Abstract
Background
Mutations in IRF6, CHUK (IKKA) and RIPK4 can lead to a disease spectrum that includes cutaneous, limb and craniofacial malformations. Loss of these alleles in the mouse leads to perinatal lethality and severe cutaneous, limb and craniofacial defects. Genetic rescue in the mouse has been shown for Ikka and Ripk4.
Results
Here, we show partial genetic rescue of Irf6 knockout embryos using the KRT14 promoter to drive Irf6 expression in the basal epithelium. In contrast to Irf6 knockout embryos, rescue embryos survive the immediate perinatal period. Macroscopic examination reveals rescue of skin adhesions between the axial and appendicular skeleton. Unexpectedly, KRT14-driven Irf6 expression does not completely rescue orofacial clefting and adhesions between the palate and tongue, suggesting the importance of cell-autonomous IRF6 expression in periderm. Like knockout embryos, Irf6 rescue embryos also have persistent esophageal adhesions, which likely contribute to postnatal demise.
Conclusion
Together, these data suggest that targeted expression of IRF6 can significantly reduce disease severity, but that a minimum level of Irf6 in both periderm and basal epithelial cells is necessary for orofacial development. Therefore, homologous human and mouse phenotypes are observed for IRF6, IKKA and RIPK4. In this work, we show that altering the expression level of IRF6 dramatically modified this phenotype in utero.
Keywords: IRF6, GRHL3, cleft lip and palate, Van der Woude Syndrome, Popliteal Pterygium Syndrome, limb defects, genetic rescue, mouse models, epithelium, epidermis, oral periderm
Introduction
Mutations in IRF6 lead to Van der Woude Syndrome (VWS OMIM #119300) and Popliteal Pterygium Syndrome (PPS OMIM #119500). VWS and PPS are rare orofacial clefting syndromes (Kondo et al., 2002). Common variants within the IRF6 locus also increase risk for isolated or nonsyndromic orofacial clefting (iCLP) (Zucchero et al., 2004). Loss of IRF6 expression in epithelial cells is also associated with skin and head and neck squamous cell carcinoma (Botti et al., 2011, Stransky et al., 2011). Loss of Irf6 in the mouse leads to skin, limb and craniofacial defects (Ingraham et al., 2006). Loss of Ikka (Takeda et al., 1999, Hu et al., 1999), Sfn (14-3-3-σ) (Richardson et al., 2006), Ripk4 (Holland et al., 2002), and Kdf1 (Lee et al., 2013) lead to similar phenotypes in the mouse. Furthermore, mutations in RIPK4 result in a similar but more severe form of PPS, called Bartsocas-Papas Syndrome (BPS OMIM # 263650). Mutations in CHUK result in a similar but more severe form of BPS, called Severe Fetal Encasement Malformation or Cocoon Syndrome (OMIM # 613630) (Leslie et al., 2015). The similarity in type and degree of affected tissues between mouse and human phenotypes suggests that the mouse models are relevant to the human disease process for these loci and diseases.
During embryonic development, the epithelium is composed of two cell types, basal and periderm cells. The basal layer contains the progenitor cells for more differentiated and superficial epithelial cells, including the periderm. The basal cell layer robustly expresses Keratins 14 and 5 (KRT14 and KRT5), intermediate filaments that contribute to the cellular cytoskeleton. As a result, the KRT14 and KRT5 promoters have been used to drive gene expression in the basal epithelium of transgenic mice (Sil et al., 2004, Liu et al., 2011). For instance, the KRT14 promoter was used to drive Ikka in an Ikka knockout embryo and rescued skin, limb and digit malformations. However, a curled tail persisted (Sil et al., 2004). A very similar experiment using the KRT5 promoter completely rescued the Ikka knockout phenotype only when the transgene was over-expressed (Liu et al., 2011). In both instances, rescued embryos had persistent esophageal adhesions and the pups died in the postnatal period. Similarly, the KRT14 promoter was used to drive Ripk4 expression in a Ripk4 knockout mouse. The KRT14-Ripk4 transgene rescued epidermal development but did not rescue the esophagus. Lack of esophageal rescue was attributed to weaker KRT14 promoter activity (Rountree et al., 2010). Interestingly, the KRT14-Ripk4 transgene did not rescue Ikka and Sfn knockout embryos (Rountree et al., 2010).
In addition to its role in skin development, IRF6 function is essential during early palatogenesis. In fact, palate development in Irf6 knockout mice arrests after palatal elongation (Ingraham et al., 2006, Richardson et al., 2006, Kousa and Schutte, 2016). As a result, Irf6 knockout mice have vertically oriented palatal shelves from E13.5 – E17.5 (Richardson et al., 2006, Ingraham et al., 2006). The palatal shelves remain vertically oriented because of adhesions between the lingual and palatal epithelium (Richardson et al., 2014). The importance of IRF6 in palatal elongation limits our understanding of its function in later stages of palatogenesis. Therefore, rescuing palatal adhesion in Irf6 knockout mice could produce an intermediate phenotype and allow for a more nuanced understanding of IRF6 in palatal development, including elevation of the palatal shelves, apposition and adhesion. The final stage of palatal development is fusion, which is marked by loss of epithelial cells and a breakdown of the basement membrane at the medial edge of palatal shelves (Kousa and Schutte, 2016).
Like Ikka and Ripk4, epithelial adhesions in Irf6 knockout embryos obliterate the esophageal lumen and fasten all appendages to the body wall (Ingraham et al., 2006). Therefore, like palate development, the severity of epidermal defects in Irf6 knockout mice has limited our ability to identify additional Irf6 functions in vivo. Additional functions are plausible because they have already been reported in a different cell and tissue type, i.e. tongue (Goudy et al., 2013).
Prior work showed the expression of Irf6 using the KRT14 promoter (TgKRT14::Irf6) (Iwata et al., 2013). Here, we use this transgenic mouse line to rescue epithelial defects in Irf6 knockout mice. Like Ikka and Ripk4, basal epithelial Irf6 expression in Irf6 knockout embryos delays perinatal lethality but does not prevent it, in part, due to esophageal adhesions. However, unlike Ikka and Ripk4 rescue experiments, limb, tail and craniofacial defects are not rescued by epithelial Irf6 expression. In addition, we find that Irf6 expression in basal epithelium of the oral cavity is not sufficient to completely rescue oral adhesions and palatal clefting. Importantly, tongue-palate oral adhesions appear to mechanically disrupt elevation of palatal shelves. Mechanistically, this work suggests that Irf6 has additional cell autonomous functions in vivo. This work is significant because we developed an animal model that appears more similar to the VWS-PPS syndromic disease spectrum, with cleft palate and less severe limb and epidermal defects.
Results
We wanted to determine if Irf6 expression into the basal epithelial layer of skin and the oral cavity could rescue Irf6 null embryos. To perform this experiment, we crossed mice that carried a null allele of Irf6 with mice that carried a transgene that uses the Keratin 14 (KRT14) promoter to drive the Irf6 cDNA into the basal epithelial layer. Genotyping of embryos from the experimental cross (Irf6+/−;TgKrt14::Irf6 × Irf6+/−) revealed a significant difference between the expected and predicted genotypic distribution (N=179; p-value = 0.000017) (Table 1). These differences were driven by fewer than expected Irf6 knockout (predicted 22; actual 9) and heterozygous embryos (predicted 44; actual 25). In addition, while prior work showed spontaneous resorptions of 1–3% in C57BL/6 mice (Krishnan et al., 1996), we detected a significant increase in embryonic resorptions (N=11, P-value = 0.048, Table 1). The number of Irf6 knockout embryos that also had the KRT14-Irf6 transgene (Irf6−/−;TgKrt14::Irf6, herein referred to as “rescue embryo/pups”) did not differ from the predicted genotypic distribution (predicted 22; actual 23).
Table 1.
First, we intercrossed mice hemizygous for the TgKRT14::Irf6 transgene (over-expressing Irf6 under the control of the KRT14 promoter) with mice heterozygous for the Irf6 genetrap allele (Irf6+/gt from here on referred to as Irf6+/−) to generate Irf6+/+; TgKRT14::Irf6 mice. We than intercrossed Irf6+/+;TgKRT14::Irf6 with littermates that are heterozygous for the genetrap allele (Irf6+/−). We examined embryos at E15.5 (13 litters) and pups at parturition (P0) (13 litters). We detected a significant number of embryonic resorptions at E15.5. Furthermore, we found a significant under-representation of Irf6−/− genotypes and corresponding phenotypes.
| E15.5 | P0 | Total | |
|---|---|---|---|
| Irf6+/+ | 12 | 10 | 22 |
| Irf6+/− | 8 | 17 | 25 |
| Irf6−/− | 1 | 8 | 9 |
| Irf6+/+; TgKRT14::Irf6 | 26 | 13 | 39 |
| Irf6+/−; TgKRT14::Irf6 | 31 | 19 | 50 |
| Irf6−/−; TgKRT14::Irf6 | 11 | 12 | 23 |
| Resorbing | 11 | N/A | 11 (P-value 0.049) |
| Total | 100 | 79 | 179 |
| P-value | 2.7×10−8 | 0.25 | 1.68×10−5 |
Rescue pups (Fig. 1B,E,H) had an intermediate phenotype (N=12) compared with wildtype (Fig. A,D, G) and Irf6 knockout littermates (Fig. 1G,F, I). Most strikingly, while Irf6 knockout pups died, rescue pups survived the perinatal period. Rescue pups were active and responsive to stimuli. However, rescue pups appeared to have periods of irregular breathing, including apnea events, with severe subcostal retractions (Supp Movie) and ultimately did not survive. We did not observe any gross phenotypic variation among the rescue pups.
Figure 1.
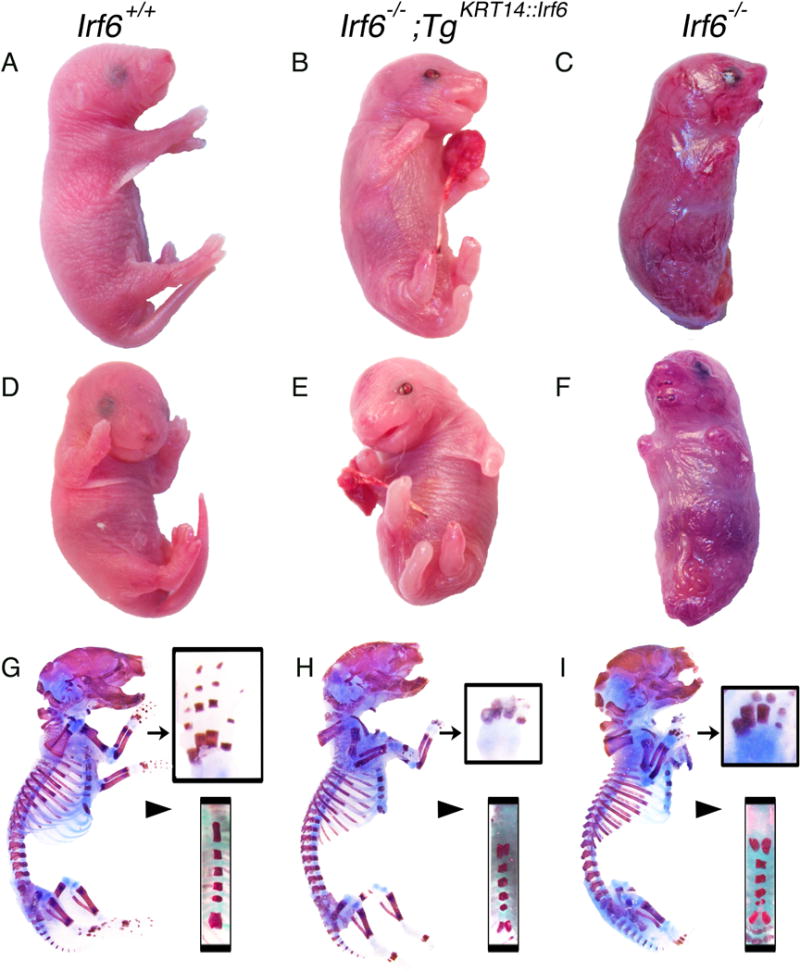
Transgenic KRT14::Irf6 delays perinatal lethality but does not rescue limb skeletal defects. Profile (A–C and G–I) and Frontal (D–F) views of representative P0 pups with the following genotypes; (A, D, G) Irf6+/+left column; (B, E, H) Irf6−/−;TgKRT14::Irf6 center column; (C, F, I) Irf6−/− right column. Gross analysis of Irf6−/−;TgKRT14::Irf6 (rescue pups) reveals perinatal survival, limb clubbing and syndactyly, a curled tail and a somewhat taut, shiny skin compared to wildtype littermates. Unlike Irf6−/− (knockout) littermates, the appendages of rescue embryos are not attached to the body wall and an open oral cavity is visible. (G–I) Profile views of skeletal preparations of P0 pups. Analysis of rescue pups reveals limb clubbing and syndactyly, in a manner highly analogous to Irf6−/− (arrow, top inset). In contrast, the sternum (arrow head, bottom inset) and in particular the xiphoid process appears less bifurcated in rescue pups.
Compared to wildtype littermates (Fig. 1A), rescue pups had more taut skin. Unlike knockout embryos (Fig. 1C,F), the tail and limbs of rescue embryos were not attached to the body wall (Fig. 1B,E). Despite rescue of skin and limb adhesion, clubbing of both upper and lower limbs persisted and digits could not be discerned (Fig. 1G–I). In addition, rescue pups had a curled tail but it was not attached to the body wall (Fig. 1A,E). Therefore, while the skin phenotype improved, and the tail and limbs were not attached to the body wall, appendage malformations persisted.
Like Ikka, Irf6 knockout embryos have severe skeletal malformations, which are thought to be secondary to cutaneous defects (Richardson et al., 2006, Ingraham et al., 2006). We analyzed the skeletons of wildtype, rescue and knockout pups to assess how Irf6 expression in epithelium affects the skeleton. The skeleton of rescue pups appeared different in two ways (Fig. 1B). First, despite preventing limb adhesion to the abdomen and thorax, rescue pups had abnormal digit ossification with fully penetrate clubbing of the upper and lower limbs (Fig. 1G–I). Second, while prior work showed Irf6 knockout embryos have a bifid xiphoid (Ingraham et al., 2006), rescue pups had an intermediately affected xiphoid, suggesting partial rescue (Fig. 1G–I). Therefore, Irf6 expression in basal epithelium did not completely rescue skeletal defects. These experiments suggest that IRF6 has a role in skeletal formation that is independent of its role in skin development.
In the epidermis, loss of Irf6 leads to an expanded, hyper-proliferative epidermis that lacks stratification and differentiation (Ingraham et al., 2006, Richardson et al., 2006). During epidermal development, IRF6 is expressed in basal and periderm cell layers (de la Garza et al., 2013, Fakhouri et al., 2012). In mature epidermis, IRF6 is primarily expressed in the spinous cell layer (Ingraham et al., 2006). We wanted to test how replacing Irf6 expression in the epidermis using the KRT14 promoter would affect epidermal development. First, we isolated mature murine skin at birth and isolated RNA. Consistent with the genotype and phenotype, rescue pups expressed more Irf6 than Irf6 knockout littermates (Fig. 2A). Irf6 expression level was not statistically different between rescue and wildtype pups. Therefore, rescue pups had a curled tail and clubbing of both upper and lower limbs despite epidermal Irf6 expression.
Figure 2.
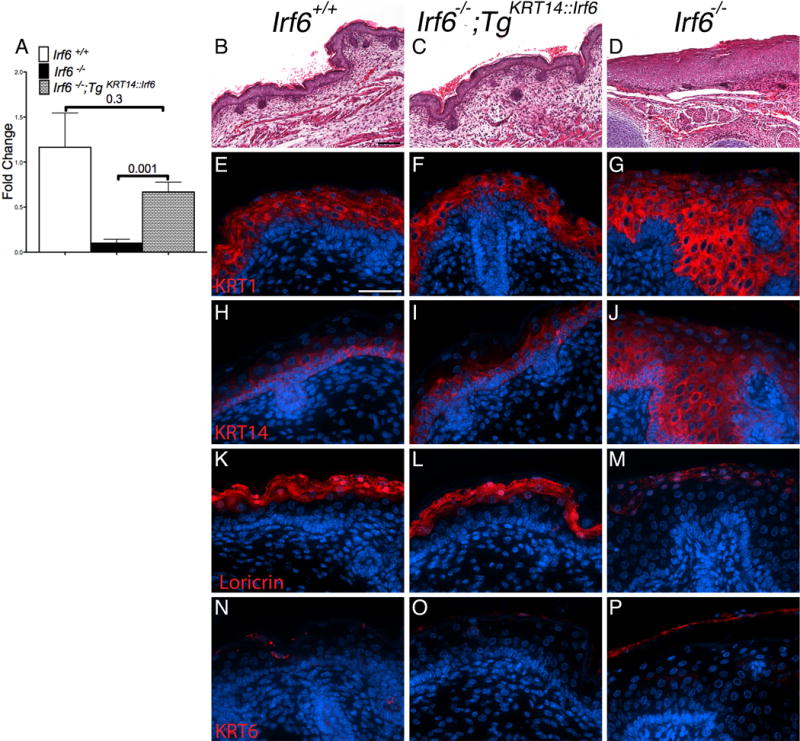
Irf6 expression using the KRT14 promoter rescues skin defects in knockout embryos. A) Analysis of skin mRNA levels reveals a significant increase in Irf6 expression in rescue (TgKRT14::Irf6) compared to knockout pups (Irf6−/−). No statistical differences are detected between rescue and wildtype pups (Irf6+/+). (B–D) Skin histological analysis (Hematoxylin and Eosin) reveals epidermal hypertrophy in (D) knockout but not in rescue (B) and wildtype (C) pups. E–P) Immunostaining of KRT1, KRT14, Loricrin and KRT6. (E–J) Rescue pups have epithelial stratification, without ectopic KRT1 and KRT14 expression. (K–M) Rescue pups demonstrate epithelial differentiation (Loricrin), similar to wild type embryos. (N–P) Irf6 knockout embryos have markers of cellular stress in mature skin (KRT6). Scale bars: (B–C) =100 μm; (E–P) = 50 μm.
The epidermis of rescue pups (Fig. 2C) was more similar to wildtype (Fig. 2A) than knockouts littermates (Fig. 2D). We used markers of epidermal differentiation to examine epithelial stratification in rescue embryos. At birth, these markers were KRT14 for the basal cell layer, KRT1 for the spinous cell layer, Loricrin for the granular layer (Richardson et al., 2006, Ingraham et al., 2006) and KRT6 for hyperproliferation and injury (Stoler et al., 1988). Rescue pups had a stratified epidermis, as shown by expression of KRT1, KRT14 and Loricrin (Fig. 2F,I,L). Irf6 knockout pups (Fig. 2G,J,M) had ectopic expression of KRT14, an expanded spinous layer and absences of stratified epidermis, as shown previously (Richardson et al., 2006, Ingraham et al., 2006). Furthermore, Irf6 knockout embryo (Fig. 2P) had expression of KRT6, a marker of stress, in contrast to wildtype (Fig. 2N) and rescue pups (Fig. 2O).
Prior work shows that Irf6 knockout embryos have cleft palate with ubiquitous oral and esophageal adhesions (Ingraham et al., 2006, Richardson et al., 2006). We predicated that replacing epithelial Irf6 would rescue oral adhesions and cleft palate. At birth, rescue embryos had a cleft palate in the anterior, middle and posterior oral cavity (Fig. 3B,E,H). In contrast to knockout littermates (Fig. 3C,F,I), rescue pups had less extensive oral adhesions throughout the oral cavity. In the anterior and middle palate, rescue embryos had adhesions superficial to the tooth germ between maxillary and mandibular epithelium (Fig. 3B,E). More posteriorly, rescue embryos had adhesions between the tongue and palate but not at the tooth germ (Fig. 3H). Unexpectedly, the posterior palate of rescue embryos also had oral fusions between the tongue and palate (Fig. 3H), a phenotype previously undocumented in Irf6 knockout embryos.
Figure 3.
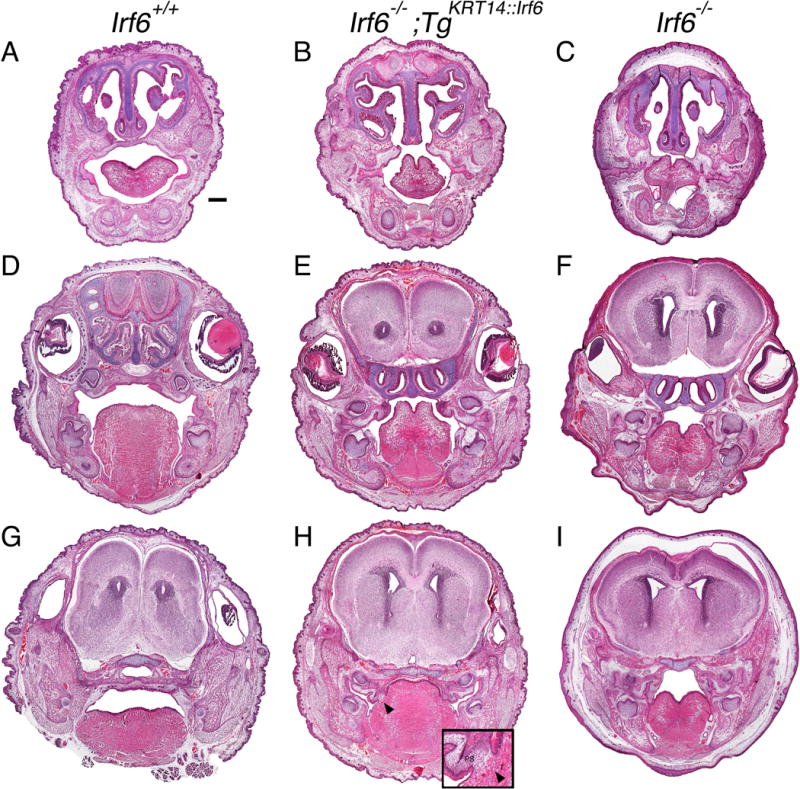
Rescue embryos have oral adhesions and cleft palate at birth. Head coronal sections of perinatal pups stained with H&E. A–C) anterior, D–F) middle and G–I) posterior oral cavities of wildtype (A, D, G), rescue (B, E, H) and knockout (C, F, I) pups. The oral cavity of rescue pups is less severely affected overall but oral adhesions persist bilaterally in the anterior oral cavity (B) and in the middle oral cavity between the maxilla and mandible at the tooth germ (E). In the posterior palate of rescue embryos (H), adhesions and a fusion (black arrow head, enlarged with inset) is observed between the palate and the tongue. (A) Scale bar = 500 μm. T, Tongue; PS, palatal shelves.
To identify the pathophysiological mechanism contributing to oral clefts in rescue pups, we examined an earlier timepoint in palatal development. We chose E15.5 because it marks the conclusion of palatal closure in wildtype embryos. At E15.5, rescue embryos had bilateral oral adhesions at the tooth germ in anterior and middle portions of the palate (N = 11) (Fig. 4B,E). We also observed adhesion between the nasal (medial) surface of the palatal shelves and the tongue (Fig. 4B). However, we did not observe oral adhesions between the oral (lateral) surface of the palatal shelves and the mandibular epithelium (Fig. 4E). As a result of this pattern of palatal adhesion, the palatal shelves elevated in the anterior and middle portions of the oral cavity (Fig. 4B,E). In many histological sections, the palatal shelves appeared to be moving toward midline despite adhering to tongue. In some section, the palatal shelves appeared to be pulling the adhered tongue epithelium medially (Fig. 4B,E). In the posterior oral cavity, palatal shelves adhered to the mandible and did not appear to elevate (Fig. 4H).
Figure 4.
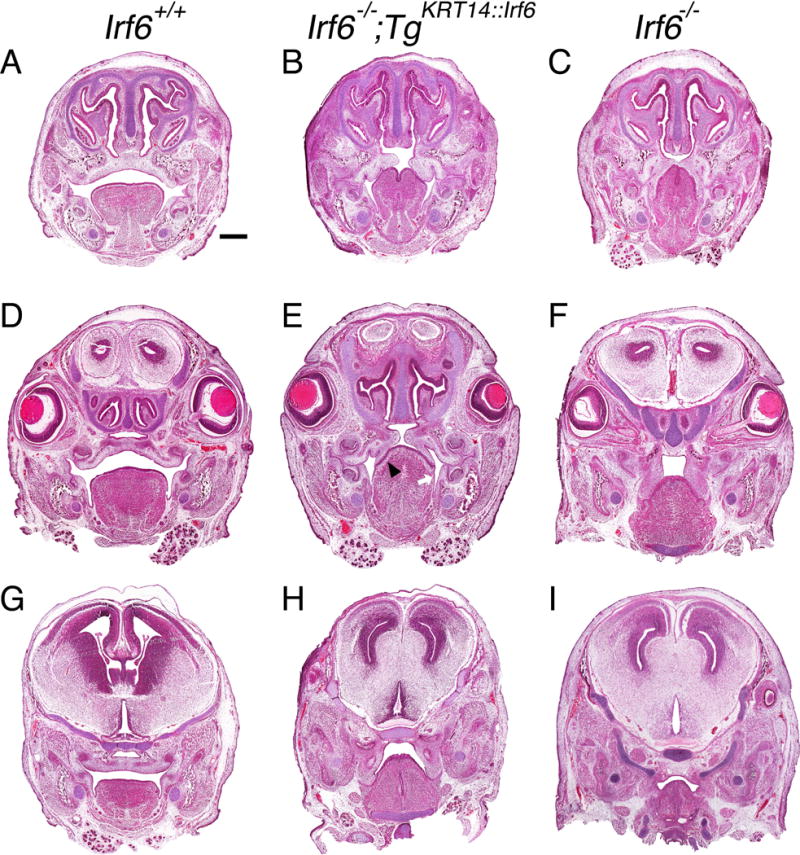
Tongue-palate oral adhesions hinder palatal development in rescue embryos at E15.5. Head coronal sections stained with H&E in (A–C) anterior, (D–F) middle, (G–I) posterior sections of the oral cavity. Three comparison groups are illustrated, including (A, D, G) wildtype (Irf6+/+), (B, E, H) rescue (Irf6−/− ;TgKRT14::Irf6) and (C, F, I) knockout (Irf6−/−) embryos. Rescue embryos had a cleft palate in anterior (B), middle (E) and posterior (H) sections of the oral cavity. Oral adhesions between the mandible and maxilla were similar between rescue (E) and knockout embryos (F). In contrast, oral adhesions between the palate and the tongue were less severe in rescue embryos (E, black arrow head). We did not detect oral adhesions between palatal shelves and the mandible (E, white arrow). In posterior oral cavity, oral adhesions between the palatal shelves and tongue were less severe but the palatal shelves did not reorient toward midline or elevate. (A) Scale bar = 500 μm.
To investigate the molecular mechanism leading to cleft palate in rescue embryos, we tested for markers of the oral epithelium in the middle oral cavity at E15.5 (Fig. 5). Previous work showed Irf6 regulates development of the periderm and that periderm prevents pathological epithelial adhesions (Richardson et al., 2014). We used KRT6, KRT17 and GRHL3 as periderm markers (de la Garza et al., 2013, Peyrard-Janvid et al., 2014). Unexpectedly, we detected KRT6 expression in rescue (Fig. 5E) and knockout (Fig. 5F) embryos at sites of oral adhesion between the tongue and palate. We also detected KRT14 and IRF6 expression at these sites in rescue embryos (Fig. 5H,K). In contrast to KRT6, we did not detect GRHL3 expression in periderm at sites of oral adhesions in rescue embryos (Fig. 5N). Rescue embryos showed more Activated Caspase 3 staining along the nasal surface of the palatal shelves (Fig. 5Q) when compared to knockout littermates (Fig. 5R).
Figure 5.
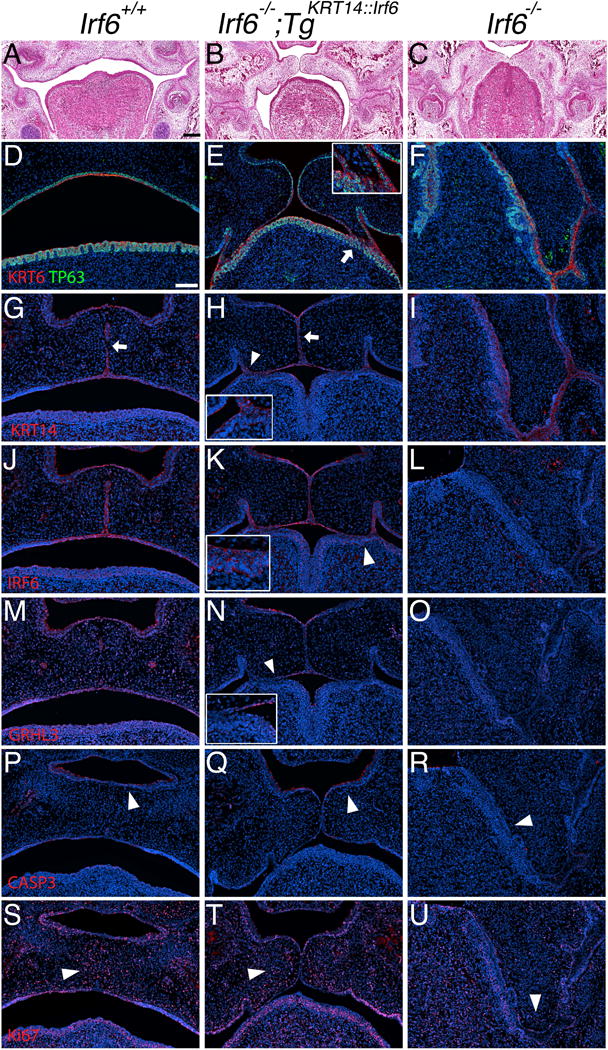
Oral adhesions between lingual and palatal epithelium prevents re-orientation and apposition of the palatal shelves. (A–U) Coronal section of E15.5 middle oral cavity with H&E (A–C) and immunostaining (D–U). Left column (images: A, D, G, J, M, P, S) shows wildtype embryos (Irf6+/+). Middle column (images: B, E, H, K, N, Q, T) shows rescue embryos (Irf6−/−;TgKRT14::Irf6). Right column (images: C, F, I, L, O, R, U) shows knockout embryos (Irf6−/−). (A) Wildtype embryos have a formed palate, whereas palatal shelves in (C) knockout embryos do not elevate. Palatal shelves in (B) rescue embryos (Irf6−/−;TgKRT14::Irf6) are able to re-orient and reach midline but do not fuse. (D–U) Immunostaining of (D–F) KRT6 (red) and TP63 (Green), (G–I) KRT14 (red), (J–L) IRF6 (red), (M–O) GRHL3 (red), (P–R) Activated Caspase 3 (red), (S–U) Ki- 67 (red). (D–F) In rescue embryos, TP63 marks the basal cell layer and KRT6 staining highlights the superficial epithelial layer. KRT6 marks periderm but was detected in areas of adhesion between the palate and the tongue (white arrow, enlarged with inset in top, right corner for rescue embryo) (E). (G–I) Unlike rescue embryos, loss of KRT14 immunostaining in wildtype embryos (G) indicates loss of the medial edge epithelium (white arrow). In rescue embryos, palatal shelves do not fuse even when there is apposition of the palatal shelves (H). Areas of epithelial adhesion show a KRT14 signal (white arrow head, enlarged with inset in bottom, left corner for rescue embryo). (J–L) Immunostaining shows a signal for IRF6 in the oral epithelium of rescue embryos (K), including sites of adhesion between the palate and tongue (white arrow head, enlarged with inset in bottom, left corner for rescue embryo). (M–O) In contrast to KRT6 expression in rescue embryos (compare E vs. N), we did not detect a GRHL3 signal in areas with palate-lingual oral adhesions (white arrow head, image N, enlarged with inset in bottom, left corner of rescue embryo). (P–R) A signal for Activated Caspase 3 was detected on the nasal surface of the palatal shelves in both (P) wildtype and (Q) rescue embryos but not in knockout littermates (white arrow head in each image). (S–U) Examination of proliferation shows a cluster of Ki-67-positive cells in the mesenchyme of (S) wildtype and (T) rescue embryos near the middle edge epithelium but not in (U) knockout littermates (white arrow head in each image). Scale bar (A) = 200 μm, (D) = 100 μm.
Immunostaining for Ki-67, a marker of cellular proliferation, appeared to show mesenchymal proliferating cells localizing in the palatal shelves near the medial edge epithelium of wildtype (Fig. 5S) and rescue embryos (Fig. 5T) but not knockout littermates (Fig. 5U). In addition, immunostaining for KRT17, like KRT6 a marker of oral periderm, showed a discontinuous expression pattern in the epithelium of rescue embryos (Fig. 6B), compared to a continuous expression pattern in wildtype (Fig. 6A) and knockout embryos (Fig. 6C). JAG2 expression was detected in areas of oral adhesion between the lingual and palatal epithelium of rescue embryos (Fig. 6E). In contrast, we did not detect JAG2 expression in areas of adhesion in knockout embryos (Fig. 6F). Similarly, MMP13 expression was detected in areas of adhesion between the lingual and palatal epithelium in rescue embryos (Fig. 6H) but not in knockout embryos (Fig. 6I).
Figure 6.
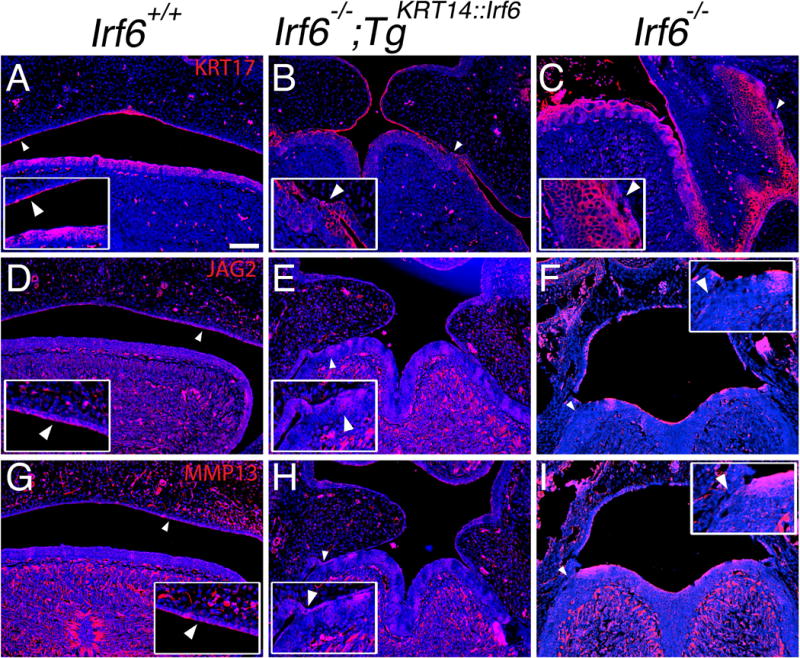
JAG2 and MMP13 expression in sites of oral adhesions between lingual and palatal epithelium. (AI) Coronal section of E15.5 middle oral cavity with immunostaining. Left column (images: A, D, G) shows wildtype embryos (Irf6+/+). Middle column (images: B, E, H) shows rescue embryos (Irf6−/− ;TgKRT14::Irf6). Right column (images: C, F, I) shows knockout embryos (Irf6−/−). Immunostaining of (AC) KRT17 (red), (D–F) JAG2 (red), (G–I) MMP13 (red), with nuclear staining (blue). (A–C) Compared to wildtype (A) and knockout (C) embryos, we found a discontinuous KRT17 expression pattern in rescue embryos (B) (white arrow head, enlarged with inset). (D–F) Unlike knockout embryos (F), we found increased JAG2 expression at sites of oral adhesions in rescue embryos (E) (white arrow head, enlarged with inset). (G–I) Similar to JAG2, MMP13 expression was increased at sites of oral adhesions in rescue embryos (H) compared to knockout embryos (I) (white arrow head, enlarged with inset in bottom, left corner for rescue embryo). Scale bar (A) =100 μm.
Like Ikka and Ripk4 rescue embryos, we wanted to examine the esophagus of Irf6 rescue embryos for persistent esophageal adhesions. Histological examination of the thoracic cavity revealed fully penetrant esophageal adhesions in rescue pups (Fig. 7B). Surprisingly, these adhesions were highly similar to, but morphologically distinct from, Irf6 knockout embryos (Fig. 7C). Consistent with prior work (Xie et al., 1998), we detected KRT14 expression in wildtype esophageal epithelium (Fig. 7D–F), suggesting transgene promoter activity. However, previous reports also showed that the KRT14 promoter is >1000-fold less active in the esophagus (Rountree et al., 2010). Consistent with this finding, we found that KRT14 immunostaining in the esophagus required 10-fold more exposure time relative to the skin.
Figure 7.
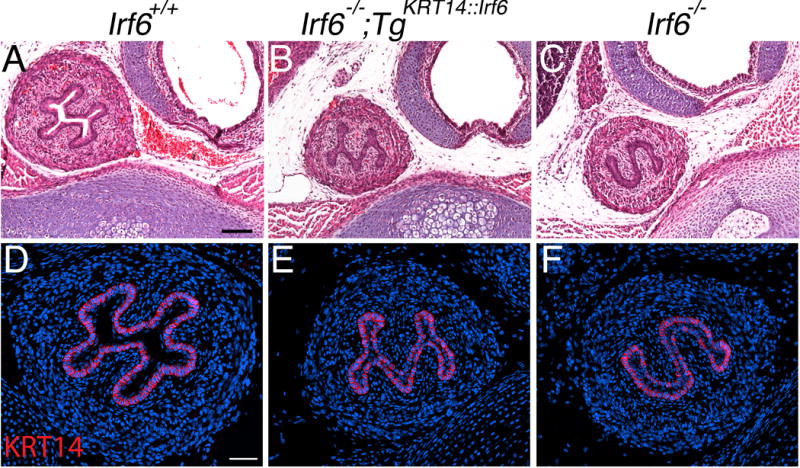
Rescue embryos have esophageal adhesions that contribute to delayed perinatal lethality. (A–C) H&E and (D–F) immunostaining staining of transverse sections from P0 pups of (A, D) wildtype (Irf6+/+), (B, E) rescue (Irf6−/−;TgKRT14::Irf6) and (C, F) knockout (Irf6−/−) sections. In stark contrast to the open lumen in wildtype pups (A, D), both rescue (B, E) and knockout (C, F) littermates have extensive esophageal adhesions. However, there was a distinct difference in the shape of the adhesion, with rescue pups having an “M” shaped adhesion while knockout embryos have had an “S” shaped adhesion. (D–F) Immunostaining for KRT14 revealed a signal but required a 10-fold greater fluorescent exposure time compared to skin. Scale bars (A) = 100 um, (B) = 50 um.
Discussion
We used a transgenic mouse line expressing IRF6 under the control of the KRT14 promoter to test the role of IRF6 in basal epithelial cells of Irf6 knockout embryos. In the palate, our data suggest that KRT6 expression is not a reliable marker for functional periderm during epithelial contact because a signal for KRT6 can be detected at sites of oral adhesions in rescue and knockout embryos. Importantly, prior work shows that IRF6 regulates GRHL3 and that GRHL3 is expressed in periderm (de la Garza et al., 2013). In contrast to KRT6, we detected epithelial GRHL3 expression in rescue embryos but not in knockout embryos. Therefore, basal IRF6 expression was at least partially sufficient for GRHL3 expression in oral periderm in vivo.
Interestingly, despite continuous epithelial IRF6 expression in rescue embryos (Fig. 5K, arrowhead), GRHL3 expression was discontinuous (Fig. 5N, arrowhead). It is not clear why GRHL3 expression was discontinuous despite continuous IRF6 expression. Sites lacking epithelial GRHL3 expression also had oral adhesions. It is not known if GRHL3 expression can rescue oral adhesions. Nonetheless, these data suggest that GRHL3 is a more specific marker for functional periderm whereas KRT6 might be a more sensitive indictor for the presence of a peridermal stress response (Stoler et al., 1988). like KRT6, KRT17 expression in knockout embryos might also be a marker of cellular stress. These data also suggest IRF6 expression in basal epithelial cells is sufficient for periderm development but cell-autonomous IRF6 expression is necessary for complete restoration of periderm function. Expression of JAG2 and MMP13 in adhered oral epithelium between lingual and palatal epithelium might explain the oral fusion in the posterior palate of rescue embryos (Fig. 3H) and further elucidate why oral fusions are not observed in knockout embryos (Fig. 3C,F,I) {Richardson, 2009 #261}. Importantly, loss of GRHL3 can lead to Van der Woude Syndrome (Peyrard-Janvid et al., 2014) and these results might provide some mechanistic insight into how a cleft palate forms in vivo.
Palatal shelve adhesion to the lingual or mandibular epithelium provided a striking contrast between knockout and rescue embryos. In general, replacing basal IRF6 expression rescued adhesions between the palatal shelves and mandibular epithelium. In contrast, adhesions between the palatal shelves and lingual epithelium persisted but were less severe. These findings indicate important biological implications for palatal development. First, it shows that not all palatal epithelium responds to basal IRF6 expression in the same way. Second, palatal-lingual adhesions allowed us to capture a dramatic moment in palatal development. Strikingly, oral adhesions between the tongue and palate seemed to physically prevent reorientation of the palatal shelves toward midline. Despite lateral attachment to the tongue, the palatal shelves moved medially. This process was not uniform, as posterior aspects of the palatal shelves did not elevate.
There are two important implications to these findings. First, it suggests that oral adhesions to the tongue can physically restrain palatal adhesion. These data invoke a Pierre Robin-like sequence, mediated by physical attachment to the tongue (adhesion) as opposed to obstruction (micrognathia). In support of this model, we recently showed that all Grhl3 knockout embryos have bilateral oral adhesions between the mandible and maxilla but that clefting only resulted when lingual-palatal adhesions were also present (Peyrard-Janvid et al., 2014). Second, this data is consistent with palatal reorientation, rather than elevation, in the development of anterior and middle sections of the palatal shelves (Bush and Jiang, 2011, Walker, 1956). Similarly, these data also support a more fluid determination of the medial edge epithelium rather than a single cohort of cells along the palate. Finally, this work suggests that not all oral adhesions are contributing to the same disease process in the oral cavity. Modulating epithelial attachment may be a pre-clinical target for future preventative strategies.
Importantly, our findings at E15.5 and P0 suggest that earlier stages of palatal development are not perturbed. For instance, at E15.5, we see that palatal shelves have surpassed the stage of being a flat epithelium (E12.5) and undergoing mesenchymal proliferation to enlarge (E13.5) (Kousa and Schutte, 2016). In addition, at E15.5, we also observed that palatal shelves were elevating (E14) and adhering (E14.5) in several rescue embryos. Despite adhering, palatal shelves failed to fuse (E15.5 to E16). Therefore, while an examination of earlier timepoints might show a delay in these milestones, the phenotype observed at P0 can be traced back to a defect at E15.5. In fact, we designed our experiments so that we could trace back from P0 pups, using each timepoint as a “mile marker” in development. We concluded our experiments at E15.5 because we observed adhered palatal shelves that did not fuse.
In addition, we found that replacing IRF6 in basal epithelial cells is sufficient for cutaneous epidermal stratification. In contrast, replacing epithelial IRF6 delayed but did not prevent perinatal lethality. Like Ikka and Ripk4, replacing IRF6 using the KRT14 promoter did not prevent esophageal adhesions, suggesting that endogenous expression is critical. These esophageal adhesions likely played a role in perinatal demise in rescue pups. Unlike Ikka and Ripk4, expression of IRF6 in basal epithelium was not sufficient to completely rescue limb and tail defects or cleft palate. This work suggests that either endogenous epithelial IRF6 expression is playing a non-cell autonomous role in the development of these tissues or that IRF6 is expressed in and regulates other tissues. Consistent with the latter hypothesis, the IRF6 enhancer (MCS9.7) is active in the hindlimbs, cartilage primordium of the humerus (forelimb) and metacarpals (digits) (Fakhouri et al., 2012). DNA variants in MCS9.7 are associated with risk for nonsyndromic cleft lip and palate (Rahimov et al., 2008) and VWS (Fakhouri et al., 2014).
Locus heterogeneity has been reported for Ripk4, Ikka, Irf6, Sfn and Kdf1. However, unlike Ikka and Ripk4, limb and tail defects were not rescued with epithelial Irf6 expression (Rountree et al., 2010). Furthermore, epithelial Ripk4 did not rescue either Ikka or Sfn knockout embryo. Together, these results suggest that either Ripk4 1) is involved in an independent parallel pathway, 2) requires Ikka and/or Sfn for activation or 3) is peripherally involved in this network. Consistent with a peripheral role in this gene regulatory network, Ripk4 knockouts are less severely affected when compared to Ikka, Irf6, Sfn and Kdf1 knockout mice. Therefore, the common knockout phenotype for these fives alleles might be mediated through multiple cellular and molecular means as opposed to a single molecular pathway. Consistent with this model, Sfn and Irf6 interact in vivo while Ikka and Irf6 do not (Richardson et al., 2006). Interestingly, it might be possible to at least partially rescue Sfn knockout embryos using the KRT14::Irf6 transgenic allele reported here.
Experimental Procedures
Murine crosses
Use of, husbandry and procedures involving research animals were approved by the Michigan State University Institutional Animal Care and Use Committee (AUF # 05/12-093-00). Harem matings (4 females with a single breeder male) were used to enhance pregnancy rates, and presence of a copulation plug was denoted at E0.5. We used a recently characterized transgene that drives Irf6 expression under the control of the KRT14 promoter (Iwata et al., 2013) to rescue Irf6 knockout embryos (Irf6+/gt, referred to as Irf6+/−) (Ingraham et al., 2006). We first inter-crossed Irf6+/− and TgKrt14::Irf6 to produce Irf6+/−;TgKrt14::Irf6. We than inter-crossed Irf6+/−;TgKrt14::Irf6 with Irf6+/−. Rescue embryos (Irf6−/−;TgKrt14::Irf6) had an expected yield of 12.5%. We examined embryos at two developmental time points, E15.5 and just upon birth (P0). Genotyping was performed as described previously (Ingraham et al., 2006, Iwata et al., 2013).
Morphological and histological analysis
All embryos and pups were grossly examined upon parturition or dam euthanasia. After initial inspection, embryos and pups were placed into freshly prepared 4% paraformaldehyde (245–684, per Protocol). Upon fixation at 4°C for 16–24 hours, embryos and pups were dehydrated in 50–80% ethanol. Embryos were than paraffin embedded and sectioned at 7 μm intervals for both craniofacial and thoracic tissues. Histological analysis with hematoxylin (GHS332, Sigma) and eosin (E511-25, Fisher Chemical) staining was performed as described previously (Ingraham et al., 2006). Briefly, we removed the paraffin with a series of short xylene incubations. We than hydrated the tissues using a series of increasingly diluted ethanol solutions. Following short incubations in Eosin (90 seconds) and hematoxylin (90 seconds), we dehydrated the tissue using a series of decreasingly diluted ethanol solutions. Following xylene incubations, the tissue was mounted (Permount, SP15-100, Fisher Scientific), visualized and obtained images. We used the eyes as a landmark to determine anterior (anterior to eyes), middle (at eyes) and posterior (posterior to eyes) sections of the oral cavity.
Molecular analyses of murine tissues
Similar to hematoxylin and eosin staining, we performed a series of tissue incubations in xylene and ethanol to remove paraffin and hydrate the tissue. Following this step, we performed antigen retrieval in sodium citrate (pH6.0) and permeabilization in Triton X-100 (VWR). We than performed a series of washing steps to remove the detergent. We then performed two blocking steps, each lasting one hour. The first blocking step was 10% BSA in PBS. After two brief washing steps in PBS, we than incubated the slides in 40 μg/ml of goat anti-mouse Fab fragment in PBS (Jackson ImmunoResearch Laboratories, 115-007-003). Primary antibodies were incubated for 18–24 hours at 4°C. Primary antibodies included TP63 (Santa Cruz, 4A4, sc-8431), Keratin 6 (Covance, PRB-169P), Keratin 14 (Novocastra, NCL-L-LL002), IRF6 (Sigma-Aldrich, SAB2102995), Keratin 1 (NCL-CK1, Novocastra), Loricrin (PRB-145P, Covance), Ki-67 (ab15580, Abcam), GRHL3 (a kind gift from Dr. B. Andersen, UCI, Irvine, CA), Activated Caspase 3 (Abcam, Ab13847), KRT17 (Sigma-Aldrich, HPA000453) JAG2 (ThermoFisher, PI701287) and MMP13 (Sigma-Aldrich, HPA030636). Secondary antibodies (goat anti-mouse and goat anti-rabbit (Molecular Probes)) were incubated for 1–2 hours. Nuclei were labeled with DAPI (Invitrogen).
Skeletal Preparation
Skeletons were processed as described previously (Ingraham et al., 2006). Skin and subcutaneous fat was removed from fixed samples. Following gross dissection, the samples were incubated in 70% ethanol for 24 hours and then 95% ethanol for 24 hours. Samples were than incubated for 72 hours in 2% KOH. Following incubation, skeletons were stained in Alcian blue for cartilage (Sigma, A5268-10G). De-staining of Alcian blue in 95% ethanol was followed by Alizarin Red staining for bone (Sigma-Aldrich, A5533-25G) for 24–36 hours. Skeletal tissue was than placed in 1%KOH/20% Glycerol solution until images were obtained.
Bioimaging using Upright/Fluorescent microscope and Stereomicroscope
We imaged tissue on an upright microscope (Nikon Eclipse 90i upright) with a 4×, 10× and 40× objectives. NIS Elements Advanced Research v3.10 imaging software was used to obtain and to analyze images. Enhancement was limited to program algorithms, applied evenly to all samples, and only included deconvolution and sharpening with Gauss-LaPlace. To capture whole mount embryo images, we used a SMZ1000 Nikon microscope with both Fiber Optic Gooseneck and Ring Light sources and NIS-Elements Software 4.11. We used Adobe Photoshop Elements v9.0 to assemble and produce the figures.
Transcriptional profiling using quantitative-PCR
Dorsal skin was collected from pups after euthanasia. We snap froze the tissue in liquid nitrogen and used a TRIzol RNA extraction kit (15596-026, Ambion). To prevent DNA contamination, we treated the samples with RNase-Free DNase (79254, Qiagen) for 30 minutes, which was followed by heat inactivation at 65°C. To purify the RNA, we used acidic phenol and chloroform extraction. After purification, we resuspended the RNA in RNase-free H2O and incubated at 55°C for 10 minutes. To make cDNA, we used Oligo dT primers (18418-012, Invitrogen), dNTP mix (18427-013, Invitrogen), SuperScriptIII Reverse Transcriptase (18080-093, Invitrogen) and Recombinant RNasin Ribonuclease Inhibitor (N2511, Promega). The negative control for this reaction did not include either the SuperScriptIII Reverse Transcriptase or the Recombinant RNasin Ribonuclease Inhibitor. We used SYBR Green (4309155, Applied Biosystems) to quantify transcript levels from total starting material of 5.5 ng of cDNA. We quantified fold change using the delta-delta Ct method relative to beta-actin. All reactions were performed with three technical replicates per biological sample. Irf6 primers (5′ to 3′) were AGTGTGGCCCAAAACAGAAC (forward) and GGGTTGCTCACCGTCATAGT (reverse).
Statistics
We used Excel (version 2010) and GraphPad Prism Software (version 5) to analyze data. All tables and histograms were constructed within GraphPad. We used a Student’s t-test to determine significance and rejected the null hypothesis with a P-value equal to or below 0.05.
Supplementary Material
Acknowledgments
Financial support to YAK (F31DE022696-01) came from the NIH National Institute of Dental and Craniofacial Research. Additional support to BCS was provided from NIH (DE13513) and Michigan State University grants.
Footnotes
Author Contributions
YAK and BCS conceived of the work. YAK designed and performed all murine crosses, completed statistical analyses, analyzed all data, prepared the figures and tables and wrote the manuscript. YAK performed and analyzed morphological phenotyping and histological analysis, immunostaining, qPCR, skeletal preps and captured images. DM performed histological stains and analysis, conducted and analyzed immunostaining and captured and collected images. YAK supervised all mouse genotyping and statistical analyses. BCS supervised the project. All co-authors reviewed the manuscript. Edits were reviewed and incorporated by YAK and BCS.
References
- BOTTI E, SPALLONE G, MORETTI F, MARINARI B, PINETTI V, GALANTI S, DE MEO PD, DE NICOLA F, GANCI F, CASTRIGNANO T, PESOLE G, CHIMENTI S, GUERRINI L, FANCIULLI M, BLANDINO G, KARIN M, COSTANZO A. Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proc Natl Acad Sci U S A. 2011;108:13710–5. doi: 10.1073/pnas.1110931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSH JO, JIANG R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2011;139:231–43. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA GARZA G, SCHLEIFFARTH JR, DUNNWALD M, MANKAD A, WEIRATHER JL, BONDE G, BUTCHER S, MANSOUR TA, KOUSA YA, FUKAZAWA CF, HOUSTON DW, MANAK JR, SCHUTTE BC, WAGNER DS, CORNELL RA. Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of grainyhead-like 3. J Invest Dermatol. 2013;133:68–77. doi: 10.1038/jid.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAKHOURI WD, RAHIMOV F, ATTANASIO C, KOUWENHOVEN EN, FERREIRA DE LIMA RL, FELIX TM, NITSCHKE L, HUVER D, BARRONS J, KOUSA YA, LESLIE E, PENNACCHIO LA, VAN BOKHOVEN H, VISEL A, ZHOU H, MURRAY JC, SCHUTTE BC. An etiologic regulatory mutation in IRF6 with loss- and gain-of-function effects. Hum Mol Genet. 2014;23:2711–20. doi: 10.1093/hmg/ddt664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAKHOURI WD, RHEA L, DU T, SWEEZER E, MORRISON H, FITZPATRICK D, YANG B, DUNNWALD M, SCHUTTE BC. MCS9.7 enhancer activity is highly, but not completely, associated with expression of Irf6 and p63. Dev Dyn. 2012;241:340–9. doi: 10.1002/dvdy.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUDY S, ANGEL P, JACOBS B, HILL C, MAININI V, SMITH AL, KOUSA YA, CAPRIOLI R, PRINCE LS, BALDWIN S, SCHUTTE BC. Cell-autonomous and non-cell-autonomous roles for IRF6 during development of the tongue. PLoS One. 2013;8:e56270. doi: 10.1371/journal.pone.0056270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND P, WILLIS C, KANALY S, GLACCUM M, WARREN A, CHARRIER K, MURISON J, DERRY J, VIRCA G, BIRD T, PESCHON J. RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation. Curr Biol. 2002;12:1424–8. doi: 10.1016/s0960-9822(02)01075-8. [DOI] [PubMed] [Google Scholar]
- HU Y, BAUD V, DELHASE M, ZHANG P, DEERINCK T, ELLISMAN M, JOHNSON R, KARIN M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–20. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- INGRAHAM CR, KINOSHITA A, KONDO S, YANG B, SAJAN S, TROUT KJ, MALIK MI, DUNNWALD M, GOUDY SL, LOVETT M, MURRAY JC, SCHUTTE BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–40. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWATA J, SUZUKI A, PELIKAN RC, HO TV, SANCHEZ-LARA PA, URATA M, DIXON MJ, CHAI Y. Smad4-Irf6 genetic interaction and TGFbeta-mediated IRF6 signaling cascade are crucial for palatal fusion in mice. Development. 2013;140:1220–30. doi: 10.1242/dev.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONDO S, SCHUTTE BC, RICHARDSON RJ, BJORK BC, KNIGHT AS, WATANABE Y, HOWARD E, DE LIMA RL, DAACK-HIRSCH S, SANDER A, MCDONALD-MCGINN DM, ZACKAI EH, LAMMER EJ, AYLSWORTH AS, ARDINGER HH, LIDRAL AC, POBER BR, MORENO L, ARCOS-BURGOS M, VALENCIA C, HOUDAYER C, BAHUAU M, MORETTI-FERREIRA D, RICHIERI-COSTA A, DIXON MJ, MURRAY JC. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–9. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUSA YA, SCHUTTE BC. Toward an orofacial gene regulatory network. Dev Dyn. 2016;245:220–32. doi: 10.1002/dvdy.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHNAN L, GUILBERT LJ, RUSSELL AS, WEGMANN TG, MOSMANN TR, BELOSEVIC M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–52. [PubMed] [Google Scholar]
- LEE S, KONG Y, WEATHERBEE SD. Forward genetics identifies Kdf1/1810019J16Rik as an essential regulator of the proliferation-differentiation decision in epidermal progenitor cells. Dev Biol. 2013;383:201–13. doi: 10.1016/j.ydbio.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESLIE EJ, O’SULLIVAN J, CUNNINGHAM ML, SINGH A, GOUDY SL, ABABNEH F, ALSUBAIE L, CH’NG GS, VAN DER LAAR IM, HOOGEBOOM AJ, DUNNWALD M, KAPOOR S, JIRAMONGKOLCHAI P, STANDLEY J, MANAK JR, MURRAY JC, DIXON MJ. Expanding the genetic and phenotypic spectrum of popliteal pterygium disorders. Am J Med Genet A. 2015;167A:545–52. doi: 10.1002/ajmg.a.36896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU B, WILLETTE-BROWN J, LIU S, CHEN X, FISCHER SM, HU Y. IKKalpha represses a network of inflammation and proliferation pathways and elevates c-Myc antagonists and differentiation in a dose-dependent manner in the skin. Cell Death Differ. 2011;18:1854–64. doi: 10.1038/cdd.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEYRARD-JANVID M, LESLIE EJ, KOUSA YA, SMITH TL, DUNNWALD M, MAGNUSSON M, LENTZ BA, UNNEBERG P, FRANSSON I, KOILLINEN HK, RAUTIO J, PEGELOW M, KARSTEN A, BASEL-VANAGAITE L, GORDON W, ANDERSEN B, SVENSSON T, MURRAY JC, CORNELL RA, KERE J, SCHUTTE BC. Dominant mutations in GRHL3 cause Van der Woude Syndrome and disrupt oral periderm development. Am J Hum Genet. 2014;94:23–32. doi: 10.1016/j.ajhg.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHIMOV F, MARAZITA ML, VISEL A, COOPER ME, HITCHLER MJ, RUBINI M, DOMANN FE, GOVIL M, CHRISTENSEN K, BILLE C, MELBYE M, JUGESSUR A, LIE RT, WILCOX AJ, FITZPATRICK DR, GREEN ED, MOSSEY PA, LITTLE J, STEEGERS-THEUNISSEN RP, PENNACCHIO LA, SCHUTTE BC, MURRAY JC. Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008;40:1341–7. doi: 10.1038/ng.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON RJ, DIXON J, MALHOTRA S, HARDMAN MJ, KNOWLES L, BOOT-HANDFORD RP, SHORE P, WHITMARSH A, DIXON MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–34. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- RICHARDSON RJ, HAMMOND NL, COULOMBE PA, SALORANTA C, NOUSIAINEN HO, SALONEN R, BERRY A, HANLEY N, HEADON D, KARIKOSKI R, DIXON MJ. Periderm prevents pathological epithelial adhesions during embryogenesis. J Clin Invest. 2014;124:3891–900. doi: 10.1172/JCI71946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUNTREE RB, WILLIS CR, DINH H, BLUMBERG H, BAILEY K, DEAN C, JR, PESCHON JJ, HOLLAND PM. RIP4 regulates epidermal differentiation and cutaneous inflammation. J Invest Dermatol. 2010;130:102–12. doi: 10.1038/jid.2009.223. [DOI] [PubMed] [Google Scholar]
- SIL AK, MAEDA S, SANO Y, ROOP DR, KARIN M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–4. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- STOLER A, KOPAN R, DUVIC M, FUCHS E. Use of Monospecific Antisera and Crna Probes to Localize the Major Changes in Keratin Expression during Normal and Abnormal Epidermal Differentiation. Journal of Cell Biology. 1988;107:427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANSKY N, EGLOFF AM, TWARD AD, KOSTIC AD, CIBULSKIS K, SIVACHENKO A, KRYUKOV GV, LAWRENCE MS, SOUGNEZ C, MCKENNA A, SHEFLER E, RAMOS AH, STOJANOV P, CARTER SL, VOET D, CORTES ML, AUCLAIR D, BERGER MF, SAKSENA G, GUIDUCCI C, ONOFRIO RC, PARKIN M, ROMKES M, WEISSFELD JL, SEETHALA RR, WANG L, RANGEL-ESCARENO C, FERNANDEZ-LOPEZ JC, HIDALGO-MIRANDA A, MELENDEZ-ZAJGLA J, WINCKLER W, ARDLIE K, GABRIEL SB, MEYERSON M, LANDER ES, GETZ G, GOLUB TR, GARRAWAY LA, GRANDIS JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEDA K, TAKEUCHI O, TSUJIMURA T, ITAMI S, ADACHI O, KAWAI T, SANJO H, YOSHIKAWA K, TERADA N, AKIRA S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–6. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- WALKER BE, FRASER FC. Closure of the Secondary Palate in Three Strains of Mice. J Embryol Exp Morphol. 1956;4:176–189. [Google Scholar]
- XIE W, WU X, CHOW LT, CHIN E, PATERSON AJ, KUDLOW JE. Targeted expression of activated erbB-2 to the epidermis of transgenic mice elicits striking developmental abnormalities in the epidermis and hair follicles. Cell Growth Differ. 1998;9:313–25. [PubMed] [Google Scholar]
- ZUCCHERO TM, COOPER ME, MAHER BS, DAACK-HIRSCH S, NEPOMUCENO B, RIBEIRO L, CAPRAU D, CHRISTENSEN K, SUZUKI Y, MACHIDA J, NATSUME N, YOSHIURA K, VIEIRA AR, ORIOLI IM, CASTILLA EE, MORENO L, ARCOS-BURGOS M, LIDRAL AC, FIELD LL, LIU YE, RAY A, GOLDSTEIN TH, SCHULTZ RE, SHI M, JOHNSON MK, KONDO S, SCHUTTE BC, MARAZITA ML, MURRAY JC. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004;351:769–80. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


