Abstract
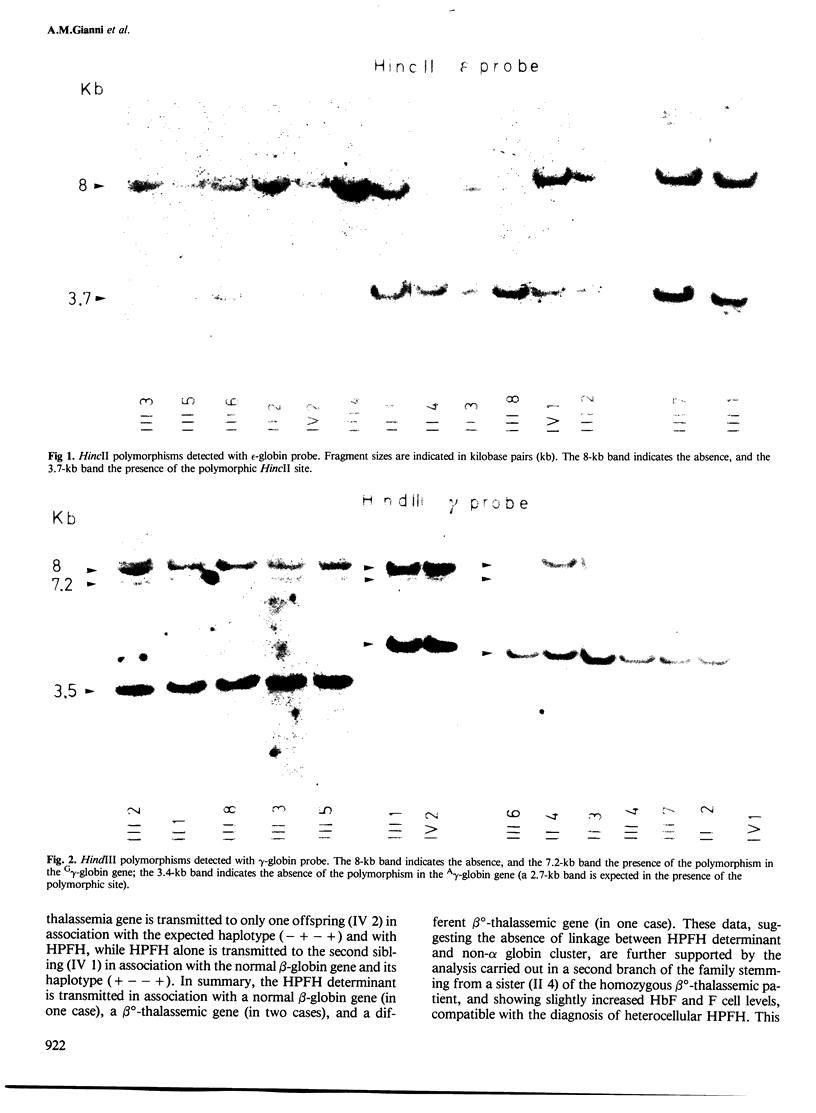
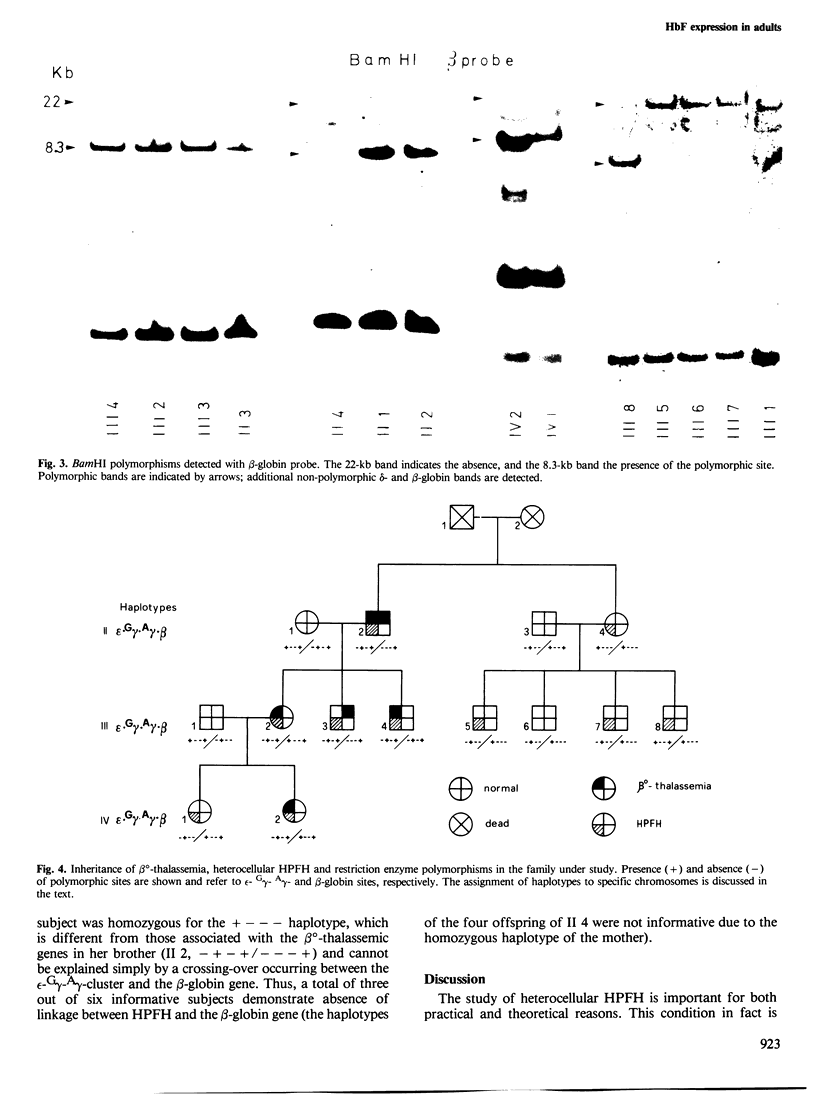
The possible linkage between a gene causing heterocellular hereditary persistence of fetal hemoglobin (HPFH) and human non-alpha globin loci has been studied in a large Sardinian family. In this family a homozygous beta o-thalassemic patient was found, with an unusually mild form of this disease, which was ascribed to the co-existence of a gene causing heterocellular HPFH. DNA polymorphisms in the non-alpha globin cluster were analyzed by restriction enzyme digestion with HincII, HindIII and BamHI and with epsilon-, gamma-and beta-globin probes; the pattern of inheritance of these polymorphisms indicates that the HPFH gene is transmitted with one beta o-thalassemic gene in a single instance, with the second beta o-thalassemic gene in three instances and with a normal beta-globin gene in two cases. These data indicate that this HPFH gene is not linked to the non-alpha globin gene cluster, in contrast to previous observations with different HPFH genes, and suggest that this gene might code for diffusible substances acting, directly or indirectly, on gamma-globin gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsley J. F., Rappaport E., Schwartz E., Surrey S. The gamma-delta-beta-globin gene region in G gamma-beta +-hereditary persistence of fetal hemoglobin. Blood. 1982 Apr;59(4):828–831. [PubMed] [Google Scholar]
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Kooter J. M., Flavell R. A. Physical mapping of the globin gene deletion in (delta beta (0)) -thalassaemia. Gene. 1979 Jul;6(3):265–280. doi: 10.1016/0378-1119(79)90062-3. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Comi P., Giglioni B., Ottolenghi S., Migliaccio A. R., Migliaccio G., Lettieri F., Maguire Y. P., Peschle C. Biosynthesis of Hb in individual fetal liver bursts. gamma-Chain production peaks earlier than beta-chain in the erythropoietic pathway. Exp Cell Res. 1980 Dec;130(2):345–352. doi: 10.1016/0014-4827(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Wood W. G., Clegg J. B., Weatherall D. J. Restriction endonuclease maps of the beta-like globin gene cluster in the British and Greek forms of HPFH, and for one example of G gamma beta + HPFH. Br J Haematol. 1982 Mar;50(3):415–422. doi: 10.1111/j.1365-2141.1982.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Little P., Curtis P., Coutelle C., Van den Berg J., Dalgleish R., Malcolm S., Courtney M., Westaway D., Williamson R. Isolation and partial sequence of recombinant plasmids containing human alpha-, beta- and gamma-globin cDNA fragments. Nature. 1978 Jun 22;273(5664):640–643. doi: 10.1038/273640a0. [DOI] [PubMed] [Google Scholar]
- Martinez G., Colombo B. A new type of hereditary persistence of foetal haemoglobin: is a diffusible factor regulating gamma-chain synthesis? Nature. 1974 Dec 20;252(5485):735–736. doi: 10.1038/252735a0. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old J. M., Ayyub H., Wood W. G., Clegg J. B., Weatherall D. J. Linkage analysis of nondeletion hereditary persistence of fetal hemoglobin. Science. 1982 Feb 19;215(4535):981–982. doi: 10.1126/science.6186021. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Comi P., Gianni A. M., Polli E., Acquaye C. T., Oldham J. H., Masera G. Globin gene deletion in HPFH, delta (o) beta (o) thalassaemia and Hb Lepore disease. Nature. 1979 Apr 12;278(5705):654–657. doi: 10.1038/278654a0. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Taramelli R., Comi P., Mazza U., Saglio G., Camaschella C., Izzo P., Cao A., Galanello R. Molecular comparison of delta beta-thalassemia and hereditary persistence of fetal hemoglobin DNAs: evidence of a regulatory area? Proc Natl Acad Sci U S A. 1982 Apr;79(7):2347–2351. doi: 10.1073/pnas.79.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Saglio G., Ricco G., Mazza U., Camaschella C., Pich P. G., Gianni A. M., Gianazza E., Righetti P. G., Giglioni B., Comi P. Human T gamma globin chain is a variant of A gamma chain (A gamma Sardinia). Proc Natl Acad Sci U S A. 1979 Jul;76(7):3420–3424. doi: 10.1073/pnas.76.7.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Biro P. A., deRiel J. K., Lazarus H., Forget B. G. Restriction endonuclease mapping of the human gamma globin gene loci. Nucleic Acids Res. 1979 Jun 11;6(7):2519–2544. doi: 10.1093/nar/6.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Murnane M. J., deRiel J. L., Forget B. G. Heterogeneity in the molecular basis of hereditary persistence of fetal haemoglobin. Nature. 1980 May 29;285(5763):335–337. doi: 10.1038/285335a0. [DOI] [PubMed] [Google Scholar]






