Abstract
Aim
To determine the systematic error (∑), random error (σ) and derive PTV margin at different levels of the target volumes in Nasopharyngeal Cancer (NPC).
Materials and methods
A retrospective offline review was done for patients who underwent IMRT for NPC from June 2015 to May 2016 at our institution.
Alternate day kV images were matched with digitally reconstructed radiographs to know the setup errors. All radiographs were matched at three levels – the clivus, third cervical (C3) and sixth cervical (C6) vertebra. The shifts in positions along the vertical, longitudinal and lateral axes were noted and the ∑ and σ at three levels were calculated. PTV margins were derived using van Herk's formula.
Results
Twenty patients and 300 pairs of orthogonal portal films were reviewed. The ∑ for the clivus, C3 and C6 along vertical, longitudinal and lateral directions were 1.6 vs. 1.8 vs. 2 mm; 1.2 vs. 1.4 vs. 1.4 mm and 0.9 vs. 1.6 and 2.3 mm, respectively. Similarly, the random errors were 1.1 vs. 1.4 vs. 1.8 mm; 1.1 vs. 1.2 vs. 1.2 mm and 1.2 vs. 1.3 vs. 1.6 mm. The PTV margin at the clivus was 4.4 mm along the vertical, 4 mm along the longitudinal direction and 3.2 m in the lateral direction. At the C3 level, it was 5.5 mm in the vertical, 5 mm in the lateral direction and 4.4 mm in the longitudinal direction. At the C6 level, it was 6.4 mm in the vertical, 6.9 mm in the lateral direction and 4.4 mm in the longitudinal direction.
Conclusion
A differential margin along different levels of target may be necessary to adequately cover the target.
Keywords: Nasopharyngeal cancer, PTV margin, Setup error
1. Background
Primary radiotherapy with or without concurrent chemotherapy is an effective curative treatment for Nasopharyngeal cancers (NPC). With the advent of intensity modulated radiotherapy (IMRT), it is possible to deliver highly conformal doses to the target volumes and at the same time reduce the dose to organs at risk (OAR) thereby achieving a maximum therapeutic gain.
The target volume in NPC usually extends from the skull base to the supraclavicular fossa. The accurate delivery of highly conformal dose with steep fall off obtained with the new planning techniques requires selection of an appropriate planning target volume (PTV). Often, a uniform PTV margin is chosen for the entire target volume. However, this may not be appropriate considering the movement of the skull and neck in spite of rigid immobilization. Further, these variations are often found to be differential at various regions.1
The close proximity of critical organs, like brainstem, optic apparatus and cochlea, to the target volume often necessitates stringent PTV margins. The positional accuracy during each fraction of radiation is of paramount importance in both ensuring good tumor coverage and sparing OARs. It is often difficult to attain a perfect match of the treatment portal images with the digitally reconstructed radiograph (DRR) at all levels of the target volume. Therefore, matching primary target volume at the skull base receives top priority due to the location of adjacent critical structures. This can result in positional inaccuracies in lower neck which can impact the dose delivery to this region.
2. Aim
To determine the systematic error, random error and derive PTV margin at different levels of the target volumes in NPC.
3. Materials and methods
3.1. Patient selection
20 patients with NPC (irrespective of stage), treated using volumetric arc therapy (VMAT) technique, between June 2015 and May 2016 were included in the analysis. All patients were treated using simultaneous integrated boost (SIB) protocol delivering 66, 60 and 54 Gy in 30 fractions to the corresponding PTVs with or without concurrent chemotherapy as indicated.
3.2. Immobilization and ct simulation
Patients were immobilized with the neck extended using a neck rest and use of a head and neck thermoplastic shell with 4 point fixation (Orfit). Planning CT was acquired on a wide bore CT scanner (GE optima) with the use of intravenous contrast and slice thickness of 2.5 mm. Laser markings were made on the shell at the time of CT simulation to be used for patient setup in the treatment machine.
3.3. Planning, treatment setup and execution
The clinical target volumes (CTV) was contoured on the planning CT scan. The CTV to PTV margin in all patients was 5 mm. IMRT plans using 6 MV photons were generated using the Eclipse© planning system version 13. Patients were set up in the treatment machine, aligning the treatment lasers to the markings on the shell. Necessary couch shifts as obtained from the planning system were then applied. Image verification using two orthogonal kilo voltage (kV) images (100 kV for anterior and 70 kV for lateral) were performed using the on-board imaging system. The imaging protocol followed at our institution includes daily image verification prior to treatment.
3.4. Study design
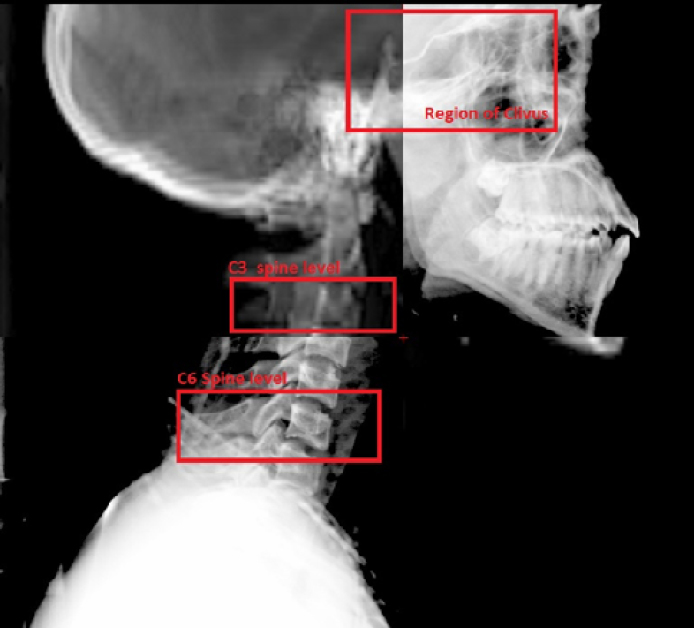
This study was planned as a retrospective offline review of the kV orthogonal images that were acquired for daily image verification. The kV images taken on alternate days were reviewed and compared with the DRR to assess the shifts at three different levels of the target volume. The three levels used for matching were the region of the clivus, third cervical vertebra (C3) and sixth cervical vertebra (C6) (Fig. 1). The corresponding shifts in positions along the vertical, longitudinal and lateral axes were collected for the data analysis.
Fig. 1.
Overlay of digitally reconstructed radiograph and lateral portal image for setup verification.
4. Data analysis
The systematic error and random error for matched positions at the clivus, C3 and C6 were calculated separately. Systematic error for the population (∑) was calculated by taking the standard deviation of the average value of individual mean setup error along the vertical, longitudinal and lateral directions, respectively.
Random error for the population (σ) was calculated by taking the root mean square value of individual standard deviations along the vertical, longitudinal and lateral directions respectively.
Further, the PTV margin for the antero-posterior direction, lateral direction and cranio-caudal direction for the levels were determined using the van Herk formula.2
5. Results
5.1. Patient characteristics
Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| Age | |
| Mean | 42 years |
| Range | 14–70 years |
| Sex | |
| Male | 10 |
| Female | 10 |
| Stage | |
| I | 1 |
| II | 5 |
| III | 13 |
| IV | 1 |
| Chemotherapy | |
| Induction | 15 |
| Concurrent | 19 |
5.2. Setup error
For each patient, fifteen orthogonal pair portal images were reviewed off-line, amounting to a total of 300 image pairs for 20 patients. The systematic error and random error along the three axes (vertical, longitudinal and lateral) are illustrated in Table 2.
Table 2.
Calculated systematic and random errors at different levels.
| Level | Systematic error in mm |
Random error in mm |
||||
|---|---|---|---|---|---|---|
| Vertical | Longitudinal | Lateral | Vertical | Longitudinal | Lateral | |
| CLIVUS | 1.6 | 1.2 | 0.9 | 1.1 | 1.1 | 1.2 |
| C-3 | 1.8 | 1.4 | 1.6 | 1.4 | 1.2 | 1.3 |
| C-6 | 2 | 1.4 | 2.3 | 1.8 | 1.2 | 1.6 |
C-3, cervical spine -3; C-6, cervical spine-6.
Overall, the systematic error was greater in all directions at the C-6 level (the greatest in the lateral direction – 2.3 mm) and the smallest error was at the level of the clivus (lateral direction – 0.9 mm). The systematic error at the level of C-3 spine (1.8, 1.4 and 1.6 mm along the vertical, longitudinal and lateral directions) was greater when compared to that at the clivus (1.6, 1.2 and 0.9 mm along the vertical, longitudinal and lateral directions).
Similar to the systematic errors, the random errors at the C-6 level were greater in all directions compared to the level of the clivus and C-3. The greatest error was along the vertical direction (1.8 mm) at the C-6 level. The smallest random error was at the level of the clivus along the vertical and longitudinal direction (1.1 mm each). The random error at the level of C-3 was greater than at the level of the clivus in all directions (1.1 vs. 1.4, 1.1 vs. 1.2, 1.2 vs. 1.3 mm along the vertical longitudinal and lateral directions, respectively).
The calculated PTV margin according to the van Herk formula is shown in Table 3.
Table 3.
Calculated planning target volume (PTV) margins at different levels.
| PTVmargin in mm |
|||
|---|---|---|---|
| Level | Vertical/AP | Longitudinal/SI | Lateral/ML |
| CLIVUS | 4.4 | 4 | 3.2 |
| C-3 | 5.5 | 4.4 | 5 |
| C-6 | 6.4 | 4.4 | 6.9 |
C-3, cervical spine -3; C-6, cervical spine-6; AP, antero-posterior; ML, medio-lateral; SI, supero-inferior.
The calculated PTV margin was the greatest along all directions at the C-6 level, with the largest one along the lateral direction (6.9 mm) followed by the vertical direction (6 mm). At the C-3 level, the calculated margin was the largest in the vertical direction (at 5.5 mm) and the smallest in the longitudinal direction (4.4 mm).
6. Discussion
Strict immobilization and regular treatment portal verification form an integral part of IMRT. Most centers treating head and neck tumors use an uniform PTV margin of 3–5 mm with appropriate image guidance protocols to account for the setup uncertainties during treatment.3, 4 In spite of using good immobilization in the head and neck, varying degrees of movements are possible at the level of the skull and neck. Zhang et al. demonstrated this in day to day setup at selected regions of interest (palatine process, C2 and C6 vertebra) and the largest shifts were noted at the C6 level.5 Considering these movements, a uniform margin at all the levels may not be ideal in head and neck treatment.
Various publications have looked into the setup errors in different sub-regions of the head and neck.6, 7, 8 Both kV orthogonal matching and cone beam CT (CBCT) matching have been used in the evaluation of these errors. In our study, we evaluated the errors at the clivus, C3 spine and C6 spine levels. Clivus is an important landmark in nasopharynx as it forms the roof and lies in close proximity to the gross tumor volume and critical structures like the optic apparatus and brainstem. This bony landmark matching will give a close approximation of the target and OAR match. Other studies have evaluated the region of the occiput, maxilla and mandible as well.6, 9, 10 The mandible can move independently of the skull producing different positional errors, if used for matching. Since the target structure in NPC is mostly away from the region of the mandible, the errors in this region were not evaluated in our study. In evaluating the neck for positional errors, we chose the C3 vertebra since it represents the level of the hyoid which forms a landmark for the level II and level III neck node levels. For the lower neck, the C6 vertebra corresponds to the lower lymph node regions.
The positional variations at the clivus and neck have been demonstrated by Cheo et al. by using kilo voltage cone beam CT verification and correction protocol.8 The setup error was considerably small at the clivus when compared to the lower neck. In addition, the errors were most often in the lateral direction in the neck. Van Kranen et al. have evaluated the set up uncertainties at eight different regions in the head and neck (including different levels of the cervical spine) with an offline shrinking action level correction protocol.10 In this study, the systematic error at different local regions ranged from 1.1 to 3.4 mm, with random error ranging from 1.3 to 2.5 mm. The variations in day to day setup were found to be more in the lateral and the antero-posterior directions in the lower neck. Also, the setup errors were comparatively smaller at the occiput bone. In another study by Djordjevic et al., evaluating different correction protocols, a similar increase in setup errors at the lower neck were noted.9 In our patient group, the systematic error ranged from 0.9 mm to 2.3 mm and random error ranged from 1.1 mm to 1.6 mm. The maximum variations were found at the C6 spine level.
In the present study, the calculated PTV margin at the level of the clivus was small compared to the other two levels in all directions. Overall, the margin required in the vertical (antero-posterior) direction is comparatively larger than the other directions for the clivus and C3 spine level. The lower neck requires a wider margin in the lateral direction and in antero-posterior direction. Our result is in concurrence with various studies reporting on margins in the region of the skull and at different levels of the neck (Table 4).
Table 4.
Planning target volume (PTV) margins reported by different studies.
| Study | Imaging | Correction protocol | Margin (range) in mm | Largest margin direction | |
|---|---|---|---|---|---|
| van Kranen et al.10 (2009) | CBCT | SAL | Skull (occiput) | 4.6–7 | ML |
| C1–C3 spine | 3.8–4.7 | ML | |||
| C5–C7 spine | 5.4–6 | AP | |||
| Kapanen et al.7 (2013) | Orthogonal | Weekly | Skull (occiput) | 6.1(SI), 8.3(AP) | AP |
| kV image | C1–C2 spine | 4.8–7 | AP | ||
| C5–C7 spine | 4.9–5.7 | AP | |||
| Djordjevic et al.9 (2014) | Orthogonal | No | Skull (maxilla) | 5.2–5.9 | SI |
| kV image | ‘ | C2 spine | 4.5(SI), 6.5(AP) | AP | |
| C5 spine | 5–9.3 | AP | |||
| Daily | Skull (maxilla) | 4.2–5.9 | AP | ||
| C2 spine | 2.3(SI),2.6(AP) | AP | |||
| C5 spine | 2.6–5 | AP | |||
| Cheo et al.8 (2015) | CBCT | No | Skull (clivus) | 1.75–2.33 | SI |
| C4 spine | 2.61–4.33 | ML | |||
| C7 spine | 2.72–6.52 | ML | |||
| Weekly | Skull (clivus) | 0.51–1.2 | AP | ||
| C4 spine | 0.97–3.72 | ML | |||
| C7 spine | 1.2–6.08 | ML | |||
| Present study | Orthogonal | No | Skull(clivus) | 3.2–4.4 | AP |
| kV image | C3 spine | 4.4–5.5 | AP | ||
| C6 spine | 4.4–6.9 | ML | |||
CBCT, cone beam CT; SAL, shrinking action level; AP, antero-posterior; ML, medio-lateral; SI, supero-inferior.
This study has several limitations. First, the analysis was limited to kV portal images. Although the kV portal image usage can be correlated well with that of CBCT for treatment setup verification in head and neck radiation therapy, rotational errors are not corrected.11 The interplay between translational and rotational errors and their impact has been demonstrated by previous studies.12, 13 The rotational errors could have influenced our results. Second, patient weight loss during the course of radiation increases the probability of errors. This correlation was not analyzed in this study. Finally, the quality of the kV portal image and DRR is important for accurate matching. The thickness of the simulation CT slice determines the quality of the DRR.14 Also, overlap of bony structures may cause difficulty in matching the landmarks.
The setup accuracy can be improved by paying careful attention to the mask molding process. Ensuring proper patient positioning – shoulders pulled caudally, mouth closed and neck extended, removing the thermoplastic mask after cooling and reapplying before simulation, and making tattoo marks on the chest have shown to reduce the errors.15 In addition, following a daily image guidance protocol with a threshold level for correction can reduce the need for larger margins.16
Bony landmark matching and verification is followed for both kV portal image and CBCT. This matching might not always correlate with the target location, especially in the neck considering the change in patient contour due to weight loss and also response of the disease during the treatment course. These issues have led to the evolution and usage of adaptive radiotherapy practices.17
7. Conclusions
Regular treatment portal verification is necessary for maintaining accuracy in delivering radiation. In head and neck treatment, although rigid immobilization is achieved, there is still a considerable variation occurring in day to day setup of patients. This difference and its magnitude vary widely at different sub-regions and are found to be higher at the lower neck. From our study, the maximum margin required at the level of the clivus, C3 spine and C6 spine were 4.4 mm, 5.5 mm and 6.9 mm, respectively. To encompass the target structure at various levels, a differential PTV expansion may be followed.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Ove R., Cavalieri R., Noble D., Russo S.M. Variation of neck position with image-guided radiotherapy for head and neck cancer. Am J Clin Oncol. 2012;35:1–5. doi: 10.1097/COC.0b013e3181fe46bb. [DOI] [PubMed] [Google Scholar]
- 2.van Herk M., Remeijer P., Rasch C., Lebesque J. The probability of correct target dosage: dose–population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen A.M., Farwell D.G., Luu Q., Donald P.J., Perks J., Purdy J.A. Evaluation of the planning target volume in the treatment of head and neck cancer with intensity-modulated radiotherapy: what is the appropriate expansion margin in the setting of daily image guidance? Int J Radiat Oncol Biol Phys. 2011;81:943–949. doi: 10.1016/j.ijrobp.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Den R.B., Doemer A., Kubicek G. Daily image guidance with cone-beam computed tomography for head and neck cancer intensity-modulated radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2010;76:1353–1359. doi: 10.1016/j.ijrobp.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Garden A.S., Lo J. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:1559–1569. doi: 10.1016/j.ijrobp.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Yang J., Garden A.S., Zhang Y., Zhang L., Dong L. Variable planning margin approach to account for locoregional variations in setup uncertainties. Med Phys. 2012;39:5136–5144. doi: 10.1118/1.4737891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapanen M., Laaksomaa M., Tulijoki T., Peltola S., Wigren T., Hyödynmaa S. Estimation of adequate setup margins and threshold for position errors requiring immediate attention in head and neck cancer radiotherapy based on 2D image guidance. Radiat Oncol. 2013;8:212. doi: 10.1186/1748-717X-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheo T., Loh Y., Chen D., Lee K.M., Tham I. Measuring radiotherapy setup errors at multiple neck levels in nasopharyngeal cancer (NPC): a case for differential PTV expansion. Radiother Oncol. 2015;117:419–424. doi: 10.1016/j.radonc.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic M., Sjöholm E., Tullgren O., Sorcini B. Assessment of residual setup errors for anatomical sub-structures in image-guided head-and-neck cancer radiotherapy. Acta Oncol. 2014;53(5):646–653. doi: 10.3109/0284186X.2013.862593. [DOI] [PubMed] [Google Scholar]
- 10.van Kranen S., van Beek S., Rasch C., van Herk M., Sonke J.J. Setup uncertainties of anatomical sub-regions in head-and-neck cancer patients after offline CBCT guidance. Int J Radiat Oncol Biol Phys. 2009;73:1566–1573. doi: 10.1016/j.ijrobp.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Zhu X.R., Zhang L. Comparison of 2D radiographic images and 3D cone beam computed tomography for positioning head-and-neck radiotherapy patients. Int J Radiat Oncol Biol Phys. 2008;71:916–925. doi: 10.1016/j.ijrobp.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Remeijer P., Rasch C., Lebesque J.V., van Herk M. Margins for translational and rotational uncertainties: a probability-based approach. Int J Radiat Oncol Biol Phys. 2002;53:464–474. doi: 10.1016/s0360-3016(02)02749-9. [DOI] [PubMed] [Google Scholar]
- 13.Guckenberger M., Meyer J., Vordermark D., Baier K., Wilbert J., Flentje M. Magnitude and clinical relevance of translational and rotational patient setup errors: a cone-beam CT study. Int J Radiat Oncol Biol Phys. 2006;65:934–942. doi: 10.1016/j.ijrobp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Sturgeon J.D., Cox J.A., Mayo L.L. Improved human observer performance in digital reconstructed radiograph verification in head and neck cancer radiotherapy. Int J Comput Assist Radiol Surg. 2015;10:1667–1673. doi: 10.1007/s11548-014-1127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronin B., McCarthy A., Claire K. Quality improvement investigation for head and neck stabilization in radiotherapy using setup tattoos. J Med Imag Radiat Sci. 2013;44 doi: 10.1016/j.jmir.2012.11.002. 92–9.15. [DOI] [PubMed] [Google Scholar]
- 16.Kapanen M., Laaksomaa M., Tulijoki T. Effects of remedies made in patient setup process on residual setup errors and margins in head and neck cancer radiotherapy based on 2D image guidance. Rep Pract Oncol Radiother. 2015;20:292–298. doi: 10.1016/j.rpor.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz D.L., Dong L. Adaptive radiation therapy for head and neck cancer-can an old goal evolve into a new standard? J Oncol. 2011 doi: 10.1155/2011/690595. [DOI] [PMC free article] [PubMed] [Google Scholar]