Abstract
Integration of sexual and reproductive health within HIV care services is a promising strategy for increasing access to family planning and STI services and reducing unwanted pregnancies, perinatal HIV transmission and maternal and infant mortality among people living with HIV and their partners. We conducted a Phase II randomized futility trial of a multi-level intervention to increase adherence to safer sex guidelines among those wishing to avoid pregnancy and adherence to safer conception guidelines among those seeking conception in newly-diagnosed HIV-positive persons in four public-sector HIV clinics in Cape Town. Clinics were pair-matched and the two clinics within each pair were randomized to either a three-session provider-delivered enhanced intervention (EI) (onsite contraceptive services and brief milieu intervention for staff) or standard-of-care (SOC) provider-delivered intervention. The futility analysis showed that we cannot rule out the possibility that the EI intervention has a 10 % point or greater success rate in improving adherence to safer sex/safer conception guidelines than does SOC (p = 0.573), indicating that the intervention holds merit, and a larger-scale confirmatory study showing whether the EI is superior to SOC has merit.
Keywords: Integration of sexual and reproductive health and HIV services, HIV-positive women and men, Contraception, Safer conception, South Africa
Introduction
For more than 20 years there have been calls for the integration of sexual and reproductive health (SRH) and HIV services [1–11]. SRH and HIV integration was cast as a key strategy to meeting the 2015 Millennium Development Goals [12] in the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) [13] and in the 2016 United Nations General Assembly Political Declaration Zero Draft fast track agenda for sustainable development [14]. In sub-Saharan Africa, where reproductive-aged women bear a disproportionate burden of HIV, and both HIV-infected women and men are living longer, integration of SRH and HIV services in this era of combination therapy needs to be a priority. Differences in fertility intentions and fertility rates between HIV-positive and HIV-negative women are narrowing due to wider availability of antiretroviral therapy (ART), and the resulting improved health outcomes and life expectancy of people living with HIV (PLWH) [10, 15–21].
High rates of unmet contraceptive need and unintended pregnancy are common among the estimated 13 million women living with HIV in sub-Saharan Africa [22–24] Unintended pregnancies account for 14–58 % of all births in countries where the burden of HIV is the greatest [25]. One 2012 South African study found that 62 % of pregnancies among HIV-positive women at public-sector ART clinics were unintended [26], while a 2009 South African study reported that among women on ARVs, 9 % reported having been pregnant since initiating treatment, and 30 % of these pregnancies were unintentional [27]. Studies of contraceptive use among women living with HIV show contradictory findings, with some reporting low use of any form of contraception [20], others reporting increased use [28, 29]], and in some countries, there is no relationship between HIV status and contraceptive use [19].
The benefits of SRH-HIV linkage have been documented in two systematic reviews [30–32]. A growing body of research indicates that integration of SRH and HIV services has the potential to increase access to family planning and STI prevention services, uptake of effective contraception and condom use among those who do not want to conceive [29, 33, 34], ART initiation during pregnancy, as well as to improve HIV testing rates and quality of services, and reduce unwanted pregnancies, perinatal HIV transmission and maternal and infant mortality [6, 7, 17, 35–39]. However, there is little consensus on how integration is operationalized and how best to integrate these services. Some integration models are a one-stop service that combine previously separate components of care, and add new components into existing services [34], while others use referral-based models. Other programs have integrated SRH into ARV services [37, 38] immediately prior to ART initiation rather than after initial linkage to HIV care. Most notably, empirical evidence is still limited [28, 29, 33, 34, 36] and often inconsistent [39], as noted in the studies below.
The Integra Initiative, which assessed the impact of different models of delivering integrated HIV-SRH service delivery in 40 health facilities in Kenya and Swaziland on service and health outcomes, found mixed effects in terms of technical efficiency of service delivery [40, 41]. Number of HIV and STI services in maternal and child health units, public ownership, and facility type resulted in positive effects on technical efficiency of service delivery, but number of HIV services in the same clinical room, relative number of clinical staff to overall staff, proportion of HIV services provided in facility, and rural location had negative effects on technical efficiency of integrated HIV-SRH services [41]. Another analysis of these data that quantified the extent and type of integration between HIV and SRH services in Kenya and Swaziland suggested that both structural integration (infrastructure and multi-tasking providers in same facility) and functional integration (delivery of care to clients in one place at one time) are needed for effective integration of these services to clients [34]. In the Swaziland Integra Initiative sites, a clinic-level evaluation found that integrating HIV testing and treatment into family planning and postnatal care, or providing on-site SRH services at an ARV site did not address reproductive health needs of PLWH any better than stand-alone clinics, with multiple organizational and provider factors affecting integration [42].
However, other studies have found positive effects of integration. A pilot study of a one-stop shop model in Malawi comparing contraceptive prevalence in clinics before and after family planning service integration found that a clinic that integrated family planning into ART services increased non-pregnant women's use of a modern contraceptive method and cervical cancer screening compared to an ART clinic that did not implement SRH integration [43]. Similarly, a non-randomized study in Kenya found that a one-stop same-day integrated family planning and HIV care model was associated with increased use of modern contraception but not a reduction in pregnancy incidence among HIV-positive women compared to a non-integrated service delivery model [44]. Data from a rigorous randomized trial comparing integrated vs. non-integrated contraception and HIV services in public-sector HIV clinics in Nyanza, Kenya, found that integration of services was cost-effective [29], and potentially could provide an opportunity to enhance men's active involvement in contraceptive decision-making [45].
SRH-HIV integration studies that simultaneously address both the contraceptive and safer conception needs of HIV-positive women and men are lacking. In this paper, we describe the results of a Phase II futility trial that evaluated an integrated one-stop shop provider-delivered SRH and HIV intervention, Emtonjeni (spring of knowledge). The intervention aimed to increase consistent condom use among HIV-positive people seeking to avoid pregnancy and uptake of safer conception services among those seeking pregnancy within public-sector HIV care services in Cape Town, South Africa.
Methods
Study Setting
The study was conducted in South Africa, a country with one of the highest burdens of HIV in the world, with an estimated 6.4 million people living with HIV/AIDS at the end of 2012 [46]. For many, infection occurs prior to and/or during peak reproductive years, and the epidemic has continued to be marked by pronounced gender differences. HIV prevalence among women and men aged 15–49 years is 23.3 %, and 13.3 %, respectively [46]. The national contraceptive prevalence is approximately 65 % among sexually active women based on the 2003 Demographic and Health Survey data [47].
HIV prevalence among pregnant women attending public sector facilities in the Cape Town Metropole District was 20.0 % in 2012, slightly higher than the 17.8 % overall provincial prevalence among pregnant women, but lower than the 29.5 % national HIV prevalence [48]. HIV prevalence in Cape Town among pregnant women was 24.6 % among those 30-34 years old, 22 % among those 25–29 years old, and 13 % among those 20–24 years old [49]. Risk of mother-to-child transmission has continued to decline in South Africa, from approximately 20 % a decade ago to 2.4 % at birth in 2012 [50]. South Africa's fertility rate has declined as well, from 2.92 in 2001 to 2.35 in 2011, and in the Western Cape, from 2.36 between 2001 and 2006, to 2.27 between 2006 and 2011, and to 2.19 between 2011 and 2016 [51].
Interventions
Description of the Enhanced and Standard of Care Interventions
The Enhanced Intervention (EI) was targeted to persons newly diagnosed with HIV, who often are highly dependent upon health care providers (including physicians, nurses and counselors) for guiding them to optimal health. As gate-keepers of information, providers can help to ensure that clients have access to effective contraception and safer conception strategies [52]. However, numerous studies have reported that HIV providers do not routinely engage PLWH in discussions about contraception and fertility desires [35, 36, 53–61], while clients rarely initiate such discussions with providers [27, 60]. Barriers to providers’ discussion of safer conception options include assumptions of HIV sero-concordance, limited knowledge of safer conception, weighing reproductive rights versus personal views toward PLWH having children, and concerns about delivering customized safer sex messages within the context of time constraints [61]. Therefore, we designed and tested a provider-delivered intervention in which HIV providers were trained to deliver integrated SRH and HIV care services.
The Standard of Care (SOC) intervention condition consisted of three 30–45 min-counseling sessions delivered by trained peer HIV counselors. Sessions were held within the first few months of entry into HIV medical monitoring. At the time the study was conducted, this was a routine clinic procedure for orienting patients to HIV treatment, fostering disclosure prior to receipt of ARV, and promoting the importance of adherence to medication and safer sex guidelines. Following HIV testing and post-test counseling, HIV-positive individuals were referred to an HIV care clinic. The interval between receiving HIV test results and seeking HIV care ranged from one week to six months for the majority of HIV-positive persons. Upon entering HIV care, clients attended two “triage” visits. At the initial visit, clients had their blood drawn for CD4 testing, received a brief introduction to HIV services, and returned to the clinic approximately 3–4 weeks later for clinical staging and receipt of their CD4 lab results.
The EI was designed to enable HIV-positive women and men to make informed decisions regarding the risks, benefits and options associated with safer sex, contraceptive use, fertility, and parenting options. It also consisted of three sessions and covered HIV basics, treatment issues, the importance of adherence and suggestions for increasing adherence, safer sex, and disclosure of status to significant others. The structure of the EI in part was determined by our decision to construct an intervention that would use existing staff and involve a similar time commitment (number of sessions) to match the organization of services in the SOC, thus minimizing system burden and optimizing potential sustainability. With the myriad needs confronting newly-diagnosed HIV-positive individuals, a three-session intervention, conducted within the context of a strained health care system, cannot address all issues. Our decision to involve both nurses experienced in family planning and HIV peer counselors was determined collaboratively during our intervention developmental work with them, particularly with regard to their comfort and sensitivity about topics. In the EI, the nurses provided participants with contraceptive methods requiring a medical provider to dispense (oral and injectable contraceptives; emergency contraception). The nurses also provided basic SRH counseling to all participants, and focused on topics that entailed more medical information such as types of contraception, termination of pregnancy and safer conception strategies. Participants interested in termination of pregnancy and voluntary sterilization would be referred for services. Men were invited to refer or come with their partner for these services, and all men received individual SRH counseling, whether or not a partner attended. Clients saw the nurse at the time of their first and third sessions and had the option whether or not to see the nurse at the time of their second peer counseling session. HIV counselors also delivered three sessions of the same duration following a scripted protocol, with the addition of modules addressing mental health issues, partner violence, and availability of supportive services. With the use of a standardized counseling flip chart designed for the study, we provided counseling tailored to participants’ reproductive goals, whether contraception or safer conception, that took into account participants’ partnership types and, if known, awareness of partner(s’) HIV status.
At the first visit, the point of entry into the HIV care system, all clients received a basic information and education package to orient them to future health (e.g., TB, nutrition, mental health issues), and the HIV peer counselor reviewed issues pertinent to staying healthy and treatment options, as well as explored clients’ interpersonal situations (family structure and sexual relationships and ease with which to disclose status), including sources of support and referral needs. Clients provided a blood specimen for CD4 testing and clinical staging. At the second visit (3 weeks after the first visit), in addition to triage to further medical services based on the results of the CD4 test, clients received individualized specific SRH counseling by the peer counselors using an interactive provider-client flip chart tool designed for the study. These initial sessions with the HIV counselors concluded with the development of an ‘Action Plan’ to address client-identified needs. After a goal was identified, and possible barriers and solutions explored, peer counselors worked with the client to concretize behavioral steps needed to attain their goals (e.g., a goal might be disclosing their status to their mother; the Action Plan would include consideration of when, where and how this would be done). At the end of Session 2, and during the entire third session, the peer counselor reviewed and supported progress on the prior Action Plans, helped clients revise the plans if needed, and develop additional plans as new issues emerged.
Male and female condoms were freely available to participants in both conditions. Progestogen-only injectables are the most commonly provided contraceptives in South Africa [62]. Implants, intrauterine devices, the diaphragm, and combined hormonal transdermal contraceptive patches, and diaphragms were not available in the public sector in South Africa at the time of our study [62].
All EI participants were given basic information on safer conception methods and asked to consult further with the counselor and nurse if they were planning to conceive. EI participants interested in conceiving were counseled by the nurses on various “lower-tech” approaches for minimizing risk, including stabilizing health; timed intercourse (unprotected sex limited to the ovulatory period and thus peak fertility time); and manual vaginal insemination of semen [63–66] by partner or self via sterile needle-free syringes, with participants instructed on use and provided with materials and offered syringes, where relevant. Periconception [66, 67] and post-exposure prophylaxis [68, 69] and treatment as prevention, i.e., use of ART to suppress viral load of the positive partner [70], were not available in the public sector during the study period.
We addressed the structural context in which HIV care was delivered in the EI in two ways. First, we provided a one-stop shop for contraception and conception services. The co-location of contraceptive services within an HIV care clinic aimed to mitigate the structural barriers to study participants’ access to contraception. Second, the EI also included a milieu intervention with clinic staff to foster an HIV and SRH friendly-environment. Technical support with clinic staff was ongoing and provided where needed during the intervention. The milieu training aimed to help staff confront personal biases and misinformation that can stigmatize and alienate their HIV-positive clients, and to ensure that the clinic was characterized by a compassionate stance toward clients; it was grounded in a human rights perspective and an understanding of the challenges faced by HIV-positive persons. The milieu intervention involved all staff—from cleaners and clerks to physicians—and was framed as a half-day staff ‘retreat’. A critical focus involved values clarification to explore beliefs and values about HIV-positive clients and their treatment, and ways to decrease barriers and address continued challenges in providing non-judgmental, supportive services. Following this training, technical and ongoing SRH training was provided to the peer HIV counselor-family planning nurse teams who would deliver the EI to clients and to medical providers at the clinic, covering contraceptive methods, safer conception and sexual risk-reduction counseling.
Table 1 provides a comparison of EI and SOC interventions.
Table 1.
Comparison of the EI and SOC interventions at the clinic- and client-level
| EI | SOC | |
|---|---|---|
| Clinic-level | ||
| Consultation and support | Systematic, ongoing technical support | No systematic, ongoing technical support |
| On-site availability of non-barrier contraception | Dedicated Department of Health (DOH) public sector nurse available to dispense non-barrier contraception (oral and, injectable contraceptives; emergency contraception) | Non-barrier contraception not available within HIV clinic Emergency contraception is available |
| On-site availability of condoms | On-site distribution of free male and female condoms | On-site distribution of free male and female condoms |
| SRH training | Standard and follow-up training and support provided to all clinic staff | No standard training or support provided to all staff |
| Client-level | ||
| SRH counseling; adherence; disclosure | Provided to all clients enrolled in the study by peer HIV counselors and nurses in systematic manner following protocol via flip chart | Provided by trained peer HIV counselors, with non-systematic content coverage |
Development and Content of Flip-Chart Tool
The client and milieu components of the EI intervention were designed by the HIV Center, University of Cape Town, and City of Cape Town and Western Cape Departments of Health investigators and subsequently reviewed by an Intervention Development Work Group (IDWG) comprising individuals from the Departments of Health and non-governmental organizations (NGOs) with expertise in HIV prevention, HIV treatment and care, gynecology and obstetrics, and nursing and clinical and program staff from several NGOs. Peer HIV counselors similar to those who would deliver the EI counseling also were involved in the IDWG. We adapted information from the World Health Organization Decision-Making Tool for family planning women clients and providers [71] and the Western Cape AIDS Training, Information and Counselling Centre (ATICC) for HIV-positive women and men. Separate flip-chart tools were developed for women and men. Materials were subsequently reviewed by IDWG teams, based on internal consensus regarding their relevance and applicability. Comments were sent and discussed at a meeting.
A Scientific Expert Panel comprising seven international experts on contraceptive technologies, HIV treatment, prevention and care, obstetrics, assisted reproduction, and bioethics was charged with critically reviewing and advising on intervention messages, and served as the final arbiter in determining the appropriate outcomes for HIV-positive persons who want or are open to the possibility of having children. They also reviewed all intervention materials developed by the IDWG to ensure that they adhered to stipulated guidelines and reflected up-to-date medical information. Safer conception counseling, rooted in a patients’ rights perspective, focused on options for reducing transmission risk to partner and child, including pre- and post-exposure prophylaxis [68, 69, 72], treatment as prevention [70], and ‘lower-tech’ safer conception approaches (timed intercourse and manual self-insemination with partner sperm) [35, 64, 65], reflecting best practices for resource-constrained public-sector settings. Following the development of the flip-chart tool, the tool was reviewed and further edited by several provincial health department nurses and the study's HIV counselors.
In the EI, peer HIV counselors used a standardized flip-chart to counsel both women and men, and nurses used separate, gender-specific, flip charts to counsel women and men. Flip-charts had graphic illustrations on the side viewed by clients and “scripted” counseling messages on the side viewed by the counselors and nurses. Scripts were tailored to the needs of individual HIV-positive clients, and aimed to enhance the counseling skills of the peer HIV counselors and nurses. Content areas included living a healthy lifestyle with HIV, disclosure strategies, stigma, psychosocial challenges and potential violence and SRH issues such as: (1) HIV safer-sex strategies, including communication and sexual negotiations with partner(s); (2) contraception and HIV, including interactions between some ART medications and hormonal methods; (3) fertility decision-making options, including effects of HIV on pregnancy and vice versa (4) infertility in HIV-positive women and men; and (5) sexual functioning and health. The tool was translated from English into isiXhosa, and back-translated into English. We developed a ‘Tracking Sheet’ that allowed counselors and nurses (tailored to issues they dealt with) to easily identify and follow-up on participants’ Action Plans at subsequent visits.
Study Design
We used a Phase II non-superiority or futility proof-of-concept design to evaluate whether the EI has the potential for being superior to the SOC so that we could bring it forward for a Phase III confirmatory study. We chose the futility trial design because it allows for screening out “futile” or non-promising interventions, i.e., those that are unlikely to be feasible or to demonstrate effectiveness in a Phase III trial [73], in a fairly rapid and economical manner, or to recommend further study of the target intervention when findings provide encouraging evidence in support of an intervention, a goal that cannot be achieved with a typical pilot study design. Results of Phase II non-superiority studies enable one to determine, using fewer study participants and shorter-term outcomes, whether a more costly and far larger Phase III trial should be undertaken [74].
In this study, we defined the “superiority of interest” of the EI as improvement in our major outcome—adherence to safer sex/safer conception guidelines—exceeding that of SOC by 10 % points. We chose this value because we believe 10 % is the minimal clinically worthwhile improvement that would result in reductions in HIV transmission were the EI to roll out on a larger scale. With this design, the null hypothesis is that EI is superior to SOC (i.e., the differential in improvement between EI and SOC is at least 10 % points). Therefore, if the null hypothesis is rejected, we conclude that there is no evidence of a potential benefit of the EI, and a more rigorous test should not be pursued. Conversely, if the null hypothesis is not rejected, we cannot rule out the possibility that the EI is truly worthwhile, and that further confirmatory testing would be of interest. This is a relatively strong position to be in: with a conventional test of the null hypothesis of no efficacy, when we do not reject the null hypothesis, possibly due to inadequate sample size, typically one would say that we could not rule out “chance” as the explanation for any observed differences; and enthusiasm for further testing would be sharply dampened. In the futility trial, however, failure to reject the null hypothesis suggests that the intervention is either promising enough on the face of it to continue testing, or it is at least insufficient to disqualify the intervention from further examination [75, 76].
We did not choose the typical pilot study design for this study because it usually aims to understand the feasibility and acceptability of the intervention as well as to collect mission-critical parameters, such as the recruitment rate, retention rate, and standard deviation for the primary outcome, etc. Without requiring a large sample size, a typical pilot study allows us to locate the mission-critical parameter approximately, but adequately, for planning the subsequent trial; however, statistical power will generally be inadequate for testing study hypotheses in a pilot study.
Our study sites were four public-sector HIV care clinics in Cape Town. These clinics serve low-income individuals from the surrounding townships. Clinics were pair-matched on size [medium and large], HIV-positive client caseload, geographic location and demographic characteristics of clients. The two clinics within each pair were randomized to either the three-session provider-delivered EI or SOC provider-delivered intervention condition by the study's statistician, who was blinded to the participating clinics, using a computer-generated random numbers table.
To minimize the potential for contamination, we asked the EI interventionists (nurses and peer HIV counselors) to refrain from sharing intervention materials or discussing specific content outside of their clinics for the duration of the study. Since EI and SOC sites were geographically separate, we expected minimal, if any, dissemination of information by either staff or clients across clinics. Even in the event that information was shared, the information alone could not replicate the training and support provided to EI clinics in the context of provider training and the milieu intervention.
Statistical Analyses
Following intent-to-treat principles, we tested the null hypothesis H0:PEI – PSOC ≥ 0.10 using a generalized linear model, with identity link function and the method of generalized estimating equations (GEE) was employed to account for the effect of clustering within clinic. We used Rubin's [77] multiple imputation method, with five repeated imputations, to impute the missing endpoints. The final estimate of intervention effect was the average of the estimate obtained from the five completed data sets, and the standard error of the final estimate was calculated using Rubin's formula. In secondary analyses, we explored the impact of EI on key secondary outcome variables, such as uptake of contraceptive use, to help us understand in what ways the intervention affected such outcomes. For these analyses, we calculated the means and standard deviations for continuous variables and proportions for categorical variables; we did not conduct hypothesis testing since the main focus in this case was to highlight information that can be used to plan a subsequent Phase III trial rather than to conduct confirmatory comparisons, for which this study was not powered. Analyses for this study were conducted using the PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) and SAS (version 9.3; SAS Inc., Cary, NC).
Sample Size and Statistical Power
As noted above, we defined superiority as an improvement of 10 % points in the intervention success rate (i.e., PEI – PSOC ≥ 0.10, or Δ = 0.10) in increasing adherence to safer sex/safer conception guidelines of EI over SOC. We anticipated the intra-cluster correlation coefficient (ICC) was no greater than 0.003 and calculated the statistical power for the study under the design alternative of no difference.
Under the futility design, the type I error is the probability that we mistakenly declare the EI has no impact when in reality it is truly beneficial. We set the type I error at 0.10 as is conventional in proof-of-concept trials [75]. Note that this choice of type I error is conservative, as it increases the probability of rejecting the null hypothesis and therefore it becomes more difficult to conclude there is potential effectiveness of EI over SOC (compared to the 0.05 level typical for Phase III trials) [76].
The statistical power for the primary analysis under various assumptions regarding the SOC success rate, the intervention effect size, and attrition rate are shown in Table 2. With those assumptions, a total sample size of 200 (100 per group) allowed for adequate power (i.e., 80 % or more) to declare the EI futile if the success rate for EI equals that of SOC.
Table 2.
Statistical power with various success and attrition rates and effect assumptions
| Δ a |
SOC success rate = 0.02 |
SOC success rate = 0.03 |
||||
|---|---|---|---|---|---|---|
| Attrition |
Attrition |
|||||
| 5% | 10 % | 15% | 5% | 10 % | 15% | |
| 0.09 | 0.86 | 0.85 | 0.83 | 0.82 | 0.81 | 0.80 |
| 0.10 | 0.89 | 0.88 | 0.87 | 0.86 | 0.85 | 0.84 |
| 0.11 | 0.92 | 0.91 | 0.90 | 0.90 | 0.89 | 0.87 |
(Type I error rate = 0.10, one-tailed; total sample size = 200)
Δ defines the boundary of superiority. That is to say, we would consider EI to be superior to SOC only if the differential in improvement between EI and SOC is at least Δ
Study Population and Recruitment
We targeted our intervention to recently diagnosed HIV-positive persons as they entered the HIV care system. Since ART had only begun to be extensively rolled out at the time of intervention start-up, there were more ARV-naïve people in HIV care medical monitoring at that time. Due to the Department of Health expanding opportunities for HIV testing at all public sector health visits, numbers of clients entering HIV care pre-ART increased, but there was no concomitant in-depth counseling or ongoing service provision at this entry point into the HIV care continuum compared to settings such as ART services. We recruited HIV-positive women and men in the clinic waiting room between August 2010 and August 2011. Prior to their receiving their CD4 cell count results, a clinic nurse gave clients an Information Sheet describing the study (as one about SRH services for HIV-positive women and men aimed at increasing understanding of how to improve the quality of these services within the HIV care system). Those who were interested were referred to research staff who were not employed by the clinic for additional information.
To be eligible for the trial, participants had to be HIV-positive, ≥ 18 years, attending the clinic to receive their first CD4 cell count results since testing HIV-positive, not on ARVs, not pregnant, reporting unprotected vaginal or anal sex in the prior three months and/or considering conceiving within the next six months. These criteria were selected because our intervention focused on both avoidance of pregnancy and adherence to safer conception guidelines among HIV-positive individuals who were trying to conceive.
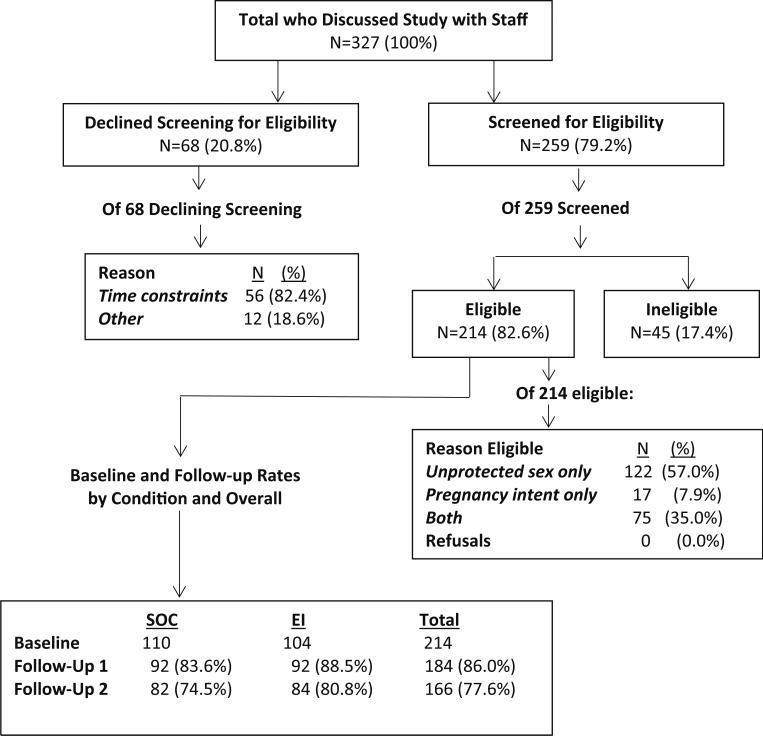
Figure 1 provides an overview of study flow. Interested individuals discussed the study with research staff (N = 327); of those, 68 (20.8 %) declined to be screened for eligibility. This was primarily due to time constraints (N = 56; 82.4 %). There were no gender differences between those who agreed to be screened and those who did not (p = 0.45). Of 259 HIV-positive women and men who were screened, 17.4 % were ineligible. We enrolled 108 women and 106 men (N = 214) who completed the Baseline (BL) interview; 184 (86.0 %) completed the Follow-Up 1 interview (FU1) and 166 (77.6 %) completed the Follow-Up 2 interview (FU2). One hundred and ten participants received counseling in a clinic randomized to the SOC, and 104 received counseling in a clinic randomized to the EI. There were no significant differences in attrition by condition.
Fig. 1.
Overview of study flow
Informed consent (including access to medical records) was obtained from all interested eligible clients. The study was approved by the Institutional Review Board at the New York State Psychiatric Institute-Columbia University Department of Psychiatry and the Human Research Ethics Committee of the University of Cape Town.
Data collection
Face-to-face interviews were conducted in isiXhosa or English, according to client preference, in privacy in the clinic, by experienced gender-matched interviewers who were blind to condition. The BL interview was administered after participants’ receipt of CD4 cell count results, except for 8 participants, who completed this interview within one month of receiving their results. FU interviews were conducted at three months (FU1) and six months (FU2) after the initial interview. Participants received 50 rand ($7.00 US at time of study) for completing the BL interview, 80 rand ($11 at time of study) for the three-month FU1 interview, and 100 rand ($14 at time of study) for the six-month FU2 interview.
To evaluate the impact of the intervention on clinic milieu, we also conducted anonymous exit interviews with clinic clients not enrolled in the trial in the waiting area to assess their perceptions of the clinic environment at baseline before the intervention started (N = 376; approximately 94 patients per clinic), and one year later (N = 306, approximately 76 per clinic). No reimbursement was provided to participants for these exit interviews.
Measures
Primary Outcome
The goal of this study was to foster safer conception among those seeking to conceive and to promote dual protection—simultaneous prevention of pregnancy and STI transmission—among those wishing to avoid pregnancy. Therefore, the primary outcome, specified a priori, was captured by a binary indicator reflecting a participant's safer sex/safer conception behavior. We considered the outcome as a “success” if, at FU2, a participant wishing to avoid pregnancy reported no condom-unprotected sex, or if a participant wishing to conceive followed safer conception guidelines. Intent to conceive was based on a ‘yes’ response to the question, “Are you thinking about trying to have a child in the next 6 months?”
Our decision to define the pregnancy prevention outcome in this way was because relying on a hormonal contraceptive without condom use ignores the health risk of STI transmission between partners, a sub-optimal public health outcome. The intervention emphasized safer conception approaches among those wishing to conceive that would minimize STI/HIV exposure for the uninfected partner.
Clinic-Level Outcome
Perceptions of the SRH-‘friendliness’ of the clinic milieu among non-enrolled clients were assessed using a 40-item measure with agree or disagree response options (sample items: It's not safe for clients to discuss their personal problems around here; There are good educational materials on HIV available for free; Staff members dislike the clients who use this service in this clinic; Clients are rarely kept waiting when they have appointments with the staff in this service; The staff go out of their way to help clients (Cronbach's alpha = 0.77).
Secondary Outcomes
These included sexual behavior and contraceptive use variables focused on behavior during the prior three-month period: number of condom-unprotected sex occasions, percentage of condom-protected sex occasions, whether dual method protection was used (i.e., condom plus an additional contraceptive), and whether all vaginal sex occasions were protected by an effective contraceptive (condoms, sterilization and/or hormonal contraceptives). We also examined changes in intention for consistent condom use (How likely is it that you will use a male or female condom every single time you have vaginal sex in the next 3 months? Would you say very unlikely, unlikely, likely, or very likely?); safer-sex self-efficacy, a 13-item scale with a four-point Likert response format assessing confidence in ability to engage in safer sex behaviors (sample item: How confident are you that you could use a male or female condom every time you have sexual intercourse with (a/your) regular partner(s) in the next 3 months? Would you say very unconfident, somewhat unconfident, somewhat confident, or confident? (Cronbach's alpha = 0.79); and self-efficacy for communicating with partner about safer sex and SRH, a 9-item scale using a four-point Likert response format (sample item: How confident are you that you could convince (a/your) regular partner(s) in the next 3 months to use condoms? (Cronbach's alpha = 0.61) using the same response format. All three measures had a range of 1-4, with higher scores reflecting the more desirable outcome. We also evaluated whether participants disclosed their HIV-positive status to their primary partner.
Reliability and Validity of Self-Reported Sexual Behavior and Contraceptive Use Measures
Although much debate has centered on the reliability and validity of self-reported sexual behavior to evaluate intervention effectiveness, there is evidence supporting the validity of self-reported condom use from studies using biological markers of STI incidence or HIV sero-conversion [see 78–82]. We took particular care in constructing sexual risk behavior and contraceptive use measures to minimize social desirability bias (see [83, 84]). We used a semi-structured interview format that builds on interviewing techniques identified by sexual science that initially emerged from Kinsey's work [85]. Participants were interviewed by gender-matched interviewers, and we used normalizing prefaces that frame sensitive behavior as normal, which evidence suggests can result in greater disclosure of unsafe behavior [86]. Specifically, questions about actual behavior were preceded by short paragraphs providing prefaces that normalized occasional non-use of condoms, and normalized desire to have children among PLWH. After the normalizing preface on condom use, participants were asked if they had ever failed to use or misused condoms for each of several reasons (e.g., there was no condom available; you got ‘carried away’; the condom was put on after sex started—before ejaculation, but after penetration), and, if so, whether that had occurred in the prior three months.
Results
Demographic and Reproductive Health Characteristics of Sample
Demographic and reproductive health characteristics of the baseline sample by intervention condition are shown in Table 3. Median age was 29, and median number of years of schooling was 11. Slightly more than half of participants reported some form of employment, predominantly self-employment in the informal sector, and 70 % resided in an informal dwelling. About three-fourths had been diagnosed with HIV within the prior year, and median CD4 count at baseline was 414. Almost all participants had someone they identified as a current main partner, with less than half living with that partner. Nearly two-thirds (64.1 %) had disclosed their HIV-positive status to their main partner. Median number of biological children was 1, and median number of children cared for was 2. Almost all (98.1 %) participants were sexually active, and 82.5 % reported unprotected sex in the prior three months. Nearly two-fifths indicated that they/their partner used a hormonal contraceptive (pill or injectable) in the prior three months, 32.2 % had used dual-method protection, and 52.1 % reported that all vaginal sex occasions were protected by effective contraception. Forty-three percent were considering conceiving a child in the near future, and nearly a third indicated that their main partner was similarly interested. Self-efficacy for safer sex and for communicating with a partner about safer sex and reproductive health was fairly high (3.7 on a 4-point scale). There were no significant differences in any sample characteristics between conditions.
Table 3.
Demographic and reproductive health characteristics of recently-diagnosed HIV-positive women and men at the start of a clinic-based SRH integration trial by intervention condition, Cape Town, 2010–2011 (N = 214)
| Characteristics | Total sample | SOC | EI | p value |
|---|---|---|---|---|
| Total, n (%) | 214 (100.0 %) | 110 (51.4 %) | 104 (48.6 %) | NA |
| Median age (Q1–Q3)d | 29 (25–35) | 30 (26–35) | 29 (24–35) | 0.272c |
| Median education level median (Q1–Q3)d | 11 (9–11) | 11 (9–12) | 10 (9–11) | 0.128c |
| Female | 108 (50.5 %) | 56 (50.9 %) | 52 (50.0 %) | 0.894a |
| Employed full-time/part-time/self-employed | 116 (54.2 %) | 64 (58.2 %) | 52 (50.0 %) | 0.288a |
| Informal dwelling | 149 (70.1 %) | 77 (70.0 %) | 73 (70.2 %) | 1.000a |
| Diagnosis within one year | 159 (74.3 %) | 81 (73.6 %) | 78 (75.0 %) | 0.943a |
| Median CD4 count (Q1–Q3)d | 414.5 (326.5–538.5) | 419.0 (331–560) | 405.0 (316–525) | 0.557c |
| Had current main partner | 209 (97.7 %) | 107 (97.3 %) | 102 (98.1 %) | 1.000a |
| Lives with main partner | 98 (46.9 %) | 53 (49.5 %) | 45 (44.1 %) | 0.519a |
| Disclosed HIV status to partner | 134 (64.1 %) | 71 (66.4 %) | 63 (61.8 %) | 0.584a |
| Median number of biological children (Q1–Q3)d | 1.48 (0–2) | 1.42 (0–2) | 1.54 (0–2) | 0.372c |
| Median number of children caring for (Q1–Q3)d | 2.61 (1–4) | 2.44 (1– 4) | 2.79 (1–4) | 0.346c |
| Sexually active | 210 (98.1 %) | 108 (98.2 %) | 102 (98.1 %) | 0.955a |
| Had condom-unprotected vaginal sex in prior 3 months | 172 (82.5 %) | 94 (86.2 %) | 78 (76.5 %) | 0.068a |
| Relied on 100 % effective contraception during all vaginal sex occasions in prior 3 months | 110 (52.1 %) | 58 (53.2 %) | 52 (51.0 %) | 0.746a |
| Pill/injectable use in prior 3 months | 84 (39.3 %) | 48 (43.6 %) | 36 (34.6 %) | 0.226a |
| Used dual method protection in prior 3 months | 69 (32.2 %) | 42 (38.2 %) | 27 (26.0 %) | 0.056a |
| Participant considering pregnancy immediately | 92 (43.0 %) | 47 (42.7 %) | 45 (43.3 %) | 0.936a |
| Partner considering pregnancy immediately | 61 (29.6 %) | 31 (29.5 %) | 30 (29.5 %) | 1.000a |
| Mean self-efficacy for communicating with partner re: safer sex and SRH | 3.71 (0.32) | 3.69 (0.36) | 3.72 (0.28) | 0.501b |
| Mean self-efficacy for safer sex | 3.74 (0.32) | 3.72 (0.36) | 3.64 (0.53) | 0.324b |
p value calculated from Pearson's χ2 test
p value calculated from independent two sample t-test
p value calculated from Mood's median test
Q1: first quartile, 25 %-tile; Q3: third quartile, 75 %-tile
Primary Analysis
In the primary analysis, we did not reject the null hypothesis that the “success” rate for EI exceeded by 10 % or more the success rate of SOC, i.e. PEI – PSOC ≥ 0:10 (PSOC = 0.72, PEI = 0.83, PEI – PSOC = 0.11, 95 % CI −0.04, 0.27), p = 0.58). This indicates that we cannot rule out the possibility of EI being a truly effective intervention for promoting safer sex and safer conception. Findings were similar for men (PSOC = 0.76, PEI = 0.87, PEI – PSOC = 0.11, 95 % CI (−0.05, 0.28), p = 0.57) and women (PSOC = 0.67, PEI = 0.78, PEI – PSOC = 0.11, 95 % CI (−0.11, 0.33), p = 0.53).
Secondary Analyses
We did not conduct hypothesis testing for secondary outcomes; therefore, no p values are reported. Instead, we provide descriptive statistics (i.e., the means and proportions) to help understand the direction of EI intervention effect on key secondary outcomes and we did not and should not declare or suggest any findings from secondary analysis as statistically significant. Considering the clinic-level outcome (perceptions about the clinic milieu), the following cross-sectional estimates were observed at baseline and 12-month follow-up: baseline: SOC = 3.13, EI = 3.11; FU2: SOC = 2.82, EI = 3.16). Longitudinal (individual-level) data are reported for secondary outcomes, according to condition and time point in Table 4. (The change over time for those outcomes can be obtained by simply subtracting BL from FU values and therefore are not presented in the table.) Participants receiving EI counseling reported greater improvement from baseline to FU1 and from baseline to FU2 on percentage of condom-protected sex occasions (SOC: 33 % increase from BL to FU1 and 39 % increase from BL to FU2 vs. EI: 51 % increase from BL to FU1 and 53 % increase from BL to FU2); fewer unprotected sex occasions (SOC: decrease of approximately 10 unprotected sex occasions at FU1 and FU2 vs. EI: decrease of approximately 17 unprotected sex occasions at FU1 and FU2); and intention for consistent condom use (group difference in average change over time on a four-point scale: SOC: .07 increase from BL to FU1 and .24 increase from BL to FU2 vs. EI: .52 increase from BL to FU1 and .49 from BL to FU2). EI participants also showed greater safer-sex self-efficacy at FU1 (average change over time on a four-point scale: SOC: .05 decrease from BL to FU1 and .09 decrease from BL to FU2 vs. EI: .07 increase from BL to FU1 and .04 decrease from BL to FU2), although both groups saw a slight decrease in rating partner communication self-efficacy (average change over time on a four-point scale: SOC: .0 decrease from BL to FU1 and .11 decrease from BL to FU2 vs. EI: .06 decrease from BL to FU1 and .06 decrease from BL to FU2). There were greater increases among EI participants in the proportion of participants reporting dual-method protection (i.e., using condoms plus another contraceptive) (SOC: an additional 11 % reported use at FU1 and approximately 12 % additional users at FU2 vs. EI: an increase from BL to FU1 of 29 % and approximately 22 % increase from BL to FU2), whether all vaginal sex occasions were protected by an effective contraceptive (SOC: a 29 % increase from BL to FU1 and a 22 % increase from BL to FU2 vs. EI: a 46 % increase from BL to FU1 and a 43 % increase from BL to FU2). More participants in the EI condition had disclosed their HIV status to their main partner by first follow-up (SOC: 67.8 % vs. EI: 76.5 %), although by FU2 the proportion of individuals who had disclosed their HIV status was nearly identical in both groups.
Table 4.
Secondary analysis comparing EI to SOC on improvement (from baseline to FU2) for key secondary outcome variables among recently-diagnosed HIV-positive women and men, Cape Town
| Dependent variable | Group | Mean (SD) or N (%) |
||
|---|---|---|---|---|
| Baseline | FU1 | FU2 | ||
| Intention for consistent condom use | SOC | 3.61 (0.68) | 3.68 (0.68) | 3.85 (0.43) |
| EI | 3.31 (0.91) | 3.83 (0.43) | 3.80 (0.56) | |
| Percentage of condom-protected sex occasions in prior 3 months | SOC | 0.49 (0.38) | 0.82 (0.33) | 0.88 (0.29) |
| EI | 0.40 (0.37) | 0.91 (0.25) | 0.95 (0.14) | |
| Number of condom-unprotected sex occasions in prior 3 months | SOC | 13.40 (14.33) | 3.58 (9.68) | 3.21 (10.40) |
| EI | 17.59 (17.74) | 1.04 (3.82) | 0.77 (2.35) | |
| Self-efficacy for safer sex | SOC | 3.76 (0.26) | 3.71 (0.27) | 3.67 (0.25) |
| EI | 3.72 (0.36) | 3.79 (0.29) | 3.68 (0.34) | |
| Self-efficacy for communicating with partner re: safer sex and SRH | SOC | 3.71 (0.30) | 3.63 (0.34) | 3.60 (0.30) |
| EI | 3.68 (0.39) | 3.62 (0.35) | 3.62 (0.34) | |
| Pill/injectable use in prior 3 months | SOC | 48 (43.6 %) | 40 (45.5 %) | 37 (49.3 %) |
| EI | 36 (34.6 %) | 45 (48.9 %) | 35 (47.3 %) | |
| Used dual-method protection in prior 3 months | SOC | 42 (38.2 %) | 29 (49.2 %) | 30 (50.0 %) |
| EI | 27 (26.0 %) | 29 (54.7 %) | 29 (47.5 %) | |
| Relied on effective contraception during all vaginal sex occasions in prior 3 months | SOC | 58 (52.7 %) | 50 (87.7 %) | 48 (85.7 %) |
| EI | 52 (50.0 %) | 49 (96.1 %) | 49 (92.5 %) | |
| Disclosed HIV status to partner | SOC | 71 (66.4 %) | 40 (67.8 %) | 47 (81.0 %) |
| EI | 63 (61.8 %) | 39 (76.5 %) | 44 (81.5 %) | |
In Table 5, we present the variables in Table 4 by gender. Men in both conditions reported a higher percentage of condom-protected sex occasions and fewer unprotected sex occasions at all three time points than did women, and a slightly higher proportion of men than women reported use of 100 % effective contraception at both follow-up assessments. Across all time points, a greater proportion of men than women had disclosed their HIV status to their main partner. The data suggest that women and men did not respond differently to SOC or EI.
Table 5.
Secondary analysis comparing EI to SOC by gender on improvement (from baseline to FU2) for key secondary outcome variables among recently-diagnosed HIV-positive women and men, Cape Town
| Dependent variable | Group | Mean (SD)/N (%) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline |
FU1 |
FU2 |
|||||
| Female | Male | Female | Male | Female | Male | ||
| Intention for consistent condom use | SOC | 3.70 (0.62) | 3.52 (0.72) | 3.65 (0.74) | 3.76 (0.48) | 3.80 (0.49) | 3.94 (0.24) |
| EI | 3.54 (0.70) | 3.07 (1.03) | 3.81 (0.47) | 3.88 (0.34) | 3.80 (0.61) | 3.79 (0.49) | |
| Percentage of condom-protected sex occasions in prior 3 months | SOC | 0.46 (0.36) | 0.52 (0.40) | 0.77 (0.36) | 0.96 (0.14) | 0.83 (0.34) | 0.99 (0.02) |
| EI | 0.38 (0.35) | 0.42 (0.40) | 0.87 (0.29) | 1.00 (0.00) | 0.93 (0.17) | 0.98 (0.09) | |
| Number of condom-unprotected sex occasions in prior 3 months | SOC | 10.77 (12.02) | 16.19 (16.06) | 4.60 (10.89) | 0.31 (1.11) | 4.56 (12.26) | 1.21 (2.93) |
| EI | 14.04 (15.64) | 21.22 (19.13) | 1.49 (4.51) | 0.00 (0.00) | 0.12 (0.49) | 0.25 (1.22) | |
| Self-efficacy for safer sex | SOC | 3.73 (0.30) | 3.79 (0.21) | 3.71 (0.26) | 3.70 (0.29) | 3.71 (0.21) | 3.58 (0.29) |
| EI | 3.79 (0.26) | 3.64 (0.45) | 3.75 (0.31) | 3.88 (0.21) | 3.65 (0.29) | 3.71 (0.36) | |
| Self-efficacy for communicating with partner re: safer sex and SRH | SOC | 3.66 (0.34) | 3.76 (0.25) | 3.63 (0.33) | 3.62 (0.39) | 3.61 (0.27) | 3.58 (0.35) |
| EI | 3.74 (0.27) | 3.62 (0.47) | 3.67 (0.37) | 3.87 (0.23) | 3.57 (0.35) | 3.69 (0.40) | |
| Pill/injectable use in prior 3 months | SOC | 27 (48.2 %) | 21 (38.9 %) | 26 (52.0 %) | 14 (36.8 %) | 23 (53.5 %) | 14 (43.8 %) |
| EI | 23 (44.2 %) | 13 (25.0 %) | 31 (63.3 %) | 14 (32.6 %) | 17 (48.6 %) | 18 (46.2 %) | |
| Used dual-method protection in prior 3 months | SOC | 22 (39.3 %) | 20 (37.0 %) | 23 (51.1 %) | 6 (42.9 %) | 22 (52.4 %) | 8 (44.4 %) |
| EI | 16 (30.8 %) | 11 (21.2 %) | 25 (67.6 %) | 4 (25.0 %) | 15 (42.9 %) | 14 (53.8 %) | |
| Relied on effective contraception during all vaginal sex occasions in prior 3 months | SOC | 32 (57.1 %) | 26 (48.1 %) | 37 (84.1 %) | 13 (100.0 %) | 32 (82.1 %) | 16 (94.1 %) |
| EI | 29 (55.8 %) | 23 (44.2 %) | 34 (94.4 %) | 15 (100 %) | 26 (89.7 %) | 23 (95.8 %) | |
| Disclosed HIV status to partner | SOC | 34 (60.7 %) | 37 (72.5 %) | 28 (63.6 %) | 12 (80.0 %) | 30 (75.0 %) | 17 (94.4 %) |
| EI | 34 (66.7 %) | 29 (56.9 %) | 25 (69.4 %) | 14 (93.3 %) | 19 (67.9 %) | 25 (96.2 %) | |
In a subgroup analysis of those not seeking pregnancy at BL (N = 134) (not tabled), those receiving EI counseling had greater improvement from baseline to FU2 on whether dual-method protection was used (SOC 44.0 to 50.0 % vs. EI 33.9 to 42.2 %), on whether all vaginal sex occasions were protected by an effective contraceptive including condoms alone (SOC 61.3 to 84.0 % vs. EI 55.9 to 94.7 %), and on consistent condom use (SOC 16.2 to 76.0 % vs. EI 10.3 to 86.8 %).
Since we lack the statistical power to do meaningful subgroup analysis of those who were interested in conceiving by condition, we provide descriptive data. We identified 80 participants at baseline with intent to conceive (17 women and 63 men). An additional three women indicated intent to conceive at the first follow-up interview. Among all individuals reporting intent to conceive in the coming 12 months at either baseline or first follow-up, we had no follow-up data from 10 men (15.9 % of 63 men). Among those with follow-up data, nine women (45.0 %) and 52 men (82.5 %) had decided not to seek pregnancy at subsequent follow-up; all but 3 of these participants still desired to conceive, but not in the next 12 months (95 % of those with follow-up data). There were no discernible differences by condition. Among the 11 remaining women with pregnancy intent at baseline or first follow-up, three did not follow safer conception guidelines, three reported 100 % condom use during the subsequent period(s), one reported practicing timed intercourse, one sought consultation with a physician before attempting to conceive, one delayed attempting to conceive and was awaiting results of medical testing, one reported using self-insemination with partner sperm, and one woman's practices were not determinable. The sole man who reported continued interest in conceiving at follow-up did not follow safer-conception guidelines. Three of the four women reporting safer conception practices at follow-up had received EI counseling, and the woman who had received SOC counseling reported having consulted with a physician. Eight participants (4 men and 4 women, half of whom received the EI and half SOC) reported a pregnancy or partner pregnancy over the course of follow-up. Two of these pregnancies were unintended (1 EI woman and 1 SOC woman). Note that although being pregnant at baseline was an exclusion criterion, this was assessed by self-report, so it is possible that some of these pregnancies occurred prior to the six-month observational period.
Discussion
This is one of the first trials to assess an intervention that integrated both contraceptive and safer conception services into HIV care and treatment. Few interventions have been implemented to promote contraceptive use and/or safer conception; moreover, SRH interventions that include HIV-positive men are limited. Unlike integration studies that provide SRH services in ARV treatment settings [37, 43], our intervention was targeted to newly-diagnosed HIV-positive individuals who were not eligible for ARV treatment at time of study recruitment. Our study differs from other rigorously evaluated SRHHIV integration initiatives by our focus on both dual protection and safer conception, our inclusion of a clinic-level component to improve the ‘reproductive health friendliness’ of the clinics, and provision of SRH services to men.
Results from the primary analysis of this proof-of-concept study, using a futility design, showed that the enhanced intervention has merit. We were unable to reject the null hypothesis that EI is superior to SOC by at least 10 % points. Eighty-three percent of participants who received the EI vs. 72 % of those receiving SOC followed safer sex or safer conception guidelines at follow-up. The results indicate that a larger-scale confirmatory study to test whether the EI is indeed superior to SOC, perhaps using a clinic-based, cluster randomized controlled design, is of interest.
Since this Phase 2 trial was not powered to evaluate secondary outcomes, we reported these as exploratory findings only. Those analyses provided a suggestion of potential greater improvement among those receiving the EI (vs. SOC) in consistent condom use, percent reliance on condoms, safer sex self-efficacy, self-efficacy for communicating with partner, and decreased condom-unprotected sex. Subgroup analyses examining dual method protection and effective contraceptive use among those seeking to avoid pregnancy were also in the expected direction, with a greater proportion of those in the EI group reporting consistent use of effective contraception than the SOC group. Descriptive data on participants who were interested in getting pregnant in the near future indicated fairly high interest at baseline (43 % of participants and 29.6 % of their partners), although most (95 % of those with follow-up data) decided to postpone plans for getting pregnant to the following year. It may be that discussion about the benefits of stabilizing health before seeking pregnancy, recommendations by counselors to avoid unprotected sex, or simply that the reality of health challenges struck home after participants first enrolled in HIV care, giving pause to participants’ pregnancy timeline. Because of shifting rates of pregnancy intent at follow-up, most of the change in our major outcome (following safer sex or safer conception guidelines) was due to decreases in condom-unprotected sex and increases in condom use. Due to the small sample size, we could not meaningfully evaluate differences by condition among those seeking pregnancy at baseline or follow-up.
It is unclear what the differences in EI and SOC client perception of the clinic milieu mean, since within condition, ratings of milieu in EI clinics were stable whereas ratings at SOC sites significantly decreased.
We note a number of limitations. All persons screened for eligibility were interested in participating in a study of SRH, and among the total screened sample, only 8.6 % of men and 22.2 % of women reported neither pregnancy intent nor unprotected sex, which may be atypical, although other studies have found similar rates of pregnancy intent among HIV ? women [87, 88] and men [27]. The 76 % of screened participants reporting unprotected sex is similar to the estimate of approximately 74 % found in a prospective study of HIV-positive women in South Africa [15]. These are similar to the baseline levels of 79 to 83 % found in a study of HIV-positive women in Kenya that evaluated contraceptive service integration [29]. A further limitation is reliance on self-reported outcomes, with the attendant issue of social desirability. While bias should be similar in both study arms, given similar intensities of counseling in EI and SOC and no differences between conditions on all reproductive health characteristics at baseline, EI participants could be more likely to over-report condom use because the intervention emphasized condom use. However, we believe that the way we assessed sexual behavior and pregnancy intent helped to increase willingness to disclose stigmatized behavior. Finally, our intervention was targeted to newly-diagnosed HIV-positive individuals who were not eligible for ART at time of recruitment; with the new WHO guidelines of treatment for all, there is a need to revise the content of our counseling to fit the needs of HIV-positive people who initiate ART.
With recognition that choices about fertility among PLWH is a basic human right, strategies for helping them realize their reproductive goals must remain a priority [1–3]. Issues of childbearing and safer conception can no longer be sidelined in public health policies and clinical practice. The need for integrated SRH services for PLWH that address STI prevention, contraceptive and conception needs, as well as models that attend to multi-level patient and provider factors [89] are reflected in high HIV-related maternal mortality in South Africa, where 34.7 % of maternal deaths were due primarily to AIDS and other non-pregnancy-related infections [90, 91] and high HIV prevalence among reproductive-aged women.
Interventions to promote safer conception are in their embryonic stages of development and implementation. A safer conception service recently launched at a primary health center in Johannesburg for HIV-positive women (and their partners) who intended to conceive in the coming six months was found to be feasible [92], with most participants favoring timed unprotected intercourse, manual insemination and combined ART, and few opting for pre-exposure prophylaxis (PrEP). We are unaware of any published clinical trials that have examined integration of HIV and true comprehensive ‘family planning’ services that address both contraceptive and safer conception needs. Such service integration needs to be targeted to both the clinic- or health systems level and individual-level [39].
Since the end of our study, the landscape of fertility options in public-sector clinics for HIV-positive women and men who want to conceive in ways that reduce HIV transmission risk to uninfected partners and to infants has been changing in some sub-Saharan countries. South Africa has pioneered the development of safer conception guidelines that outline higher and lower cost options [93]. These could serve as a model for health care providers and counselors in settings where laboratory-based assisted reproduction is neither widely available nor affordable. Research in South Africa has documented high levels of interest in procreation among PLWH [27, 94, 95] and acceptability of safer conception services [64, 94–98].
“Putting integration into practice” is a priority of the South African government. This means that evidence-based SRH counseling that fosters discussion of actual reproductive intent with HIV-positive persons should be routinely offered for HIV-affected couples desiring children in conjunction with services that promote contraceptive uptake for those who do not desire children in sub-Saharan Africa. This dual focus is articulated in South Africa's 2012 National Contraception and Fertility Planning Policy and Service Delivery Guidelines [99]. However, to move from the theoretical to programmatic implementation, adequate funding to support such programs, standardized counseling protocols, and training of health care providers in contraception and safer conception will be needed. Other health system issues, such as space, workload, staff supervision, patient flow, and ongoing monitoring and evaluation and accountability, will also need to be addressed [39].
The extent to which health care providers in South African public-sector clinics have been trained in safer conception and to which safer conception guidelines have been routinely implemented in practice is unknown. However, a recent qualitative study with health care providers and HIV-affected women and men from one Johannesburg primary clinic suggests some knowledge of safer conception methods, but that discussions about fertility planning were initiated by patients, not providers, thus reflecting non-optimal implementation of the guidelines [100]. In a study in two Durban ART clinics, providers expressed discomfort in providing safer conception services to HIV-serodiscordant couples [101], and in another Durban study, none of the 35 male and female clients reported receiving counseling on specific safer conception methods [60]. Similarly, in a study in Kisumu, Kenya, providers reported that they did not routinely offer standardized preconception counseling messages to HIV-serodiscordant couples [102].
Conclusion
In an effort to achieve the UNAIDS 90-90-90 target by 2020, testing promising interventions that appropriately address fertility desires of HIV-positive individuals—both those who want to avoid or defer childbearing and those who want to conceive—within the context of real-world, overburdened and under-resourced, public-sector clinic settings is essential for determining their readiness for scale-up. Results from this trial suggest that the intervention has merit and should be more rigorously evaluated in a Phase III trial with biological endpoints in South Africa and other high HIV burden, resource-constrained settings in sub-Saharan Africa. This is all the more important as countries move toward universal treatment of HIV-positive people, irrespective of their CD4 count and HIV viral load.
Acknowledgments
This research was funded by the National Institute of Mental Health (R01-MH078770, Principal Investigator: Joanne E. Mantell, MS, MSPH, Ph.D; South African Site Principal Investigator, Diane Cooper, Ph.D) and a Center Grant [NIMH P30 MH43520; Principal Investigators: Anke A. Ehrhardt, Ph.D (1987–2013)/Robert H. Remien, Ph.D. (2013–2018)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMH and others. The authors appreciate the women and men who volunteered their time to participate in this study and support from the Departments of Health in the Western Cape and City of Cape Town. The authors acknowledge the contributions of Chelsea Morroni, Ph.D, MBChB, and Keith Cloete, MBChB. We are grateful for the contribution of Linda-Gail Bekker, MBChB, Ph.D, to nurse training in safer conception methods and to all members of the study Intervention Development Working Group and the Scientific Expert Panel for their valuable intervention input.
Footnotes
Compliance with Ethical Standards
Conflict of Interest None of the authors have declared that they have a conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Gruskin S, Ferguson L, O'Malley J. Ensuring sexual and reproductive health for people living with HIV: an overview of key human rights, policy and health systems issues. Reprod Health Matters. 2007;15(29 Suppl):4–26. doi: 10.1016/S0968-8080(07)29028-7. [DOI] [PubMed] [Google Scholar]
- 2.Gruskin S. Reproductive and sexual rights: do words matter? Am J Public Health. 2008;98(10):1737. doi: 10.2105/AJPH.2008.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcher R, Cates W, Jr, Baeten JM. Family planning and HIV: two steps forward. AIDS. 2013;27(Suppl 1):S1–4. doi: 10.1097/QAD.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 4.Collins PY, Holman AR, Freeman M, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS. 2006;20:1571–82. doi: 10.1097/01.aids.0000238402.70379.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedland G, Harries A, Coetzee D. Implementation issues in tuberculosis/HIV program collaboration and integration: 3 case studies. J Infect Dis. 2007;196(Suppl 1):S114–23. doi: 10.1086/518664. [DOI] [PubMed] [Google Scholar]
- 6.Mayhew S. Integration of STI services into FP/MCH services: health service and social contexts in rural Ghana. Reprod Health Matters. 2000;8:112–24. doi: 10.1016/s0968-8080(00)90193-9. [DOI] [PubMed] [Google Scholar]
- 7.Maharaj P, Cleland J. Integrating sexual and reproductive health services: lessons from KwaZulu-Natal, South Africa. Health Policy Plan. 2005;20:310–9. doi: 10.1093/heapol/czi038. [DOI] [PubMed] [Google Scholar]
- 8.Paiva V, Santos N, Franca-Junior I, Filipe E, Ayres JR, Segurado A. Desire to have children: gender and reproductive rights of men and women living with HIV: a challenge to health care in Brazil. AIDS Patient Care STDs. 2007;21(4):268–77. doi: 10.1089/apc.2006.0129. [DOI] [PubMed] [Google Scholar]
- 9.Williams CD, Finnerty JJ, Newberry RN, West RW, Thomas TS, Pinkerton JV. Reproduction in couples who are affected by human immunodeficiency virus: medical, ethical and legal considerations. Am J Obstet Gynecol. 2013;189(2):333–41. doi: 10.1067/s0002-9378(03)00676-8. [DOI] [PubMed] [Google Scholar]
- 10.Soto TA, Bell J, Pillen MB. Literature on integrated HIV care: a review. AIDS Care. 2014;16(1):43–55. doi: 10.1080/09540120412331315295. [DOI] [PubMed] [Google Scholar]
- 11.Joint UN Program on HIV/AIDS (UNAIDS). Global report UNAIDS on AIDS epidemic. 2008 http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp.
- 12.MDG. United Nations [14 Oct 2015];Millennium Development Goals. 2010 http://www.un.org/millenniumgoals/.
- 13.US Department of State [06 Apr 2016];The Office of the Global AIDS Coordinator PEPFAR Blueprint: Creating an AIDS-free Generation. 2012 Nov; http://www.pepfar.gov/documents/organization/201386.pdf.
- 14.United Nations UN General Assembly 2016 political declaration zero draft. [24 Apr 2016];On the fast-track to end AIDS in the age of sustainable development. 2016 http://www.icaso.org/media/files/24060-ZeroDraftPDfinal.pdf.
- 15.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams EJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in sub-Saharan Africa: a cohort study. Plos Med. 2010;7(2):1–10. doi: 10.1371/journal.pmed.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaba B, Calvert C, Marston M, et al. Effect of HIV infection on pregnancy-related mortality in sub-Saharan Africa: secondary analyses of pooled community-based data from the network for analysing longitudinal population-based HIV/AIDS data on Africa (ALPHA). Lancet. 2013;381:1763–71. doi: 10.1016/S0140-6736(13)60803-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav. 2009;13:949–68. doi: 10.1007/s10461-009-9537-y. [DOI] [PubMed] [Google Scholar]
- 18.Dube AL, Baschieri A, Cleland J, et al. Fertility intentions and use of contraception among monogamous couples in northern Malawi in the context of HIV testing: a cross-sectional analysis. Plos One. 2012;7:e51861. doi: 10.1371/journal.pone.0051861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bankole A, Biddlecom AE, Dzekedzek K. Women's and men's fertility preferences and contraceptive behaviors by HIV status in 10 sub-Saharan African countries. AIDS Educ Prev. 2011;23:313–28. doi: 10.1521/aeap.2011.23.4.313. [DOI] [PubMed] [Google Scholar]
- 20.Oladapo OT, Daniel OJ, Odusoga OL, et al. Fertility desires and intentions of HIVpositive patients at a suburban specialist centre. J Natl Med Assoc. 2005;97:1672–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Marston M, Nakiyingi-Miiro J, Hosegood V, Lutalo T, Mtenga B, Zaba B, et al. Measuring the impact of antiretroviral therapy roll-out on population level fertility in three African countries. Plos One. 2016;11(3):e0151877. doi: 10.1371/journal.pone.0151877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand A, Shiraishi RW, Bunnell RE, Jacobs K, Solehdin N, Abdul-Quader AS, et al. Knowledge of HIV status, sexual risk behaviors and contraceptive need among people living with HIV in Kenya and Malawi. AIDS. 2009;23(12):1565–73. doi: 10.1097/QAD.0b013e32832cb10c. [DOI] [PubMed] [Google Scholar]
- 23.KNBoS, Macro I. [March 10, 2015];Kenya Demographic and Health Survey 2008–09. 2010 http://www.measuredhs.com/pubs/pdf/fr229/ fr229.pdf.
- 24.Smart T. PEPFAR: unexpected and unwanted pregnancies in women on ART highlights family planning gap. Aidsmap News; 2006. [Google Scholar]
- 25.World Health Organization (WHO) and the United Nations Population Fund (UNFPA) The Glion call to action on family planning and HIV/AIDS in women and children. http://www.unfpa.org/sites/default/files/pub-pdf/glion_rhandhiv.pdf.2004.
- 26.Schwartz SR, Rees H, Mehta S, Venter WDF, Taha TE, Black V. High incidence of unplanned pregnancy after antiretroviral therapy initiation: Findings from a prospective cohort study in South Africa. Plos One. 2012;7(4):e36039. doi: 10.1371/journal.pone.0036039. doi:10.1371/journal.pone.0036039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper D, Moodley J, Zweigenthal V, Bekker LG, Shah I, Myer L. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009;13:38–46. doi: 10.1007/s10461-009-9550-1. [DOI] [PubMed] [Google Scholar]
- 28.Grossman D, Onono M, Newmann SJ, Blat C, Bukusi EA, Shade SB, et al. Integration of family planning services into HIV care and treatment in Kenya: a cluster-randomized trial. AIDS. 2013;27(Suppl 1):S77–85. doi: 10.1097/QAD.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 29.Shade SB, Kevany S, Onono M, Ochieng G, Steinfeld RL, Grossman D, Newmann SJ, Blat C, Bukusi EA, Cohen CR. Cost, cost-efficiency and cost-effectiveness of integrated family planning and HIV services. AIDS. 2013;27(Suppl 1):S87–92. doi: 10.1097/QAD.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy CE, Spaulding AB, Brickley DB, Almers L, Mirjahangir J, Packel L, et al. Linking sexual and reproductive health and HIV interventions: a systematic review. J Int AIDS Soc. 2010;13:26. doi: 10.1186/1758-2652-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy CE, Medley AM, Sweat MD, O'Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ. 2010;88:615–23. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindegren ML, Kennedy CE, Bain-Brickley D, Azman H, Creanga AA, Butler LM, et al. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services (Review). The Cochrane Collaboration. 10. The Cochrane Library; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcher R, Hoke T, Adamchak SE, Cates W., Jr Integration of family planning into HIV services: a synthesis of recent evidence. AIDS. 2013;27(Suppl 1):S65–75. doi: 10.1097/QAD.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 34.Mayhew SH, Ploubidis GB, Sloggett A, Church K, Obure CD, Birdthistle I, et al. Innovation in evaluating the impact of integrated service-delivery: the Integra indexes of HIV and reproductive health integration. Plos One. 2016;11(1):e0146694. doi: 10.1371/journal.pone.0146694. doi:10.1371/journal.pone.0146694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantell J, Smit J, Stein Z. The right to choose parenthood among HIV-infected women and men. J Public Health Policy. 2009;30(4):367–78. doi: 10.1057/jphp.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper D, Mantell JE, Moodley J, Mall S. The HIV epidemic and sexual and reproductive health policy integration: views of South African policymakers. BMC Public Health. 2015;15:217. doi: 10.1186/s12889-015-1577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamchak SE, Grey TE, Otterness C, Katz K, Janowitz B. [04 Apr 2016];Introducing family planning services into antiretroviral programs in Ghana: an evaluation of a pilot intervention. 2007 file:///E:/My%20Documents/Integration%20of%20RH%20and%20HIV%20Services/Adamchak%20Introducing%20FP%20into%20ARV%20Programs%20in%20Ghana.pdf.
- 38.McCarraher DR, Vance G, Gwarzo U, Taylor D, Chabikuli ON. Changes in contraceptive use following integration of family planning into ART services in Cross River State, Nigeria. Stud Fam Plan. 2011;42(4):283–90. doi: 10.1111/j.1728-4465.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 39.Hope R, Kendall T, Langer A, Bärnighausen T. Health systems integration of sexual and reproductive health and HIV services in sub-Saharan Africa: a scoping study. J Acquir Immune Defic Syndr. 2014;67:S259–70. doi: 10.1097/QAI.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obure CD, Guinness L, Sweeney S, Vassall A. Integra initiative. Does integration of HIV and SRH services achieve economies of scale and scope in practice? A cost function analysis of the Integra Initiative. Sex Transm Infect. 2016;92:130–4. doi: 10.1136/sextrans-2015-052039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obure CD, Jacobs R, Guiness L, Mayhew S, Vassall A. Integra initiative. Does integration of HIV and sexual and reproductive health services improve technical efficiency in Kenya and Swaziland? An application of a two-stage semi parametric approach incorporating quality measures. Soc Sci Med. 2016;151:147–56. doi: 10.1016/j.socscimed.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Church K, Wringe A, Lewin S, Ploubidis GB, Fakudze P. Integra Initiative, Mayhew SH. Exploring the feasibility of service integration in a low-income setting: a mixed methods investigation into different models of reproductive health and HIV care in Swaziland. Plos One. 2015;10(5):e0126144. doi: 10.1371/journal.pone.0126144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phiri S, Feldacker C, Chaweza T, Mlundira L, Tweya H, Speight C, Samala B, Kachale F, Umpierrez D, Haddad L, for the Lighthouse Group Integrating reproductive health services into HIV care: strategies for successful implementation in a low-resource HIV clinic in Lilongwe, Malawi. J Fam Plann Reprod Health Care. 2016;42:17–23. doi: 10.1136/jfprhc-2013-100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosgei RJ, Lubano KM, Shen C, Wools-Kaloustina KK, Musick BS, Siika AM, Mabeya H, Carter EJ, Mwangi A, Kiaroe J. Impact of integrated family planning and HIV care services on contraceptive use and pregnancy outcomes: a retrospective cohort study. J Acquir Immune Defic Syndr. 2011;58(5):e121–6. doi: 10.1097/QAI.0b013e318237ca80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman SJ, Grossman D, Blat C, Onono M, Steinfeld R, Bukusi EA, Shade S, Cohen CR. Does integrating family planning into HIV care and treatment impact intention to use contraception? Patient perspectives from HIV-infected individuals in Nyanza Province, Kenya. Int J Gynecol Obstet. 2013;123:e16–23. doi: 10.1016/j.ijgo.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, Labadarios D, Onoya D, et al. South Africa national HIV prevalence, incidence and behaviour survey 2012. HSRC Press; Cape Town: 2014. [DOI] [PubMed] [Google Scholar]
- 47.Department of Health, Medical Research Council, OrcMacro . South African demographic and household survey 2003. Department of Health; Pretoria: 2007. [Google Scholar]
- 48.Department of Health, Republic of South Africa . The 2012 national antenatal sentinel HIV and herpes simplex type-2 prevalence survey, South Africa. National Department of Health; [10 Mar 2015]. http://www.health-e.org.za/wp-content/uploads/2014/05/ASHIVHerp_Report2014_22May2014.pdf. [Google Scholar]
- 49.Western Cape Government, Department of Health National antenatal sentinel HV prevalence survey, South Africa. Internal report. 2014 Jun; [Google Scholar]
- 50.Sherman GG, Lilian RR, Bhardwaj S, Candy S, Barron P. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. SAMJ. 2014;104(3):235–8. doi: 10.7196/samj.7598. [DOI] [PubMed] [Google Scholar]
- 51.Statistics South Africa Mid-year population estimates. [10 Apr 2016];Statistical release P0302. 2015 http://www.statssa.gov.za/publications/P0302/P03022015.pdf.
- 52.Kwale P, Mindry D, Phoya A, Jansen P, Hoffman RM. Provider attitudes about childbearing and knowledge of safer conception at two HIV clinics in Malawi. Reprod Health. 2015;2:17. doi: 10.1186/s12978-015-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz SR, Mehta SH, Taha TE, et al. High pregnancy intentions and missed opportunities for patient-provider communication about fertility in a South African cohort of HIV-positive women on antiretroviral therapy. AIDS Behav. 2012;16:69–78. doi: 10.1007/s10461-011-9981-3. [DOI] [PubMed] [Google Scholar]
- 54.van Der Spuy Z. HIV and reproductive health: a South African experience. Reprod Biomed Online. 2009;18:3–10. doi: 10.1016/s1472-6483(10)60441-5. [DOI] [PubMed] [Google Scholar]
- 55.Schaan MM, Taylor M, Puvimanasinghe J, Busang L, Keapoletswe K, Marlink R. Sexual and reproductive health needs of HIV-positive women in Botswana—a study of health care worker's views. AIDS Care. 2012;24:1120–5. doi: 10.1080/09540121.2012.672814. [DOI] [PubMed] [Google Scholar]
- 56.Mindry DL, Crankshaw TL, Maharaj P, Munthree C, Letsoalo T, Milford C, Greener RM, Rambally L, Carpenter S, Smit JA. “We have to try and have this child before it is too late”: missed opportunities in client–provider communication on reproductive intentions of people living with HIV. AIDS Care. 2014;27(1):25–30. doi: 10.1080/09540121.2014.951311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moodley J, Cooper D, Mantell JE, Stern E. Health care provider perspectives on pregnancy and parenting in HIV-positive individuals in South Africa. BMC Health Serv Res. 2014;14:384. doi: 10.1186/1472-6963-14-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goggin K, Finocchario-Kessler S, Staggs V, Woldetsadik MA, Wanyenze RK, Beyeza-Kashesya J, Mindry D, Khanakwa S, Wagner GJ. Attitudes, knowledge, and correlates of self-efficacy for the provision of safer conception counseling among Ugandan providers. AIDS Patient Care STDs. 2015;29(12):651–60. doi: 10.1089/apc.2015.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marlow HM, Maman S, Groves AK, Moodley D. Fertility inset and contraceptive decision-making among HIV positive and negative antenatal clinical attendees in Durban, South Africa. Health Care Women Int. 2012;33(4):342–58. doi: 10.1080/07399332.2012.655390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews LT, Moore L, Milford C, Greener R, Mosery FN, Rifkin R, Psaros C, Safren SA, Harrison A, Wilson IB, Bangs-berg DR, Smit JA. “If I don't use a condom ... I would be stressed in my heart that I've done something wrong”: routine prevention messages preclude safer conception counseling for HIV-infected men and women in South Africa. AIDS Behav. 2015;19(9):1666–75. doi: 10.1007/s10461-015-1026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matthews LT, Crankshaw T, Giddy J, Kaida A, Psaros C, Ware NC, et al. Reproductive counseling by clinic healthcare workers in Durban, South Africa: perspectives from HIV-infected men and women reporting serodiscordant partners. Infect Dis Obstet Gynecol. 2012:146348. doi: 10.1155/2012/146348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Department of Health, Republic of South Africa . National contraception clinical guidelines. Department of Health; Pretoria: 2012. [23 Feb 2016]. www.doh.gov.za. [Google Scholar]
- 63.Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol. 2012 doi: 10.1155/2012/587651. doi:10.1155/2012/5876511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chadwick R, Mantell JE, Moodley J, Harries J, Zweigenthal V, Cooper D. A review of safer conception interventions for HIV-affected couples: implications for resource-constrained settings. Top Antivir Med. 2011;19(4):148–55. [PMC free article] [PubMed] [Google Scholar]
- 65.Matthews LT, Crankshaw T, Giddy J, Kaida A, Smit JA, Ware NC, Bangsberg DR. Reproductive decision-making and peri-conception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav. 2013;17(2):461–70. doi: 10.1007/s10461-011-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matthews LT, Heffron R, Mugo NR, Cohen CR, Hendrix CW, Celum C, Bangsberg DR, Baeten JM, Partners PrEP Study Team High medication adherence during periconception periods among HIV-1-uninfected women participating in a clinical trial of antiretroviral pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2014;67(1):91–7. doi: 10.1097/QAI.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matthews LT, Milford C, Kaida A, Ehrlich MJ, Ng C, Greener R, Mosery FN, Harrison A, Psaros C, Safren SA, Bajunirwe F, Wilson IB, Bangsberg DR, Smit JA. Lost opportunities to reduce periconception HIV transmission: safer conception counseling by South African providers addresses perinatal but not sexual HIV transmission. J Acquir Immune Defic Syndr. 2014;67(Suppl 4):S210–7. doi: 10.1097/QAI.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 70.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Health Organization, Department of Reproductive Health and Research . Decision-making tool for family planning clients and providers. A resource for high-quality counselling. WHO; Geneva: [26 Feb 2016]. http://apps.who.int/iris/bitstream/10665/43225/2/9241593229_eng.pdf. [Google Scholar]
- 72.Moorhouse M, Bekker LG, Black V, Conradie F, Harley B, Howell, et al. Guideline on the management of occupational and non-occupational exposure to the human immunodeficiency virus and recommendations for post-exposure prophylaxis: 2015 Update. [05 Apr 2016];S Afr J HIV Med. 2015 16(1) doi: 10.4102/sajhivmed.v16i1.399. Art. #399. http://dx.doi.org/10.4102/sajhivmed.v16i1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilley BC, Palesch YY, Kieburtz K, Ravina B, Huang P, Elm JJ, Shannon K, Wooten GF, Tanner CM, Goetz GC. NET-PD investigators. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66(5):628–33. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 74.Elm JJ, Goetz CG, Ravina B, Shannon K, Wooten GF, Tanner CM, Palesch YY, Huang P, Guimaraes P, Kamp C, Tilley BC, Kieburtz K, for the NET-PD Investigators Responsive outcome for Parkinson's disease neuroprotection futility studies. Ann Neurol. 2005;57:197–203. doi: 10.1002/ana.20361. [DOI] [PubMed] [Google Scholar]
- 75.Levin B. The futility study—progress over the last decade. Contemp Clin Trials. 2015;45(Pt A):69–75. doi: 10.1016/j.cct.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levin B. The utility of futility (Editorial). Stroke. 2005;36:2331–2. doi: 10.1161/01.STR.0000185722.99167.56. [DOI] [PubMed] [Google Scholar]
- 77.Rubin DB. Inference and missing data. Biometrics. 1976;63:581–92. [Google Scholar]
- 78.Catania JA, Binson D, Van der Straten A, Stone V. Methodological research on sexual behavior in the AIDS era. In: Rosen RC, Davis CM, Ruppel H, editors. Annual review of sex research. Volume 6. The Society for the Scientific Study of Sexuality; Phoenix: 1995. [Google Scholar]
- 79.Allen S, Tice J, van de Perre P, Serufilira A, Hudes E, Nsengumuremyi F, Bogaerts J, Lindan C, Hulley S. Effect of serotesting with counseling on condom use and seroconversion among HIV discordant couples in Africa. Br Med J. 1992;304:1605–9. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doll LS, Harrison JS, Frey RL, McKirnan D, Bartholow BN, Douglas JJM, Joy D, Bolan G, Doetsch J. Failure to disclose HIV risk among gay and bisexual men attending sexually transmitted disease clinics. Am J Prev Med. 1994;10:125–9. [PubMed] [Google Scholar]
- 81.Vincenzi De. I for the European Study Group on Heterosexual Transmission of HIV. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. NEJM. 1994;331:341–6. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 82.Saracco A, Musicco M, Nicolosi A, et al. Man-to-woman sexual transmission of HIV: a longitudinal study of 343 steady partners of infected men. J Acquir Immune Defic Syndr. 1993;6:497–502. [PubMed] [Google Scholar]
- 83.Agnew CR. The role of social desirability in self-reported condom use attitudes and intentions. AIDS Behav. 1998;2(3):229–39. [Google Scholar]
- 84.Pequegnat W, Fishbein M, Celentano D, Ehrhardt A, Garnett G, Holtgrave D, Jaccard J, Schachter J, Zenilman J. NIMH/APPC Workgroup On Behavioral and Biological Outcomes in HIV/STD prevention studies: a position statement. Sex Transm Dis. 2000;27(3):127–32. doi: 10.1097/00007435-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Pomeroy WB, Flax CC, Wheeler CC. Taking a sexual history. Free Press; New York: 1982. [Google Scholar]
- 86.Catania JA, Binson D, Canchola J, Pollack LM, Hauck W, Coates TJ. Effect of interviewer gender, interviewer choice, and item wording on responses to questions concerning sexual behavior. Public Opinion Q. 1996;60(3):345–75. [Google Scholar]
- 87.Asfaw HM, Gashe FE. Fertility intentions among HIV positive women aged 18–49 years in Addis Ababa Ethiopia: a cross sectional study. Reprod Health. 2014;11:36. doi: 10.1186/1742-4755-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaida A, Laher F, Strathdee SA, Janssen PA, Money D, Hogg RS, Gray G. Childbearing intentions of HIV-positive women of reproductive age in Soweto, South Africa: the influence of expanding access to HAART in an HIV hyper endemic setting. Am J Public Health. 2011;101(2):350–8. doi: 10.2105/AJPH.2009.177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goggin K, Mindry D, Beyeza-Kashesya J, Finocchario-Kessler S, Wanyenze R, Nabiryo C, Wagner G. “Our hands are tied up”: current state of safer conception services suggests the need for an integrated care model. Health Care Women Int. 2014;35(7–9):990–1009. doi: 10.1080/07399332.2014.920023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.National Committee for Confidential Enquiry into Maternal Deaths Saving Mothers 2011–2013: sixth report on confidential enquiries into maternal deaths in South Africa. [10 Apr 2016];Short report May. 15:2014. http://www.kznhealth.gov.za/mcwh/Maternal/Saving-Mothers-2011-2013-short-report.pdf. [Google Scholar]
- 91.Moodley J, Pattinson RC, Fawcus S, Schoon MG, Moran N, Shweni PM. on behalf of the National Committee on Confidential Enquiries into Maternal Deaths in South Africa. The confidential enquiry into maternal deaths in South Africa: a case study. BJOG. 2014;121(Suppl. 4):53–60. doi: 10.1111/1471-0528.12869. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz SR, Jean Bassett J, Sanned I, Phofa R, Yende N, Van Rie A. Implementation of a safer conception service for HIV-affected couples in South Africa. AIDS. 2014;28(Suppl 3):S277–85. doi: 10.1097/QAD.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bekker LG, Black V, Myer L, Rees H, Cooper D, Mall S, et al. Guideline on safer conception in fertile HIV-infected individuals and couples. South Afr J HIV Med. 2011;12:31–44. [Google Scholar]
- 94.Schwartz SR, West N, Phofa R, Yende N, Sanne I, Bassett J, Van Rie A. Acceptability and preferences for safer conception HIV prevention strategies: a qualitative study. Int J STD AIDS. 2015 doi: 10.1177/0956462415604091. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor TN, Mantell JE, Nywagi N, Cishe N, Cooper D, Stein ZA. ‘He lacks his fatherhood’: safer conception technologies and the biological imperative for fatherhood among recently-diagnosed Xhosa-speaking men living with HIV in South Africa. Cult Health Sex. 2013;15(9):1101–14. doi: 10.1080/13691058.2013.809147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaida A, Bangsberg DR, Gray G, Hogg RS, King R, Miller CL. Editorial: introduction to the supplement on HIV, HAART, and fertility in sub-Saharan Africa. AIDS Behav. 2009;13(Suppl 1):1–4. doi: 10.1007/s10461-009-9568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaida A, Laher F, Strathdee SA, Money D, Janssen PA, Hogg RS, et al. Contraceptive use and method preference among women in Soweto, South Africa: the influence of expanding access to HIV care and treatment services. Plos One. 2010;5(11):e13868. doi: 10.1371/journal.pone.0013868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laher F, Todd CS, Stibich MA, et al. A qualitative assessment of decisions affecting contraceptive utilization and fertility intentions among HIV-positive women in Soweto, South Africa. AIDS Behav. 2009;13(Suppl 1):47–54. doi: 10.1007/s10461-009-9544-z. [DOI] [PubMed] [Google Scholar]
- 99.Department of Health . Republic of South Africa. National contraception and fertility planning policy and service delivery guidelines. Government of the Republic of South Africa; Pretoria: 2012. [Google Scholar]
- 100.West N, Schwartz S, Phofa R, Yende N, Bassett J, Sanne I, Van Rie A. “I don't know if this is right...but this is what I'm offering”: healthcare providers knowledge, practice, and attitudes towards safer conception for HIV-affected couples in the context of Southern African guidelines. AIDS Care. 2016;28(3):390–6. doi: 10.1080/09540121.2015.1093596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crankshaw TL, Mindry D, Munthree C, Letsoalos T, Maharaj P. Challenges with couples, serodiscordance and HIV disclosure: healthcare provider perspectives on delivering safer conception services for HIV-affected couples, South Africa. [14 Apr 2016];J Int AIDS Soc. 2014 17:18832. doi: 10.7448/IAS.17.1.18832. http://www.jiasociety.org/index.php/jias/article/view/18832 http://dx.doi.org/10.7448/IAS.17.1.18832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mmeje O, van der Poel S, Workneh M, Njoroge B, Bukusi E, Cohen CR. Achieving pregnancy safely: perspectives on timed vaginal insemination among HIV-serodiscordant couples and health-care providers in Kisumu, Kenya. AIDS Care. 2014;27(1):10–6. doi: 10.1080/09540121.2014.946385. [DOI] [PMC free article] [PubMed] [Google Scholar]