Abstract
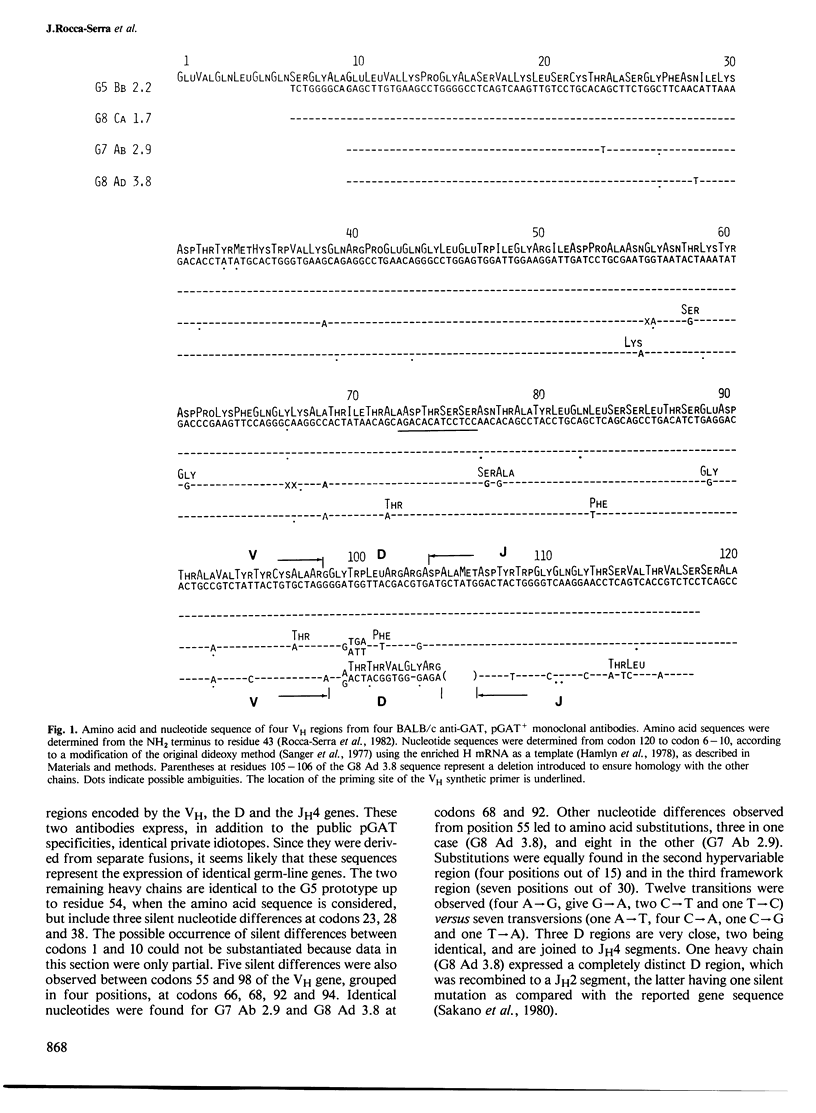
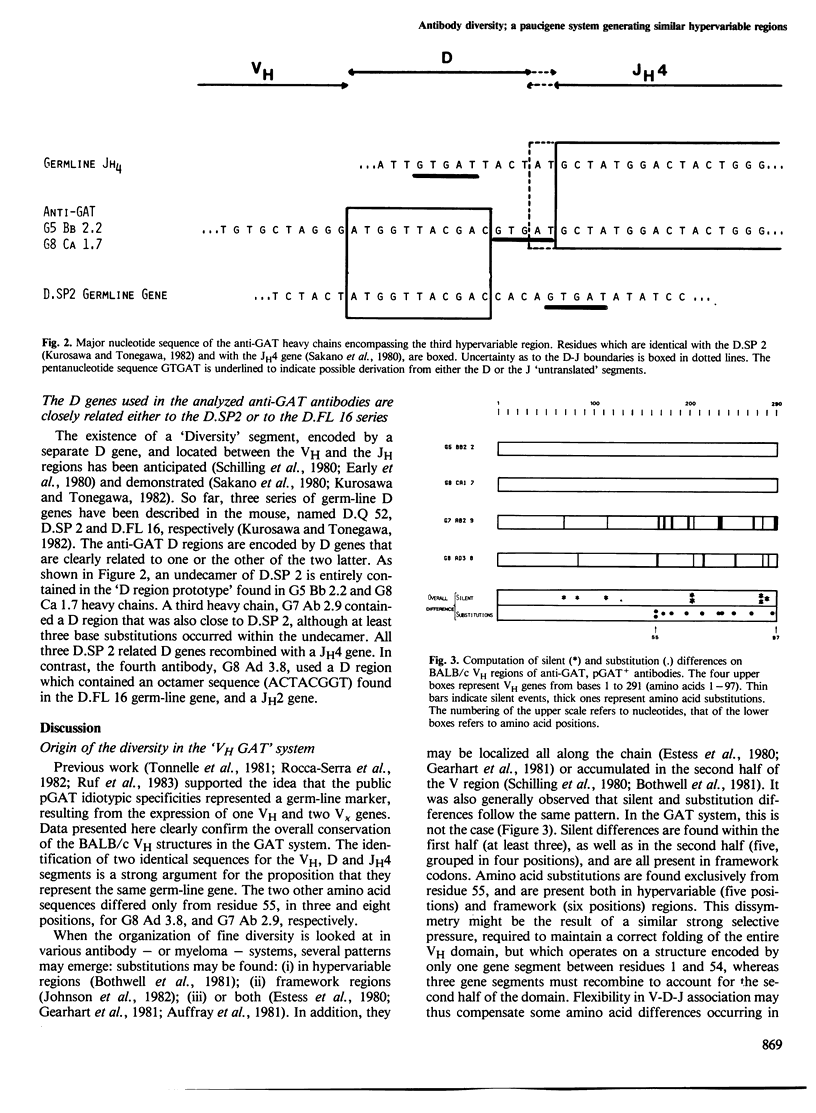
The nucleotide sequence of four anti-(Glu60-Ala30-Tyr10)n (GAT) monoclonal gamma 1 heavy chain mRNAs was determined from codon 10 to 120. This sequence overlaps with the NH2-terminal amino acid sequence, allowing elucidation of the complete protein sequence encompassing regions VH, D and JH. These sequences, which are highly conserved, indicate that anti-GAT antibodies expressing the same public idiotypic specificities represent a paucigene system, which uses at least two D-J combinations leading to functionally similar hypervariable regions involved in the recognition of the dominant Glu-Tyr determinant. D regions are encoded by D genes which are closely related either to the D-SP2 or the D.FL16 germ line gene cores.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzel L. M., Poljak R. J., Saul F., Varga J. M., Richards F. F. The three dimensional structure of a combining region-ligand complex of immunoglobulin NEW at 3.5-A resolution. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1427–1430. doi: 10.1073/pnas.71.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Auffray C., Sikorav J. L., Ollo R., Rougeon F. Correlation between D region structure and antigen-binding specificity: evidences from the comparison of closely related immunoglobulin VH sequences. Ann Immunol (Paris) 1981 Jul-Aug;132D(1):77–88. [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2701–2705. doi: 10.1073/pnas.69.9.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- De Préval C., Fougereau M. Specific interaction between VH and VL regions of human monoclonal immunoglobulins. J Mol Biol. 1976 Apr 15;102(3):657–678. doi: 10.1016/0022-2836(76)90340-5. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., OLINS D. E., GALLY J. A., ZINDER N. D. RECONSTITUTION OF IMMUNOLOGIC ACTIVITY BY INTERACTION OF POLYPEPTIDE CHAINS OF ANTIBODIES. Proc Natl Acad Sci U S A. 1963 Oct;50:753–761. doi: 10.1073/pnas.50.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Idiotype expression and the inheritance of mouse antibody clones. J Exp Med. 1973 Mar 1;137(3):603–621. doi: 10.1084/jem.137.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Sheppard R. C. Rapid synthesis of oligodeoxyribonucleotides. II. Machine-aided solid-phase syntheses of two nonanucleotides and an octanucleotide. Nucleic Acids Res. 1977 Dec;4(12):4391–4410. doi: 10.1093/nar/4.12.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Givol D., Zakut R., Effron K., Rechavi G., Ram D., Cohen J. B. Diversity of germ-line immunoglobulin VH genes. Nature. 1981 Jul 30;292(5822):426–430. doi: 10.1038/292426a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Bernard O. Sequences of the joining region genes for immunoglobulin heavy chains and their role in generation of antibody diversity. Proc Natl Acad Sci U S A. 1981 Jan;78(1):509–513. doi: 10.1073/pnas.78.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N., Slankard J., Paul L., Hood L. The complete V domain amino acid sequences of two myeloma inulin-binding proteins. J Immunol. 1982 Jan;128(1):302–307. [PubMed] [Google Scholar]
- Ju S. T., Kipps T. J., Theze J., Benacerraf B., Dorf M. E. Idiotypic analysis of anti-GAT antibodies. I. Presence of common idiotypic specificities in both responder and nonresponder mice. J Immunol. 1978 Sep;121(3):1034–1039. [PubMed] [Google Scholar]
- Ju S. T., Pierres M., Germain R. N., Benacerraf B., Dorf M. E. Idiotypic analysis of anti-GAT antibodies. VI. Identification and strain distribution of the GA-1 idiotype. J Immunol. 1979 Dec;123(6):2505–2510. [PubMed] [Google Scholar]
- Karjalainen K., Mäkelä O. A mendelian idiotype is demonstrable in the heteroclitic anti-NP antibodies of the mouse. Eur J Immunol. 1978 Feb;8(2):105–111. doi: 10.1002/eji.1830080207. [DOI] [PubMed] [Google Scholar]
- Kocher H. P., Berek C., Jaton J. C. The immune response of BALB/c mice to phosphorylcholine is restricted to a limited number of VH- and VL-isotypes. Mol Immunol. 1981 Dec;18(12):1027–1033. doi: 10.1016/0161-5890(81)90018-3. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Leclercq L., Mazié J. C., Sommé G., Thèze J. Monoclonal anti-GAT antibodies with different fine specificities express the same public idiotype. Mol Immunol. 1982 Aug;19(8):1001–1009. doi: 10.1016/0161-5890(82)90308-x. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Potter M., Humphrey W., Jr, Chien C. C. Idiotype of inulin-binding antibodies and myeloma proteins controlled by genes linked to the allotype locus of the mouse. J Immunol. 1976 Dec;117(6):2105–2111. [PubMed] [Google Scholar]
- METZGER H., WOFSY L., SINGER S. J. THE PARTICIPATION OF A AND B POLYPEPTIDE CHAINS IN THE ACTIVE SITES OF ANTIBODY MOLECULES. Proc Natl Acad Sci U S A. 1964 Apr;51:612–618. doi: 10.1073/pnas.51.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Pawlak L. L., Mushinski E. B., Nisonoff A., Potter M. Evidence for the linkage of the IGC H locus to a gene controlling the idiotypic specificity of anti-p-azophenylarsonate antibodies in strain A mice. J Exp Med. 1973 Jan 1;137(1):22–31. doi: 10.1084/jem.137.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riblet R., Blomberg B., Weigert M., Lieberman R., Taylor B. A., Potter M. Genetics of mouse antibodies. I. Linkage of the dextran response locus, VH-DEX, to allotype. Eur J Immunol. 1975 Nov;5(11):775–777. doi: 10.1002/eji.1830051109. [DOI] [PubMed] [Google Scholar]
- Rocca-Serra J., Mazie J. C., Moinier D., Leclercq L., Somme G., Theze J., Fougereau M. The limited diversity of the mouse gamma-chains anti-GAT repertoire does not seem to be noticeably amplified upon class switch. J Immunol. 1982 Dec;129(6):2554–2558. [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling J., Clevinger B., Davie J. M., Hood L. Amino acid sequence of homogeneous antibodies to dextran and DNA rearrangements in heavy chain V-region gene segments. Nature. 1980 Jan 3;283(5742):35–40. doi: 10.1038/283035a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder A., Nau M., Norman B., Leder P. Antibody diversity. Science. 1978 Oct 6;202(4363):11–17. doi: 10.1126/science.99815. [DOI] [PubMed] [Google Scholar]
- Sommé G., Serra J. R., Leclercq L., Moreau J. L., Mazie J. C., Moinier D., Fougerèau M., Thèze J. Contribution of the H- and L-chains and of the binding site to the idiotypic specificities of mouse anti-GAT antibodies. Mol Immunol. 1982 Aug;19(8):1011–1019. doi: 10.1016/0161-5890(82)90309-1. [DOI] [PubMed] [Google Scholar]
- Theze J., Kapp J. A., Benacerraf B. Immunosuppressive factor(s) extracted from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT) III. Immunochemical properties of the GAT-specific suppressive factor. J Exp Med. 1977 Apr 1;145(4):839–856. doi: 10.1084/jem.145.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Moreau J. L. Genetic control of the immune response to the GAT terpolymer. I. Interstrain and interspecies cross-reactive idiotype. Ann Immunol (Paris) 1978 Jul-Sep;129 100(5):721–726. [PubMed] [Google Scholar]
- Thèze J., Sommé G. Genetic control of the immune response to the terpolymer L-glutamic acid(60)-L-alanine(30)-L-tyrosine(10)(GAT). II. Characterization of a cross-reactive idiotype associated with anti-GAT antibodies from responder and nonresponder mice. Eur J Immunol. 1979 Apr;9(4):294–301. doi: 10.1002/eji.1830090409. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnelle C., Pierres M., Ju S. T., Moinier D., Fougereau M. NH2-terminal amino-acid sequences of poly (Glu60, Ala30, Tyr10) (GAT) and poly (Glu60, Ala40) (GA) monoclonal antibody heavy chains. Mol Immunol. 1981 Nov;18(11):979–984. doi: 10.1016/0161-5890(81)90115-2. [DOI] [PubMed] [Google Scholar]
- Vrana M., Rudikoff S., Potter M. Sequence variation among heavy chains from inulin-binding myeloma proteins. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1957–1961. doi: 10.1073/pnas.75.4.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


