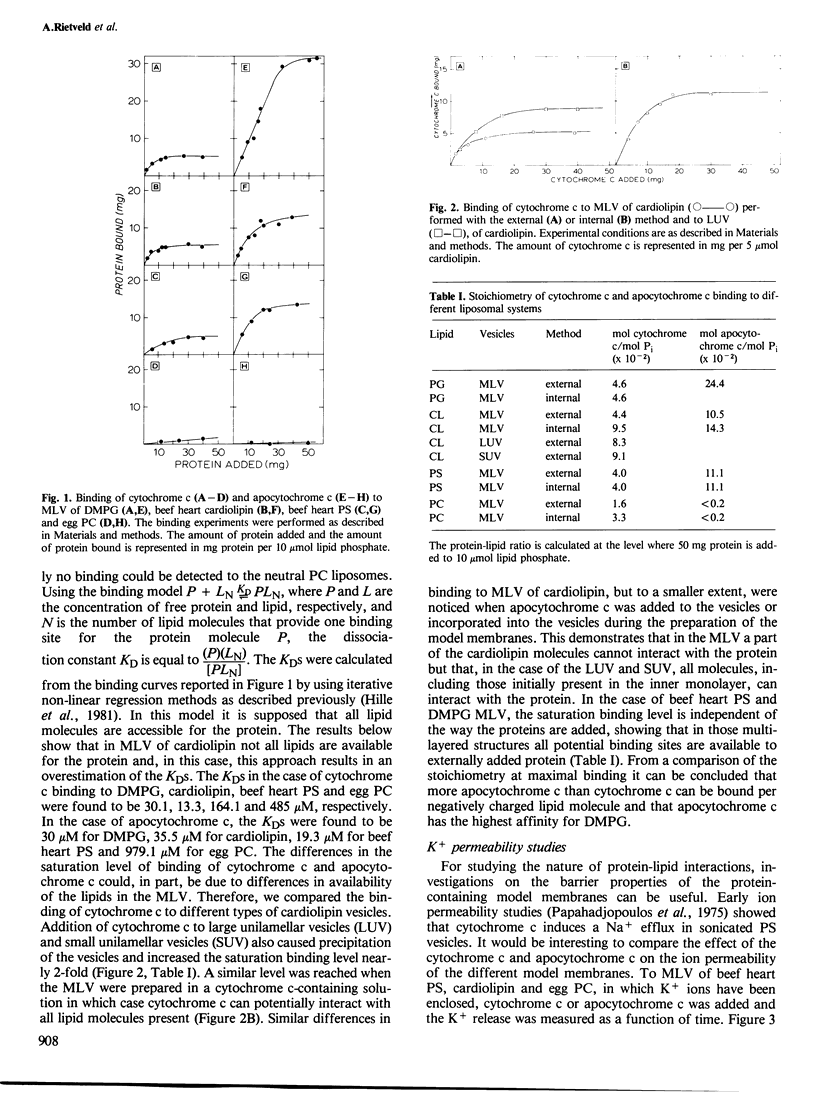
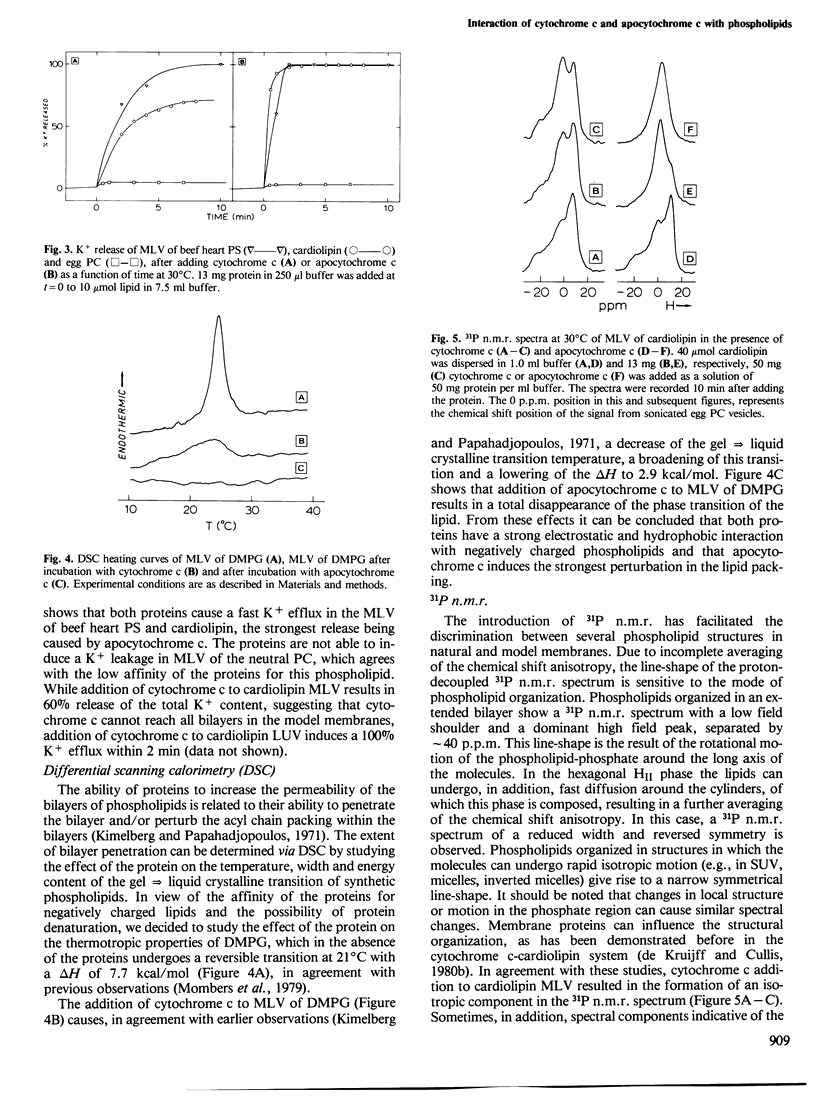
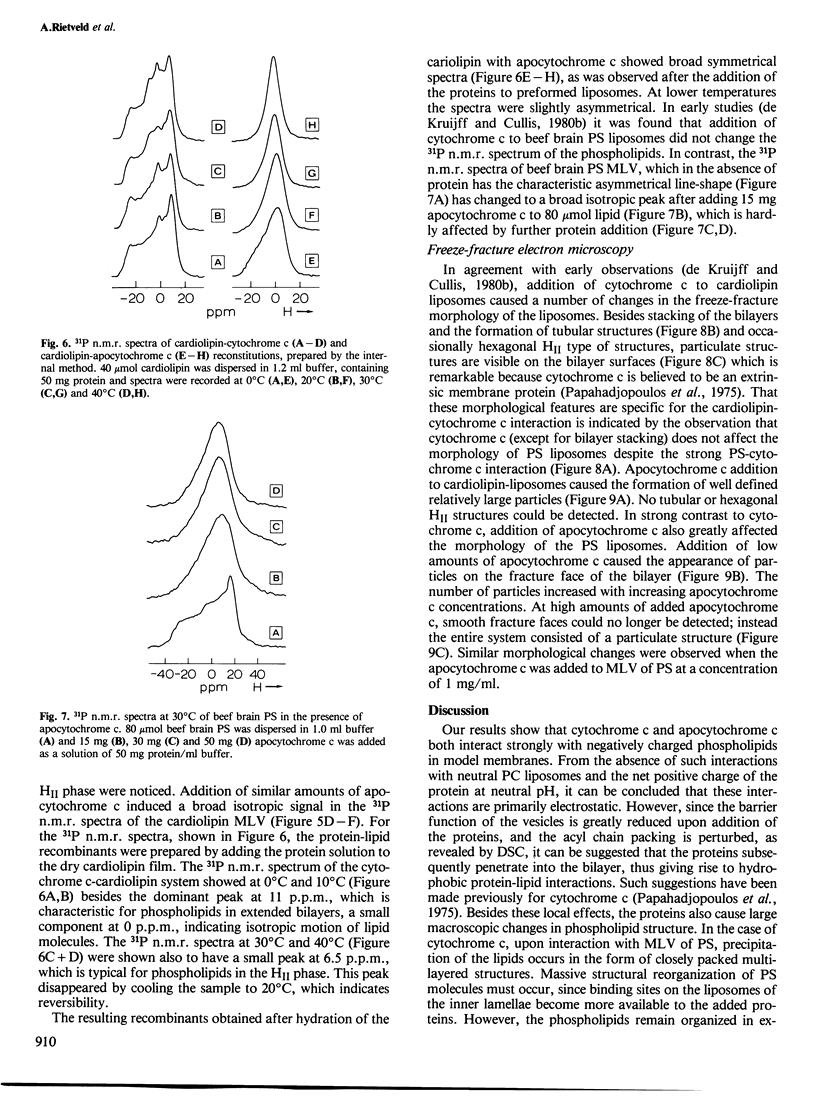
Abstract
The effects of cytochrome c and apocytochrome c on the structural properties of various membrane phospholipids in model systems were compared by binding, calorimetric, permeability, 31P n.m.r. and freeze-fracture experiments. Both cytochrome c and apocytochrome c experience strong interactions only with negatively charged phospholipids; apocytochrome c interacted more strongly than cytochrome c. These interactions are primarily electrostatic but also have a hydrophobic character. Cytochrome c as well as apocytochrome c induces changes in the structure of cardiolipin liposomes as is shown by 31P n.m.r. and freeze-fracture electron microscopy. Cytochrome c does not affect the bilayer structure of phosphatidylserine. In contrast, interaction of apocytochrome c with this phospholipid results in changes of the 31P n.m.r. bilayer spectrum of the liposomes and also particles are observed at the fracture faces. The results are discussed in relation to the import of the protein into the mitochondrion.
Keywords: apocytochrome c, cytochrome c, mitochondrial protein import, model membrane, protein-lipid interaction
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok M. C., van der Neut-Kok E. C., van Deenen L. L., de Gier J. The effect of chain length and lipid phase transitions on the selective permeability properties of liposomes. Biochim Biophys Acta. 1975 Oct 6;406(2):187–196. doi: 10.1016/0005-2736(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Wüthrich K. NMR and ESR studies of the interactions of cytochrome c with mixed cardiolipin-phosphatidylcholine vesicles. Biochim Biophys Acta. 1977 Aug 1;468(3):389–410. doi: 10.1016/0005-2736(77)90290-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Fisher W. R., Schechter A. N. Spectroscopic studies on the conformation of cytochrome c and apocytochrome c. J Biol Chem. 1974 Feb 25;249(4):1113–1118. [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Deamer D., Bangham A. D. Large volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976 Sep 7;443(3):629–634. doi: 10.1016/0005-2736(76)90483-1. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Taniuchi H., Anfinsen C. B. On the role of heme in the formation of the structure of cytochrome c. J Biol Chem. 1973 May 10;248(9):3188–3195. [PubMed] [Google Scholar]
- Gasser S. M., Ohashi A., Daum G., Böhni P. C., Gibson J., Reid G. A., Yonetani T., Schatz G. Imported mitochondrial proteins cytochrome b2 and cytochrome c1 are processed in two steps. Proc Natl Acad Sci U S A. 1982 Jan;79(2):267–271. doi: 10.1073/pnas.79.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Assembly of cytochrome c. Apocytochrome c is bound to specific sites on mitochondria before its conversion to holocytochrome c. Eur J Biochem. 1981 Dec;121(1):203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- Hille J. D., Donné-Op den Kelder G. M., Sauve P., de Haas G. H., Egmond M. R. Physicochemical studies on the interaction of pancreatic phospholipase A2 with a micellar substrate analogue. Biochemistry. 1981 Jul 7;20(14):4068–4073. doi: 10.1021/bi00517a019. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Phospholipid-protein interactions: membrane permeability correlated with monolayer "penetration". Biochim Biophys Acta. 1971 Jun 1;233(3):805–809. doi: 10.1016/0005-2736(71)90181-7. [DOI] [PubMed] [Google Scholar]
- Korb H., Neupert W. Biogenesis of cytochrome c in Neurospora crassa. Synthesis of apocytochrome c, transfer to mitochondria and conversion to Holocytochrome c. Eur J Biochem. 1978 Nov 15;91(2):609–620. doi: 10.1111/j.1432-1033.1978.tb12714.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuura S., Arpin M., Hannum C., Margoliash E., Sabatini D. D., Morimoto T. In vitro synthesis and posttranslational uptake of cytochrome c into isolated mitochondria: role of a specific addressing signal in the apocytochrome. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4368–4372. doi: 10.1073/pnas.78.7.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombers C., Verkleij A. J., de Gier J., van Deenen L. L. The interaction of spectrin-actin and synthetic phospholipids. II. The interaction with phosphatidylserine. Biochim Biophys Acta. 1979 Mar 8;551(2):271–281. doi: 10.1016/0005-2736(89)90005-9. [DOI] [PubMed] [Google Scholar]
- Müller M., Meister N., Moor H. Freezing in a propane jet and its application in freeze-fracturing. Mikroskopie. 1980 Sep;36(5-6):129–140. [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Moscarello M., Eylar E. H., Isac T. Effects of proteins on thermotropic phase transitions of phospholipid membranes. Biochim Biophys Acta. 1975 Sep 2;401(3):317–335. doi: 10.1016/0005-2736(75)90233-3. [DOI] [PubMed] [Google Scholar]
- Pscheid P., Schudt C., Plattner H. Cryofixation of monolayer cell cultures for freeze-fracturing without chemical pre-treatments. J Microsc. 1981 Feb;121(Pt 2):149–167. doi: 10.1111/j.1365-2818.1981.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Sanders H. Preparative isolation of phosphatidyl serine from brain. Biochim Biophys Acta. 1967 Oct 2;144(2):485–487. doi: 10.1016/0005-2760(67)90184-1. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Stellwagen E., Rysavy R., Babul G. The conformation of horse heart apocytochrome c. J Biol Chem. 1972 Dec 25;247(24):8074–8077. [PubMed] [Google Scholar]
- Stoffel W., Schiefer H. G. Biosynthesis and composition of phosphatides in outer and inner mitochondrial membranes. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1017–1026. doi: 10.1515/bchm2.1968.349.2.1017. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Van Dijck P. W., Ververgaert P. H., Verkleij A. J., Van Deenen L. L., De Gier J. Influence of Ca2+ and Mg2+ on the thermotropic behaviour and permeability properties of liposomes prepared from dimyristoyl phosphatidylglycerol and mixtures of dimyristoyl phosphatidylglycerol and dimyristoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Nov 3;406(4):465–478. doi: 10.1016/0005-2736(75)90025-5. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Hennig B., Neupert W. Different transport pathways of individual precursor proteins in mitochondria. Eur J Biochem. 1981 Jun 1;116(3):455–460. doi: 10.1111/j.1432-1033.1981.tb05357.x. [DOI] [PubMed] [Google Scholar]
- de Kruijff B. 13C NMR studies on [4-13C] cholesterol incorporated in sonicated phosphatidylcholine vesicles. Biochim Biophys Acta. 1978 Jan 19;506(2):173–182. doi: 10.1016/0005-2736(78)90388-7. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R. Cytochrome c specifically induces non-bilayer structures in cardiolipin-containing model membranes. Biochim Biophys Acta. 1980 Nov 18;602(3):477–490. doi: 10.1016/0005-2736(80)90327-2. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R. The influence of poly(L-lysine) on phospholipid polymorphism. Evidence that electrostatic polypeptide-phospholipid interactions can modulate bilayer/non-bilayer transitions. Biochim Biophys Acta. 1980 Sep 2;601(1):235–240. doi: 10.1016/0005-2736(80)90528-3. [DOI] [PubMed] [Google Scholar]





