Abstract
Background
Parapneumonic empyema, a serious complication of pneumonia, started increasing among U.S. children before the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000, and continued afterwards. This increase was due in part to pneumococcal serotypes not included in PCV7 that were included in the new 13-valent (PCV13) vaccine introduced in 2010. We assessed changes in the incidence of empyema hospitalizations among U.S. children after PCV13 introduction.
Methods
We calculated annualized empyema hospitalization rates among U.S. children <18 years using Nationwide Inpatient Sample and Census data (1997–2013) for four periods based on PCV7 and PCV13 introductions. Relative rates (RR) and 95% confidence intervals (CI) were calculated by age group and sex, comparing PCV7 (2001–2005 and 2006–2009) and PCV13 (2011–2013) periods with the pre-PCV7 period (1997–1999). Secondary analyses examined changes in pneumococcal, streptococcal, staphylococcal and unspecified empyema.
Results
Among children <18 years of age, annualized empyema hospitalization rates peaked at 3.6 per 100,000 in the late-PCV7 period compared with 2.1 per 100,000 in the pre-PCV7 period [RR: 1.70 (95% CI: 1.11–2.60)]. However, annualized rates in the post-PCV13 period declined to 2.0 per 100,000, similar to rates in the pre-PCV7 period. Empyema rates among children <2 years were lower in the post-PCV13 period compared to the pre-PCV7 period [RR: 0.77 (95% CI: 0.61–0.96)], but rates in the two periods among children 2–4 and 5–17 years were similar. Most empyema were of unspecified etiology. Pneumococcal and unspecified empyema declined after PCV13 introduction.
Conclusions
Although empyema hospitalization rates among U.S. children peaked after PCV7 introduction, rates decreased substantially following the introduction of PCV13.
Keywords: parapneumonic empyema, pneumococcal conjugate vaccine, invasive pneumococcal disease, epidemiology
INTRODUCTION
Parapneumonic empyema, a rare but serious complication of pneumonia, is associated with increased morbidity, mortality and length of hospital stay among patients with pneumonia [1–6]. Parapneumonic empyema among children <18 years of age in the United States (U.S.) started increasing before the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000, and continued afterwards [2, 7–9]. Greater awareness of empyema, improved diagnostics, and more complete coding may have contributed to an apparent increase in empyema [7, 8]. However, the changing ecology of Streptococcus pneumoniae after PCV7 introduction likely contributed to a real increase as well [5, 10].
The serotypes most commonly associated with parapneumonic empyema among children (1, 3, 7F and 19A) were not included in PCV7 [3, 10–15]. The incidence of pneumococcal pneumonia involving PCV7 serotypes decreased after PCV7 introduction, but with an increasing proportion of pneumococcal pneumonia attributed to non-PCV7 serotypes (such as 1, 7F and 19A) [3, 16–18]. This increased representation of serotypes historically associated with parapneumonic empyema likely contributed to the increasing incidence of parapneumonic empyema observed after PCV7 introduction [3, 10].
The 13-valent pneumococcal conjugate vaccine (PCV13) was introduced in 2010 to reduce further the incidence of invasive pneumococcal disease (IPD) due to non-PCV7 serotypes, and included serotypes 1, 3, 7F and 19A, among others. Vaccination coverage has been high and widespread among young U.S. children as coverage with ≥3 doses of PCV7 was >90% in 2007–2008 and has been >92% for ≥3 doses of PCV13 since 2010 [19, 20]. However, little information is available regarding national parapneumonic empyema rates in the U.S. after PCV13 introduction. Therefore, we assessed changes in hospitalizations for parapneumonic empyema among U.S. children after PCV13 introduction.
METHODS
Data Sources
We used the Nationwide Inpatient Sample (NIS) to obtain national hospitalization data from 1997 to 2013 for all U.S. children <18 years of age. The NIS is the largest publicly available database for all-payer inpatient healthcare in the U.S., representing over 35 million hospitalizations annually from all states that participate in the Healthcare Cost and Utilization Project (HCUP) [21]. Information on each hospitalization includes hospital characteristics, patient demographics, primary and secondary diagnoses and procedures, length of stay, and severity and comorbidity measures [21]. Prior to 2012, the NIS collected information on all discharges from a sample of hospitals in the U.S. Beginning in 2012, the NIS changed its design to include a sample of discharges from all hospitals participating in HCUP. New discharge weights generated for the 1997–2011 NIS allows assessment of trends including the 2012 and 2013 NIS data. We used NIS-provided trend weights and design elements to account for the complex sampling design and allow for the assessment of national trends over time [21]. For rate calculations, we used national population estimates from the U.S. Census (1997–2013) as denominators [22]. This study was exempt from review by the Institutional Review Board at Vanderbilt University.
Hospitalizations for Parapneumonic Empyema
We identified all hospitalizations for pneumonia as a hospitalization with a primary discharge diagnosis of pneumonia [International Classification of Disease 9th version-Clinical Modification (ICD9-CM): 480.0–486.9] or a secondary diagnosis of pneumonia with a primary diagnosis of meningitis, sepsis or empyema (ICD9-CM: 510) [8]. Hospitalizations for parapneumonic empyema were identified as any pneumonia hospitalization with a diagnosis of empyema (primary or otherwise). A separate analysis was conducted to assess a more specific outcome measure using only hospitalizations for parapneumonic empyema with a documented thoracentesis-related procedure for the diagnosis or management of empyema, including video-assisted thorascopic surgery (ICD9-CM: 34.04, 34.06, 34.09, 34.21, 34.51, 34.52, and 34.91) [23]. In addition, discharge diagnosis codes were used to classify hospitalizations for parapneumonic empyema based on the likely causative organism into mutually exclusive groups and according to the following hierarchy: pneumococcal (ICD9-CM: 481), streptococcal (ICD9-CM: 482.3, 041.0, 038.0), staphylococcal (ICD9-CM: 482.4, 041.1, 038.1) or unspecified (other/unknown etiology). The ICD9-CM codes to identify hospitalizations for pneumonia have been validated in prior studies, and the strategy to identify hospitalizations for parapneumonic empyema, including by likely causative organism, have been reported previously [7, 8, 23–25].
Data Analysis
Annualized hospitalization rates for parapneumonic empyema were calculated by pre-specified age groups (<2, 2–4 and 5–17 years), sex, and periods based on PCV7 and PCV13 introduction, excluding years of PCV introduction: pre-PCV7 (1997–1999), early-PCV7 (2001–2005), late-PCV7 (2006–2009) and post-PCV13 (2011–2013) [26]. Annualized period rates per 100,000 population were calculated by dividing the weighted number of parapneumonic empyema hospitalizations (accounting for the NIS sampling design) within each period by the average annual population in each period, and divided by the number of years within the period. Variance estimates of the weighted number of empyema hospitalizations were used to calculate 95% confidence intervals (CI) for the annualized period rates.
We compared rates of hospitalizations for parapneumonic empyema using relative rates (RR) and 95% CIs. These comparisons were conducted by age group and sex, comparing PCV7 and PCV13 periods to the pre-PCV7 period and accounting for the variance of each weighted rate [27]. Significance of the relative rates was assessed at the 5% level, considering significant differences between annualized rates when the 95% CI of the RRs excluded one [8, 26]. We conducted similar analyses using the more specific definition of parapneumonic empyema and by likely causative organism as described above.
Planned secondary analyses assessed the robustness of our findings. To determine potential changes in coding practices during the study years, we assessed changes in the mean number of discharge diagnoses and length of stay (in days) for all-cause hospitalizations and for empyema-related hospitalizations among all children. In addition, we examined the percentage of hospitalizations for parapneumonic empyema each year involving children with at least one discharge diagnosis code for certain comorbidities (asthma, neurological disorders, low birth weight, trisomy-21, liver, lung and heart disease, diabetes, cochlear implant, white blood cell disorders, congenital immunodeficiency, transplant, renal disease and HIV) to assess potential changes in coding practice or changes in the population presenting with parapneumonic empyema [28]. In addition, to assess whether parapneumonic empyema trends were specifically associated with vaccine introduction, we performed a parallel analysis of fracture hospitalizations (ICD9-CM: 800.xx-829.xx), as a control comparison disease group unlikely to be affected by PCV introduction [29]. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) and STATA 14 (StataCorp LP, College Station, TX, USA).
RESULTS
During the study period, parapneumonic empyema was rare, and accounted for 1.3% of pneumonia hospitalizations among U.S. children <18 years of age. Among all children, the annualized rate of hospitalizations for parapneumonic empyema per 100,000 persons was 2.1 (95% CI: 1.7–2.4) in the pre-PCV7 period, and progressively rose to 3.6 per 100,000 in the late-PCV7 period [RR: 1.70 (95% CI:1.11–2.60)]. Rates in the post-PCV13 period declined and were similar to rates in the pre-PCV7 period [RR: 0.95 (95% CI:0.76–1.18)] (Table 1). Similar trends were observed in the secondary analysis restricted to the 85.2% of hospitalizations with a documented thoracentesis procedure. The proportion of parapneumonic empyema with related procedures remained constant across periods (Table 1).
Table 1.
Annualized rates of parapneumonic empyema related hospitalizations per 100,000 children <18 years by vaccination era, United States, 1997–2013
| Period | All Parapneumonic Empyema | Parapneumonic Empyema with Thoracentesis | ||
|---|---|---|---|---|
|
| ||||
| Estimated Number of Hospitalizations | Rate per 100,000 (95% CI) | Estimated Number of Hospitalizations | Rate per 100,000 (95% CI) | |
| Pre-PCV7 (1997–1999) | 4,501 | 2.1 (1.7, 2.4) | 3,827 | 1.8 (1.5, 2.1) |
| Early-PCV7 (2001–2005) | 11,460 | 3.1 (2.5, 3.7) | 9,742 | 2.7 (2.1, 3.2) |
| Late-PCV7 (2006–2009) | 10,538 | 3.6 (2.9, 4.2) | 9,015 | 3.0 (2.4, 3.7) |
| Post-PCV13 (2011–2013) | 4,402 | 2.0 (1.7, 2.3) | 3,748 | 1.7 (1.4, 2.0) |
Footnote: PCV7: 7-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine; CI: Confidence Interval
Trends of hospitalization rates for empyema across vaccination periods within individual age groups appeared similar to the overall trend among all children (Figures 1, 2). Rates were higher in the late-PCV7 period compared to the pre-PCV7 period among all age groups. However, only rates among children 2–4 and 5–17 years were significantly higher [RR: 2.12 (95% CI:1.17–3.84) and RR: 1.64 (95% CI:1.08–2.49), respectively]. In contrast, rates were significantly lower in the post-PCV13 period compared to the pre-PCV7 period only among children <2 years [RR: 0.77 (95% CI:0.61–0.96)]. Rates were similar in the post-PCV13 and pre-PCV7 period among the other age groups (Table 2).
Figure 1. Annualized rates of parapneumonic empyema related hospitalizations per 100,000 children <18 years, United States, 1997–2013.
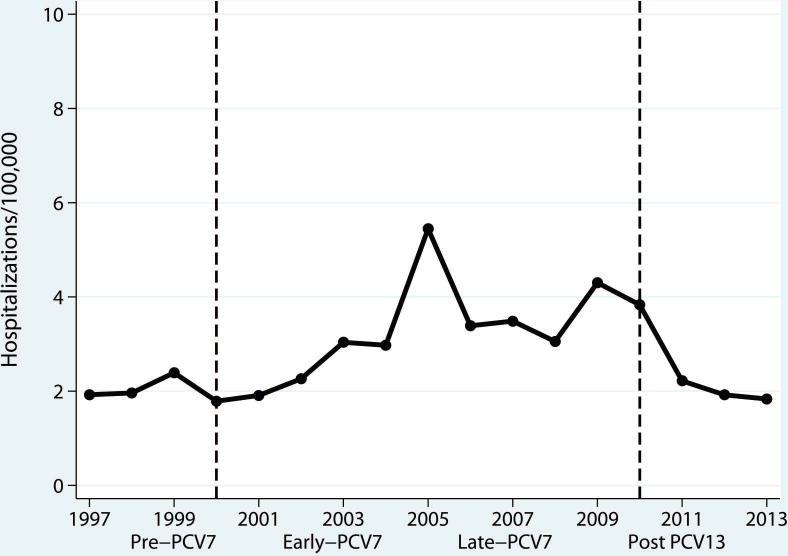
Legend: Annualized rates of empyema related hospitalizations per 100,000 children <18 years, U.S., 1997–2013. The dashed-blue line represents the years of introduction for PCV7 in 2000 and PCV13 in 2010. Periods were defined excluding years of PCV introduction: Pre-PCV7 (1997–1999), Early-PCV7 (2001–2005), Late-PCV7 (2006–2009) and Post-PCV13 (2011–2013).
Figure 2. Annualized rates of parapneumonic empyema related hospitalizations per 100,000 children <18 years (95% CI) by PCV period and age group, United States (1997–2013).
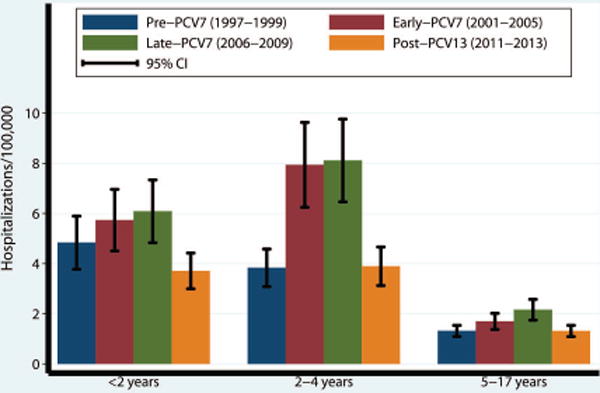
Legend: Annualized rates of hospitalization for parapneumonic empyema by age group and PCV period, per 100,000 children. Error bars represent the 95% confidence interval of the rate of hospitalizations for parapneumonic empyema by age group and period.
Table 2.
Annualized rates of parapneumonic empyema related hospitalizations per 100,000 children <18 years by age group, sex, and vaccination era, United States, 1997–2013
| Age | Sex | Hospitalization rate per 100,000 persons | Relative Rate (95% CI)1 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pre-PCV7 (1997–1999) | Early-PCV7 (2001–2005) | Late-PCV7 (2006–2009) | Post-PCV13 (2011–2013) | Post-PCV13 to Late-PCV7 | Post-PCV13 to Pre-PCV7 | ||
| <2 | All | 4.8 | 5.7 | 6.1 | 3.7 | 0.61 (0.51, 0.72) | 0.77 (0.61, 0.96) |
| Male | 5.7 | 6.0 | 6.7 | 4.1 | 0.61 (0.50, 0.74) | 0.72 (0.56, 0.91) | |
| Female | 3.9 | 5.4 | 5.3 | 3.3 | 0.63 (0.51, 0.78) | 0.85 (0.62, 1.16) | |
| 2–4 | All | 3.8 | 7.9 | 8.1 | 3.9 | 0.48 (0.42, 0.55) | 1.02 (0.77, 1.35) |
| Male | 4.0 | 8.1 | 8.7 | 3.7 | 0.43 (0.38, 0.49) | 0.94 (0.69, 1.26) | |
| Female | 3.7 | 7.8 | 7.5 | 4.1 | 0.54 (0.46, 0.64) | 1.11 (0.78, 1.57) | |
| 5–17 | All | 1.3 | 1.7 | 2.2 | 1.3 | 0.61 (0.52, 0.71) | 1.00 (0.79, 1.27) |
| Male | 1.5 | 1.8 | 2.4 | 1.5 | 0.63 (0.53, 0.75) | 1.01 (0.77, 1.31) | |
| Female | 1.2 | 1.6 | 1.9 | 1.1 | 0.59 (0.50, 0.69) | 0.98 (0.74, 1.30) | |
Statistical significance was assessed at the 5% level using the 95% confidence interval (interval including 1.0 was considered non-significant at the 5% level)
Males and females within age groups had similar trends to those observed for all children (Table 2). Males had consistently higher rates of hospitalizations for parapneumonic empyema in the pre-PCV7 and PCV7 periods compared to females, with differences that were larger in the late-PCV7 period compared to the pre-PCV7 period. However, the sex differences in each age group were more modest in the post-PCV13 period compared to the late-PCV7 period (Table 2).
Secondary analyses
In the secondary analysis examining characteristics of all hospitalizations among children during the study period, the mean length of stay increased to 3.8 days (median: 1.7) in the post-PCV13 period compared to 3.5 days (median: 1.6) in the pre-PCV7 period. The mean number of discharge diagnoses increased during each vaccination period from 2.4 (median: 1.4) in the pre-PCV7 period to 3.6 (median: 2.2) in the post-PCV13 period. The median length of stay and number of recorded diagnoses was the highest in the post-PCV13 period. Similar trends were observed for the number of recorded diagnoses in hospitalizations for parapneumonic empyema by period, although median length of stay remained stable during the study period (data not shown). In addition, the proportion of children hospitalized with parapneumonic empyema with at least one comorbidity steadily increased during the study period (1997: 9.6%, 2005: 14.9%, and 2013: 30.7%). Asthma was the most commonly diagnosed comorbid condition in each year throughout the study period.
During the study period, hospitalizations for fractures among children decreased steadily [29]. However, there were no clear changes in the observed trends of hospitalizations for fractures specifically associated with the introduction of PCV7 or PCV13 (Supplementary Figure 1).
In our assessment of probable etiologies of empyema, the most likely etiology of parapneumonic empyema among children <18 years during the study period was pneumococcal in 23.2%, staphylococcal in 11.4%, streptococcal in 9.6%, and other/unspecified organism in 55.7%. Both pneumococcal and unspecified empyema declined after PCV13 introduction (Figure 3 and supplementary table 1). A significant reduction in pneumococcal empyema rates was observed among all age groups in the post-PCV13 period compared to the late-PCV7 period, with rates also significantly lower than the pre-PCV7 period. Rates of unspecified empyema had the same pattern for children <2 years (Figure 3 and supplementary table 1). For unspecified empyema in other age groups, a significant decline was only observed in the post-PCV13 period compared with the late-PCV7 period, but with no significant change compared to the pre-PCV7 period.
Figure 3. Annualized rates of parapneumonic empyema related hospitalizations per 100,000 children <18 years (95% CI) by likely causative organism, PCV period and age group, United States (1997–2013).
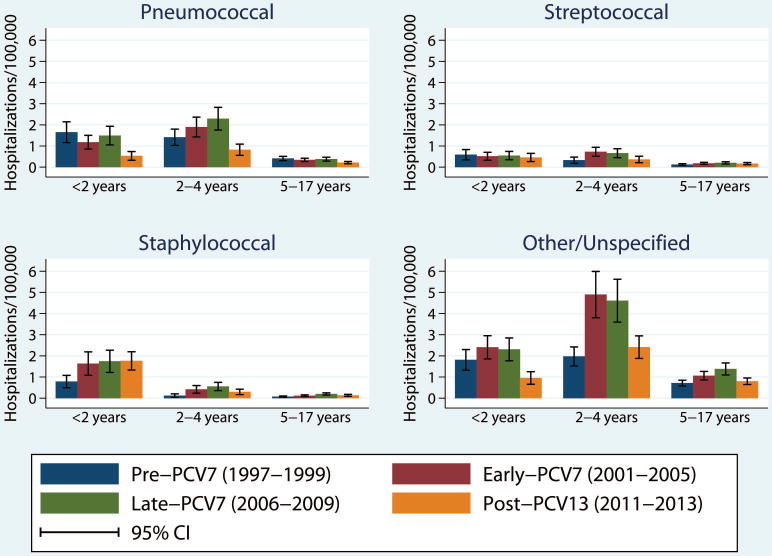
Legend: Annualized rates of hospitalization for parapneumonic empyema by organism type (pneumococcal, streptococcal, staphylococcal or other/unspecified), age group and period, per 100,000 children. Error bars represent the 95% confidence interval of the rate of hospitalizations for parapneumonic empyema by age group and period within each organism type.
Although the burden of disease was small, the pattern observed for streptococcal and staphylococcal empyemas was similar to that observed for unspecified empyema among children 2–4 and 5–17 years, as the empyema rate in the post-PCV13 period was lower than the late-PCV7 period, but not different from the pre-PCV7 period (Figure 3 and supplementary table 1). No reduction was observed for staphylococcal or streptococcal empyema among children <2 years in the post-PCV13 period. Although the rate of staphylococcal empyema was lower than the combined rate of pneumococcal and unspecified empyema in each age group during the PCV7 years, the rate of staphylococcal empyema was higher than both combined among children <2 years in the post-PCV13 period (Figure 3 and supplementary table 1).
DISCUSSION
Rates of hospitalizations for parapneumonic empyema among U.S. children continued increasing and peaked after PCV7 introduction into the U.S. childhood immunization schedule, but decreased to historical low levels after PCV13 introduction. Importantly, rates of hospitalizations for parapneumonic empyema were significantly lower in the post-PCV13 period compared to the pre-PCV7 period among children <2 years, the target population for the pneumococcal conjugate vaccination program. In addition, a reduction in the post-PCV13 period was consistently observed among different age groups and among both boys and girls, and similar declines were observed in hospitalizations for pneumococcal and unspecified parapneumonic empyema. The same pattern was not observed for fractures, a control condition unlikely to be impacted by PCV7 or PCV13 introduction. In addition, the observed changes were specific to pneumococcal and unspecified empyema, and not observed for other specified causes of empyema.
The decrease in parapneumonic empyema rates was likely driven by PCV13 introduction in 2010. Although rates of IPD specifically caused by PCV7 serotypes decreased since PCV7 introduction, rates of parapneumonic empyema increased during the post-PCV7 years. This increase was attributed, at least in part, to the increase in IPD due to serotypes 1, 7F, 3 and 19A, which were not included in PCV7 but have been consistently linked to a greater likelihood of parapneumonic empyema [3,5,10,11,13,17]. PCV13 includes serotypes 1, 3, 7F and 19A, among others, and IPD attributable to these serotypes has declined since PCV13 introduction [30–32]. Consistent with those reports, our findings provide evidence of a significant decrease in parapneumonic empyema among U.S. children after PCV13 introduction in 2010.
Similar findings among children have been observed in smaller studies conducted in selected European countries and among a subset of the U.S. population. In a regional surveillance study of hospitals in Madrid, Spain, annual parapneumonic empyema rates declined from 6.73 cases per 100,000 children in May 2009–April 2010 to 4.14 per 100,000 children in May 2010–April 2011 after PCV13 introduction in June 2010, mostly due to the reduction of serotype 1 and 19A pneumococcal disease [33]. Similarly, a large referral hospital in Central Greece reported marked reductions in childhood empyema after widespread use of PCV13; those reductions were associated with declines in empyema due to pneumococcal serotypes 19A and 7F [34]. In addition, nationally representative studies of hospital admissions for parapneumonic empyema in Scotland and England found that PCV13 introduction was associated with a relative rate reduction of 0.78 (95% CI:0.63–0.98) and 0.58 (95% CI:0.34–0.99), respectively [35, 36]. Among children in the U.S., a retrospective time series analysis, using a private insurance database of hospital discharges representing around 20% of U.S. hospitalizations and data through mid-2012, reported early declines in empyema rates after PCV13 introduction relative to pre-PCV13 years among children <2, 2–4 and 5–17 years (RR:0.76, RR:0.53, RR:0.62, respectively) [37]. However, our study is the first to demonstrate the national changes in parapneumonic empyema since introduction of the first PCVs using a representative sample of children of all insurance status and data including the full 3-year period following PCV13 introduction.
Our assessment of changes in parapneumonic empyema by likely causative organisms extended our main analysis and indicated that hospitalization rates for pneumococcal and unspecified empyema declined following PCV13 introduction. Most empyema had an unspecified etiology, reflecting the difficulty in establishing etiology with traditional diagnostics. Previous studies that applied molecular diagnostic techniques have reported that many culture negative empyemas are due to pneumococcus [3,38]. The significant declines observed in unspecified empyema following PCV13 introduction are consistent with those observations.
Several limitations should be considered when interpreting the results of our study. First, caution should be used when attributing changes in hospitalizations for parapneumonic empyema directly to PCV7 and PCV13 introduction due to the ecologic nature of our study. However, the ecologic design is the preferred method for understanding both the direct and indirect effects of PCV13 introduction, due to the rapid and high level of uptake of these vaccines after their introduction into the U.S. childhood immunization schedule. The observed changes after PCV13 introduction were more pronounced among children <2 years of age, thus providing evidence for direct benefit to the target population of the vaccination program. To address the concern of observed declines in empyema being the result of generalized changes in hospitalization patterns, we examined whether the observed declines were specific to our outcome of interest using fracture hospitalizations as a control condition. We did not find evidence of changes in this control condition associated with the introduction of PCVs. However, as previously reported, we observed a systematic decline in fracture hospitalizations among children during the study period that was similar across age groups [39]. This decline is consistent with previous reports from the Kids’ Inpatient Database that documented decreases in the rate of hospitalizations for trauma and musculoskeletal system conditions from 2000 to 2012 among US children (26% and 22.7%, respectively), as well as a reduction in specific fracture types over the study period (including femoral fractures) [39,40]. This decline in hospitalization for fractures could be partially due to a decreased incidence of injury among children (i.e. reduction in motor vehicle accidents and injury) [41–43], but could also be related to shifting patterns of care for fracture among pediatric patients from general hospitals to children’s hospitals and ambulatory surgical settings [44, 45]. Nevertheless, unlike the clear declines observed in empyema hospitalizations following PCV13 introduction, the existing declining trends of fracture hospitalizations observed throughout the study period were not clearly affected by vaccine introduction.
Second, due to the nature of the study design, the possibility that changes in coding practices for hospitalizations may have influenced the observed trends cannot be ruled out. Nevertheless, our main analyses showed that the parapneumonic empyema rates increased during PCV7 years, and decreased following PCV13 introduction, despite a steady increase in the average number of recorded diagnoses. The average length of hospital stay and the proportion of children hospitalized for parapneumonic empyema that had comorbidities also increased during the study years. It remains unclear whether these changes represent more comprehensive coding, a concentration of this complication among children with more comorbidities or a combination of these factors. Further studies would be helpful to clarify these observations. Third, the use of coded discharge diagnoses to identify hospitalizations for parapneumonic empyema allows for the possibility of misclassification of the study outcome. We did not include diagnoses for pleural effusions in our study, but a planned secondary analysis that evaluated a more specific parapneumonic empyema definition (evidence of a thoracentesis procedure in addition to the diagnosis of empyema) yielded results similar to our primary analysis. Finally, establishing the etiology of parapneumonic empyema is challenging given known limitations of current diagnostic tests, and our attempt to classify empyema by likely causative organism may be subject to misclassification. Moreover, as pneumococcal serotyping is not part of routine care nor is recorded in our data sources, we could not provide information on the specific pneumococcal serotypes involved in the observed outcomes.
CONCLUSION
In contrast to the increased incidence of hospitalizations for parapneumonic empyema observed after PCV7 introduction, PCV13 introduction into the U.S. childhood immunization schedule was associated with a substantial reduction of hospitalizations for parapneumonic empyema among U.S. children. The expanded serotype coverage of PCV13 likely contributed to the return of hospitalization rates for parapneumonic empyema closer to historical rates observed prior to PCV7 introduction.
Supplementary Material
Acknowledgments
This work was supported by the Agency for HealthCare Research and Quality (R03HS022342).
Glossary
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PCV
pneumococcal conjugate vaccine
- NIS
Nationwide Inpatient Sample
- ICD9-CM
International Classification of Disease 9th version-Clinical Modification
- RR
relative rate
- CI
confidence interval
- HCUP
Healthcare Cost and Utilization Project
Footnotes
CONFLICT OF INTEREST: Dr. Grijalva has served as consultant for Pfizer Inc. (New York, NY). Dr. Griffin received grant support from MedImmune (Gaithersburg, MD). The other authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis. 2007;45:1480–6. doi: 10.1086/522996. [DOI] [PubMed] [Google Scholar]
- 2.Brims FJ, Lansley SM, Waterer GW, Lee YC. Empyema thoracis: new insights into an old disease. Eur Respir Rev. 2010;19:220–8. doi: 10.1183/09059180.00005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher MA, Schmitt HJ, Syrochkina M, Sylvester G. Pneumococcal empyema and complicated pneumonias: global trends in incidence, prevalence, and serotype epidemiology. Eur J Clin Microbiol Infect Dis. 2014;33:879–910. doi: 10.1007/s10096-014-2062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCauley L, Dean N. Pneumonia and empyema: causal, casual or unknown. J Thorac Dis. 2015;7:992–8. doi: 10.3978/j.issn.2072-1439.2015.04.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz-Almagro C, Selva L, Pallares R. Influence of pneumococcal vaccine on the incidence of empyema. Curr Opin Pulm Med. 2010;16:394–8. doi: 10.1097/MCP.0b013e328338c19f. [DOI] [PubMed] [Google Scholar]
- 6.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 7.Grijalva CG, Zhu Y, Nuorti JP, Griffin MR. Emergence of parapneumonic empyema in the USA. Thorax. 2011;66:663–8. doi: 10.1136/thx.2010.156406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grijalva CG, Nuorti JP, Zhu Y, Griffin MR. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis. 2010;50:805–13. doi: 10.1086/650573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ampofo K, Pavia AT, Stockmann CR, Blaschke AJ, Weng HY, Korgenski KE, et al. Evolution of the epidemiology of pneumococcal disease among Utah children through the vaccine era. Pediatr Infect Dis J. 2011;30:1100–3. doi: 10.1097/INF.0b013e318232ee3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byington CL, Hulten KG, Ampofo K, Sheng X, Pavia AT, Blaschke AJ, et al. Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J Clin Microbiol. 2010;48:520–5. doi: 10.1128/JCM.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grabenstein JD, Musey LK. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine. 2014;32:2399–405. doi: 10.1016/j.vaccine.2014.02.096. [DOI] [PubMed] [Google Scholar]
- 12.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children – use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine – recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–18. [PubMed] [Google Scholar]
- 13.Krenke K, Sadowy E, Podsiadly E, Hryniewicz W, Demkow U, Kulus M. Etiology of parapneumonic effusion and pleural empyema in children. The role of conventional and molecular microbiological tests. Respiratory medicine. 2016;116:28–33. doi: 10.1016/j.rmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MF, Sheppard CL, Guiver M, Slack MP, George RC, Gorton R, et al. Emergence of pneumococcal 19A empyema in UK children. Arch Dis Child. 2012;97:1070–2. doi: 10.1136/archdischild-2012-301790. [DOI] [PubMed] [Google Scholar]
- 15.Strachan RE, Cornelius A, Gilbert GL, Gulliver T, Martin A, McDonald T, et al. Bacterial causes of empyema in children, Australia, 2007-2009. Emerging infectious diseases. 2011;17:1839–45. doi: 10.3201/eid1710.101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax. 2010;65:770–4. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- 17.Andrade AL, Toscano CM, Minamisava R, Costa PS, Andrade JG. Pneumococcal disease manifestation in children before and after vaccination: what’s new? Vaccine. 2011;29(Suppl 3):C2–14. doi: 10.1016/j.vaccine.2011.06.096. [DOI] [PubMed] [Google Scholar]
- 18.Cremers AJ, Meis JF, Walraven G, Jongh CE, Ferwerda G, Hermans PW. Effects of 7-valent pneumococcal conjugate 1 vaccine on the severity of adult 2 bacteremic pneumococcal pneumonia. Vaccine. 2014;32:3989–94. doi: 10.1016/j.vaccine.2014.04.089. [DOI] [PubMed] [Google Scholar]
- 19.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, State, and Selected Local Area Vaccination Coverage Among Children Aged 19-35 Months – United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:889–96. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 20.National, state, and local area vaccination coverage among children aged 19–35 months – United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:921–6. [PubMed] [Google Scholar]
- 21.The Healthcare Cost and Utilization Project, Overview of the Nationwide Inpatient Sample. Agency for Healthcare Research and Quality.
- 22.National Population Estimates. U.S. Census Bureau.
- 23.Shah SS, Hall M, Newland JG, Brogan TV, Farris RW, Williams DJ, et al. Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood. Journal of hospital medicine : an official publication of the Society of Hospital Medicine. 2011;6:256–63. doi: 10.1002/jhm.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM, Jr, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. American journal of epidemiology. 1999;149:282–9. doi: 10.1093/oxfordjournals.aje.a009804. [DOI] [PubMed] [Google Scholar]
- 25.Williams DJ, Shah SS, Myers A, Hall M, Auger K, Queen MA, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA pediatrics. 2013;167:851–8. doi: 10.1001/jamapediatrics.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papanicolaou A. Taylor Approximation and the Delta Method. Stanford University; 2009. [Google Scholar]
- 28.Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59:615–23. doi: 10.1093/cid/ciu348. [DOI] [PubMed] [Google Scholar]
- 29.Griffin MR, Mitchel E, Moore MR, Whitney CG, Grijalva CG. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines – Tennessee, 1998–2012. MMWR Morb Mortal Wkly Rep. 2014;63:995–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Olarte L, Barson WJ, Barson RM, Lin PL, Romero JR, Tan TQ, et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin Infect Dis. 2015;61:767–75. doi: 10.1093/cid/civ368. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shimol S, Greenberg D, Givon-Lavi N, Schlesinger Y, Somekh E, Aviner S, et al. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: an active prospective nationwide surveillance. Vaccine. 2014;32:3452–9. doi: 10.1016/j.vaccine.2014.03.065. [DOI] [PubMed] [Google Scholar]
- 32.Farnham AC, Zimmerman CM, Papadouka V, Konty KJ, Zucker JR, Nattanmai GV, et al. Invasive Pneumococcal Disease Following the Introduction of 13-Valent Conjugate Vaccine in Children in New York City From 2007 to 2012. JAMA Pediatr. 2015;169:646–52. doi: 10.1001/jamapediatrics.2015.0612. [DOI] [PubMed] [Google Scholar]
- 33.Picazo J, Ruiz-Contreras J, Casado-Flores J, Giangaspro E, Garcia-de-Miguel MJ, Hernandez-Sampelayo T, et al. Impact of introduction of conjugate vaccines in the vaccination schedule on the incidence of pediatric invasive pneumococcal disease requiring hospitalization in Madrid 2007 to 2011. Pediatr Infect Dis J. 2013;32:656–61. doi: 10.1097/INF.0b013e31827e8594. [DOI] [PubMed] [Google Scholar]
- 34.Syrogiannopoulos GA, Michoula AN, Tsimitselis G, Vassiou K, Chryssanthopoulou DC, Grivea IN. Pneumonia with empyema among children in the first five years of high coverage with 13-valent pneumococcal conjugate vaccine. Infect Dis (Lond) 2016:1–5. doi: 10.1080/23744235.2016.1192720. [DOI] [PubMed] [Google Scholar]
- 35.Nath S, Thomas M, Spencer D, Turner S. Has the incidence of empyema in Scottish children continued to increase beyond 2005? Arch Dis Child. 2015;100:255–8. doi: 10.1136/archdischild-2014-306525. [DOI] [PubMed] [Google Scholar]
- 36.Saxena S, Atchison C, Cecil E, Sharland M, Koshy E, Bottle A. Additive impact of pneumococcal conjugate vaccines on pneumonia and empyema hospital admissions in England. J Infect. 2015;71:428–36. doi: 10.1016/j.jinf.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med. 2014;2:387–94. doi: 10.1016/S2213-2600(14)70032-3. [DOI] [PubMed] [Google Scholar]
- 38.Blaschke AJ, Heyrend C, Byington CL, Obando I, Vazquez-Barba I, Doby EH, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289–94. doi: 10.1097/INF.0b013e3182002d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United States, 2012: Statistical Brief #187 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. [PubMed] [Google Scholar]
- 40.Naranje SM, Stewart MG, Kelly DM, Jones TL, Spence DD, Warner WC, Jr, et al. Changes in the Treatment of Pediatric Femoral Fractures: 15-Year Trends From United States Kids’ Inpatient Database (KID) 1997 to 2012. Journal of pediatric orthopedics. 2016;36:e81–5. doi: 10.1097/BPO.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 41.Curry AE, Elliott MR, Pfeiffer MR, Kim KH, Durbin DR. Long-term changes in crash rates after introduction of a Graduated Driver Licensing decal provision. American journal of preventive medicine. 2015;48:121–7. doi: 10.1016/j.amepre.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Vital signs: Unintentional injury deaths among persons aged 0–19 years – United States, 2000–2009. MMWR Morbidity and mortality weekly report. 2012;61:270–6. [PubMed] [Google Scholar]
- 43.Monroe K, Irons E, Crew M, Norris J, Nichols M, King WD. Trends in Alabama teen driving death and injury. The journal of trauma and acute care surgery. 2014;77:S51–4. doi: 10.1097/TA.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein DT, Chen C, Zhang W, McKay SD. National Trends in Operative Treatment of Pediatric Fractures in the Ambulatory Setting. Orthopedics. 2015;38:e869–73. doi: 10.3928/01477447-20151002-52. [DOI] [PubMed] [Google Scholar]
- 45.Fabricant PD, Seeley MA, Anari JB, Ganley TJ, Flynn JM, Baldwin KD. Medial Epicondyle Fractures in Children and Adolescents: Shifting Care from General Hospitals to Children’s Hospitals? The Journal of pediatrics. 2015;167:1116–20. doi: 10.1016/j.jpeds.2015.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


