Abstract
Reports of chronic eosinophilic pneumonia (CEP) after radiation therapy are limited to breast cancer. We herein describe a case of CEP after radiation therapy for lung cancer. The patient was a 65-year-old man who had asymptomatic peripheral blood eosinophilia but no history of asthma or allergy. One month after completion of radiation therapy, chest CT scan revealed infiltrates inside the irradiated area, leading to the diagnosis of radiation pneumonitis. His condition improved after receiving corticosteroids. However, one months after withdrawal of corticosteroids, he noticed cough and shortness of breath with patchy ground glass opacity in the contralateral lung. The WBC count was 9900/μl with 17% eosinophils and bronchoalveolar lavage showed eosinophils of 14%, leading to the diagnosis of CEP. His condition improved after receiving corticosteroids and subsequent tapering without recurrence. Asymptomatic peripheral blood eosinophilia at the initial diagnosis of lung cancer might be a trigger for developing CEP.
Keywords: Chronic eosinophilic pneumonia, Lung cancer, Radiation pneumonitis, Radiation therapy
1. Introduction
Chronic eosinophilic pneumonia (CEP) is a type of eosinophilic lung disease and characterized by the onset over a few weeks of cough, shortness of breath, malaise, and weight loss, with diffuse pulmonary infiltrates. CEP may be idiopathic or be related to various causes, such as parasitic infections or drug-induced toxicity. There are several reports of CEP after radiation therapy (RT) almost exclusively for breast cancer [1], the clinical course of which resembles radiation pneumonitis. We herein describe a case of radiation pneumonitis and subsequent CEP after RT for squamous cell lung cancer and discuss the difference between two disorders and risk factors for the onset of CEP.
2. Case report
The patient was a 65-year-old heavy smoking (86 pack-years) man without a history of asthma, allergic rhinitis, allergy to drugs, or parasitic infections. He was referred to our hospital for the investigation of abnormal chest X-ray (Fig. 1A). After examinations, he was diagnosed with squamous cell lung cancer of the right upper lobe: clinical stage IIIB (cT4N2M0). Laboratory tests revealed a white blood cell (WBC) count of 14800/μl with 9% eosinophils, total serum IgE of 43.4 IU/mL, and negative specific IgE antibody to 16 common inhalant allergens. Then, he received 6 courses of chemotherapy (carboplatin and paclitaxel) and concurrent RT (60 Gy, 2 Gy in fractions) with partial response. One month after completion of RT, chest X-ray and CT images revealed infiltrates inside the irradiated area, leading to the diagnosis of radiation pneumonitis (Fig. 1, Fig. 2A). At this time, a WBC count was 5300/μl with 4% eosinophils. His condition improved after receiving prednisolone 20 mg/day and subsequent withdrawal. However, when there was no recurrence of lung cancer 12 months after completion of RT, he noticed cough and shortness of breath persisting for more than 2 weeks. Then, he admitted to our hospital for the investigation of hypoxia and chest X-ray abnormality. Laboratory tests revealed a WBC count of 9900/μl with 17% eosinophils and serum C-reactive protein of 2.43 mg/dL. Chest X-ray and CT scan showed patchy ground glass opacity in the left lung which did not coincide with the irradiated area (Fig. 1, Fig. 2B). The arterial blood gas analysis (oxygen nasal cannula, 2 L/min) revealed PaO2 of 54.0 Torr, PaCO2 of 39.5 Torr, and pH of 7.426. Bronchoalveolar lavage from the left B3 showed alveolar macrophage of 84%, eosinophils of 14%, lymphocytes of 1%, and neutrophils of 1%, leading to the diagnosis of CEP. Transbronchial lung biopsy was not performed because of respiratory failure after bronchoalveolar lavage. He received high dose intravenous steroid therapy (methylprednisolone 1 g/day for 3 days) and subsequent oral prednisolone 30 mg/day. His symptoms and laboratory data improved rapidly. One month later, ground glass opacity on the chest X-ray and CT disappeared (Fig. 1D). He is now receiving a maintenance dose of prednisolone 5 mg/day with a WBC count of 10700/μl with 2% eosinophils without recurrence.
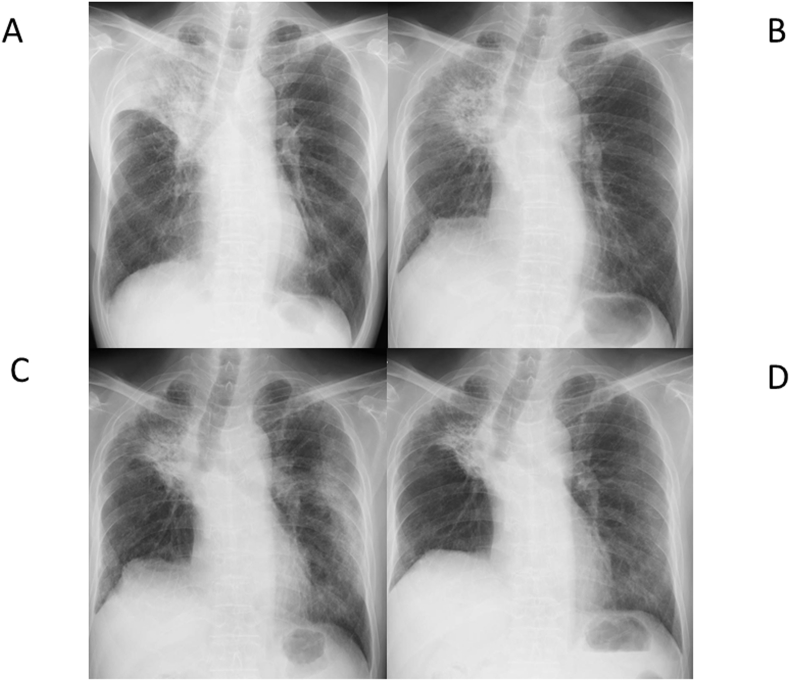
Fig. 1.
Chest X-rays showing reduced air entry of right upper lobe due to primary cancer lesion (A), primary cancer lesion and infiltrate shadows coinciding with the irradiated area (B), patchy ground grass opacity in the left lung (C), and the disappearance of the patchy ground grass opacity (D).
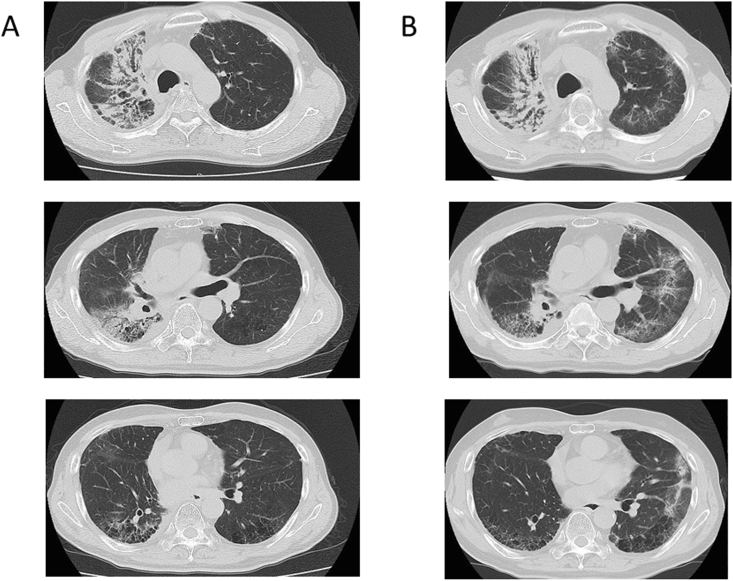
Fig. 2.
CT images showing infiltrate shadows in the right lung coinciding with the irradiated areas at 1 month (A) and patchy ground-grass opacity in the left lung at 12 months after completion of radiation therapy.
3. Discussion
Radiation-induced lung disease is a frequent complication of RT to the chest for breast or lung cancer and includes acute phase of radiation pneumonitis and chronic phase of radiation fibrosis. This lung cancer patient developed radiation pneumonitis inside the irradiated area and thereafter CEP in the contralateral lung field. We confirmed that there was not peripheral blood eosinophilia when he developed radiation pneumonitis. In contrast, not only peripheral but also pulmonary eosinophilia was found when he developed CEP. Typical CT features consist of confluent consolidations and ground glass opacities predominating in the upper lobes and peripheral subpleural areas [2]. The distribution of the shadows is usually bilateral, but can be unilateral in rare cases [1]. Recurrence of radiation pneumonitis is rare. Radiation fibrosis was excluded by the rapid improvement with oral corticosteroids without sequelae.
There are several reports of CEP following RT for breast cancer, suggesting the possibility that CEP may be facilitated by lung irradiation [1]. RT induces a diffuse bilateral and persistent lymphocytic infiltration [3]. Lymphocytes recruited to the lung mainly consist of activated CD4+ helper T cell. If Th2 cells dominate, which secrete IL-4, IL-5, and IL-13, this could lead to the development of CEP. The consequence may depend on genetic or acquired characteristics of patients, including asthma or allergy.
There is only limited number of report of radiation pneumonitis in a lung cancer patient, in which eosinophil counts increased in peripheral blood or bronchoalveolar lavage after RT (macrophage of 17%, eosinophils of 18%, lymphocytes of 60%, and neutrophils of 5%) [4]. However, reports of CEP after RT are almost exclusively limited to breast cancer and there is no report for lung cancer. This patient had asymptomatic peripheral blood eosinophilia at the initial diagnosis of lung cancer. This eosinophilia was thought to be a paraneoplastic syndrome [5], which might be a trigger for developing CEP. However, bronchoalveolar lavage revealed no malignant cells, indicating that CEP itself was not regarded as a paraneoplastic syndrome. Since the patient received no other chemotherapy after finishing 6 courses of treatment with carboplatin and paclitaxel, there was no possibility of drug-induced pneumonitis. Further accumulation of similar cases is needed to clarify the CEP after RT in lung cancer.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Cottin V., Frognier R., Monnot H. Chronic eosinophilic pneumonia after radiation therapy for breast cancer. Eur. Respir. J. 2004;23:9–13. doi: 10.1183/09031936.03.00071303. [DOI] [PubMed] [Google Scholar]
- 2.Cottin V. Eosinophilic lung diseases. Clin. Chest Med. 2016;37:535–556. doi: 10.1016/j.ccm.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Kawai S., Baba K., Tanaka H. A case of radiation pneumonitis with eosinophilia in bronchoalveolar lavage fluid. Ann. Jap Respir. Soc. 2008;45:44–49. [PubMed] [Google Scholar]
- 4.Roberts C.M., Foulcher E., Zaunder J.J. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann. Intern Med. 1993;118:696–700. doi: 10.7326/0003-4819-118-9-199305010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Pandit R., Scholnik A., Wulfekuhler L., Dimitrov N. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am. J. Hematol. 2007;82:234–237. doi: 10.1002/ajh.20789. [DOI] [PubMed] [Google Scholar]