMD loss, assessed using DXA, during the first year of RA was associated with the Genant-modified Sharp score for 6.4 years in patients with early RA with a mean disease duration of 6.0 months.19 The loss of hand BMD, determined using digital x-ray radiogrammetry, at 1 year in RA patients with a disease duration 0–4 years was an independent predictor of joint damage on the basis of the vdH-S at 5 and 10 years.20 The hand BMD loss, using DXA, was a more sensitive indicator than vdH-S in patients with early RA with a mean disease duration of 8.5 years.21 According to previous reports, in early RA, hand BMD loss was associated with joint damage.
Meanwhile in established RA, Hoff et al. reported that the hand BMD loss by DXA did not change for 2 years in RA patients with a disease duration of 9 year; furthermore, the change in the hand BMD was −0.96% with a disease duration of ≤3 years and 0.24% with a disease duration >3 years.22 In RA patients with a disease duration of 15.9 years, the Larsen score (0–5) was associated with the forearm BMD.23 According to our results, whole hand BMD was correlated with vdH-S of the hand. Periarticular bone loss in RA induces the production of osteoclasts. In synovial tissue with RA, the levels of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1, and interleukin-6, are elevated. The cytokines stimulate RANKL to activate the osteoclasts.24, 25, 26 Moreover, on the basis of our results, the hand BMD was more strongly correlated with the erosion score than the joint narrowing score of the vdH-S, especially in patients who received treatment with biological DMARDs. These findings may reflect the relationship between the synovium and bone erosion in the joints.
In our study, a strong correlation was observed between hand BMD and vdH-S in all patients as well as the patients treated with biological DMARDs. However, the correlation between hand BMD and vdH-S were weak in the patients treated with corticosteroids. Corticosteroids are known to possess a deleterious effect on the bone, increasing the risk of fracture.27, 28 In a randomized study of RA, hand BMD loss was less severe in patients treated with prednisolone compared to those who received the placebo for 2 years. Furthermore, C-reactive protein levels were correlated with hand BMD loss in the placebo patients but not in patients treated with prednisolone.29 In established RA, biological DMARD treatment was associated with increased hip BMD, whereas glucocorticoid treatment was found to be associated with decreased hip BMD. In the patients treated with glucocorticoids, changes in the profile of bone metabolic markers (bone-specific alkaline phosphatase and urinary type Ⅰ collagen cross-linked N-telopeptide) was not an independent variable.30 Corticosteroid treatment in RA has a variety of roles. Corticosteroids suppress inflammation, thus decreasing the BMD, but conversely enhancing the reduction in BMD. The effects of corticosteroid treatment on the bone in RA differ according to patients’ conditions. Hence, in our study, the correlation between hand BMD and the vdH-S in patients treated with corticosteroid was low.
The present study has a few limitations. First, this study involved a small sample size. The results are likely to change if the number of cases included increased. Second, this study was cross-sectional in nature. We could not obtain information regarding the treatment of the patients from the onset of RA to the present. Furthermore, this study could not clarify the relationship between osteoporosis, disease activity of RA, and generalized change. In order to clarify this relationship, a prospective study is required.
In conclusion, it is important to control disease activity in order to obtain structural remission. The prevalence of osteoporosis of the hand was higher than that of the lumbar spine and hip, and the hand BMD and vdH-S were correlated in established RA. Our results suggest that hand BMD is important for structural assessment of the hand, particularly for patients treated without corticosteroids. Additionally, we may be able to predict the vdH-S of the hand from the hand BMD in established RA.
Limitations
This study has limitations. This study was a cross-sectional. Accordingly, this study could not clarify RA and osteoporosis treatments from onset each diseases. In order to clarify this relationship, a prospective study is required.
Conflict of interest
None.
References
- 1.Smolen J.S., Landewé R., Breedveld F.C. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aletaha D., Neogi T., Silman A.J. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 3.Boutry N., Lardé A., Lapègue F., Solau-Gervais E., Flipo R.M., Cotten A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol. 2003;30(4):671–679. [PubMed] [Google Scholar]
- 4.Horsten N.C., Ursum J., Roorda L.D., van Schaardenburg D., Dekker J., Hoeksma A.F. Prevalence of hand symptoms, impairments and activity limitations in rheumatoid arthritis in relation to disease duration. J Rehabil Med. 2010;42:916–921. doi: 10.2340/16501977-0619. [DOI] [PubMed] [Google Scholar]
- 5.Deodhar A.A., Brabyn J., Pande I., Scott D.L., Woolf A.D. Hand bone densitometry in rheumatoid arthritis, a five year longitudinal study: an outcome measure and a prognostic marker. Ann Rheum Dis. 2003;6:767–770. doi: 10.1136/ard.62.8.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiguchi S., Goto H., Inaba M., Nishizawa Y. Preferential reduction of bone mineral density at the femur reflects impairment of physical activity in patients with low-activity rheumatoid arthritis. Mod Rheumatol. 2010;20:69–73. doi: 10.1007/s10165-009-0242-5. [DOI] [PubMed] [Google Scholar]
- 7.Xu S., Wang Y., Lu J., Xu J. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol Int. 2012;32:3397–3403. doi: 10.1007/s00296-011-2175-5. [DOI] [PubMed] [Google Scholar]
- 8.Gravallese E.M., Manning C., Tsay A. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi H., Iizuka H., Juji T. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto J., Garnero P., van der Heijde D., Miyasaka N., Yamamoto K., Kawai S. et al. A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod Rheumatol. 2009;19:273–282. doi: 10.1007/s10165-009-0170-4. [DOI] [PubMed] [Google Scholar]
- 11.Momohara S., Okamoto H., Yago T. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005;15:410–414. doi: 10.1007/s10165-005-0435-5. [DOI] [PubMed] [Google Scholar]
- 12.Van der Heijde D. Radiographic progression in rheumatoid arthritis: dose it reflect outcome? Dose it reflect treatment? Ann Rheum Dis. 2001;60:47–50. doi: 10.1136/ard.60.90003.iii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett F.C., Edworthy S.M., Bloch D.A. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;16:65–67. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura N., Muraki S., Oka H. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 15.Haugeberg G., Lodder M.C., Lems W.F. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50-70Øyear old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann Rheum Dis. 2004;63:1331–1334. doi: 10.1136/ard.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Güler-Yüksel M., Bijsterbosch J., Goekoop-Ruiterman Y.P. Changes in bone mineral density in patients with recent onset, active rheumatoid arthritis. Ann Rheum Dis. 2008;67:823–828. doi: 10.1136/ard.2007.073817. [DOI] [PubMed] [Google Scholar]
- 17.Vis M., Havaardsholm E.A., Haugeberg G. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1495–1499. doi: 10.1136/ard.2005.044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoff M., Kvien T.K., Kälvesten J., Elden A., Kavanaugh A., Haugeberg G. Adalimumab reduces hand bone loss in rheumatoid arthritis independent of clinical response: subanalysis of the PREMIER study. BMC Musculoskelet Disord. 2011;12:54. doi: 10.1186/1471-2474-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bejarano V., Hensor E., Green M. Relationship between early bone mineral density changes and long-term function and radiographic progression in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:66–70. doi: 10.1002/acr.20553. [DOI] [PubMed] [Google Scholar]
- 20.Hoff M., Haugeberg G., Odegård S. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis. 2009;68:324–329. doi: 10.1136/ard.2007.085985. [DOI] [PubMed] [Google Scholar]
- 21.Haugeberg G., Green M.J., Conaghan P.G. Hand bone densitometry: a more sensitive standard for the assessment of early bone damage in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1513–1517. doi: 10.1136/ard.2006.067652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoff M., Haugeberg G., Kvien T.K. Hand bone loss as an outcome measure in established rheumatoid arthritis: 2-year observational study comparing cortical and total bone loss. Arthritis Res Ther. 2007;9:R81. doi: 10.1186/ar2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsblad D'Elia H., Larsen A., Waltbrand E. Radiographic joint destruction in postmenopausal rheumatoid arthritis is strongly associated with generalised osteoporosis. Ann Rheum Dis. 2003;62:617–623. doi: 10.1136/ard.62.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldring S.R., Gravallese E.M. Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol. 2000;12:195–199. doi: 10.1097/00002281-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Pettit A.R., Ji H., von Stechow D. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redlich K., Hayer S., Ricci R. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Staa T.P., Leufkens H.G., Abenhaim L., Zhang B., Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 28.van Staa T.P., Leufkens H.G., Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 29.Haugeberg G., Strand A., Kvien T.K., Kirwan J.R. Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis: results from a randomized placebo-controlled trial. Arch Intern Med. 2005;165:1293–1297. doi: 10.1001/archinte.165.11.1293. [DOI] [PubMed] [Google Scholar]
- 30.Okano T., Koike T., Tada M. The limited effects of anti-tumor necrosis factor blockade on bone health in patients with rheumatoid arthritis under the use of glucocorticoid. J Bone Miner Metab. 2014;32:593–600. doi: 10.1007/s00774-013-0535-9. [DOI] [PubMed] [Google Scholar]