Abstract
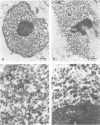
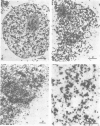
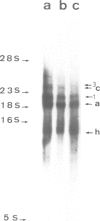
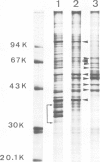
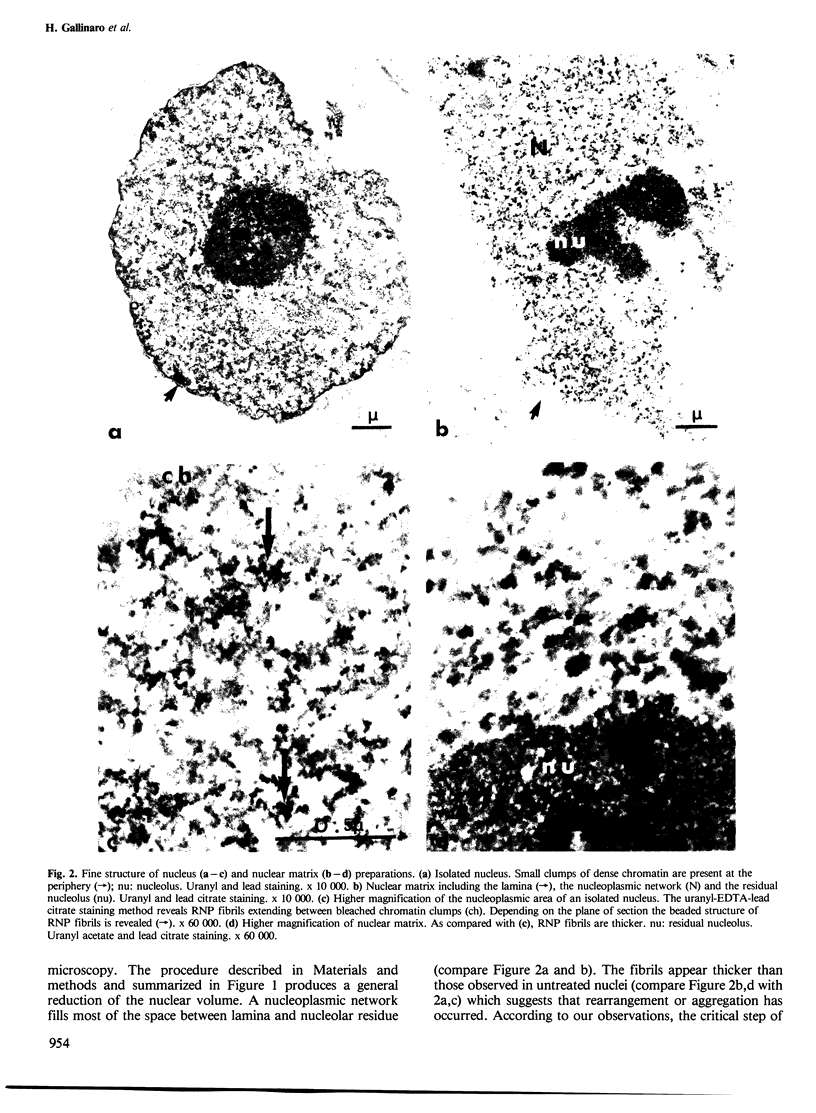
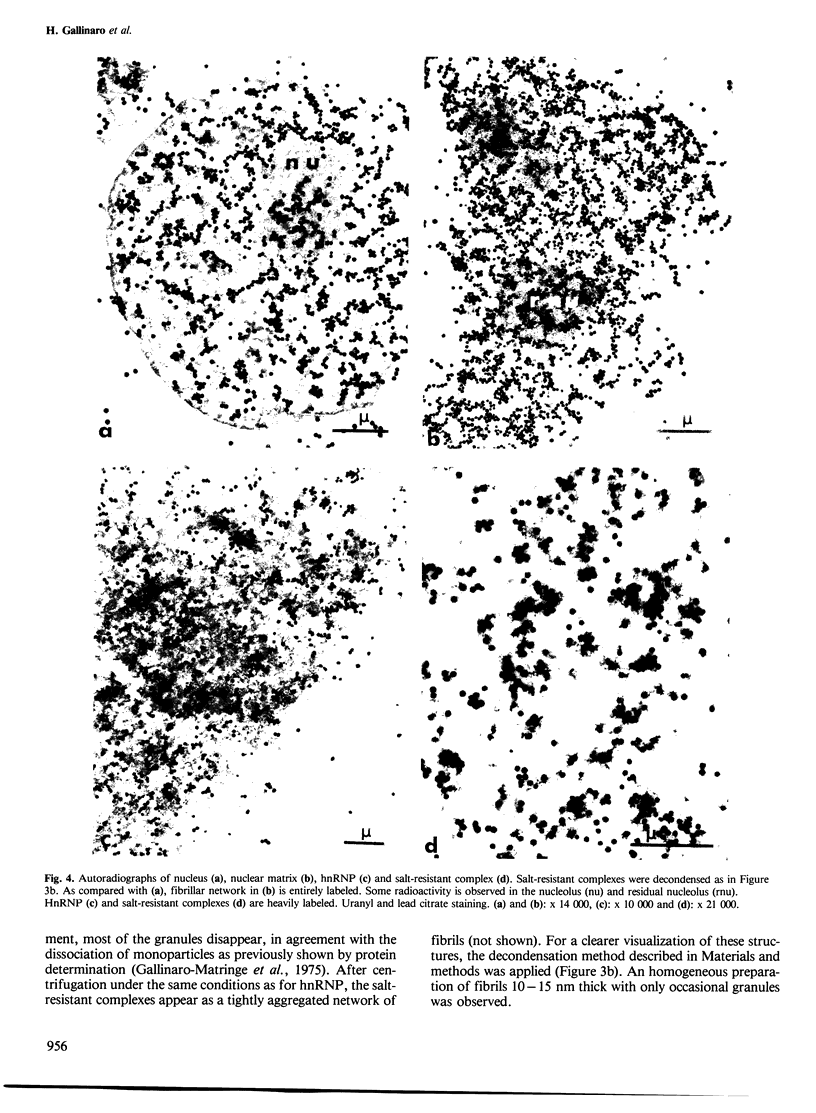
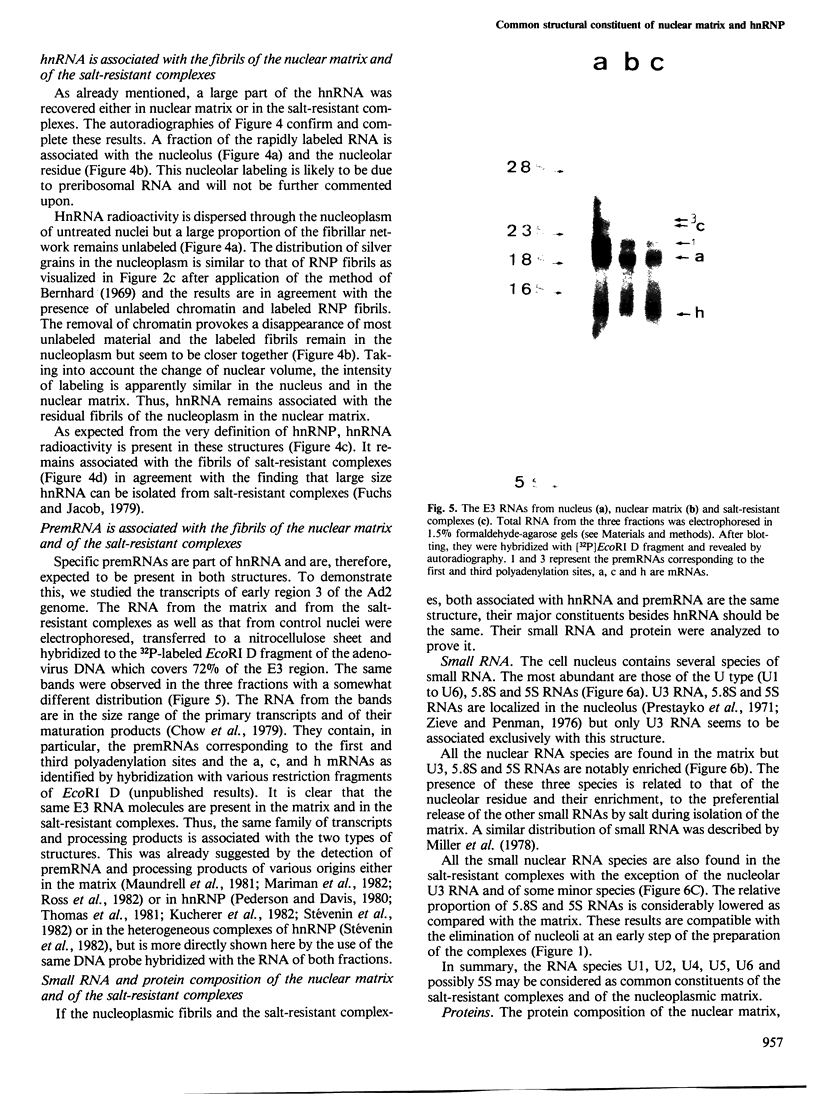
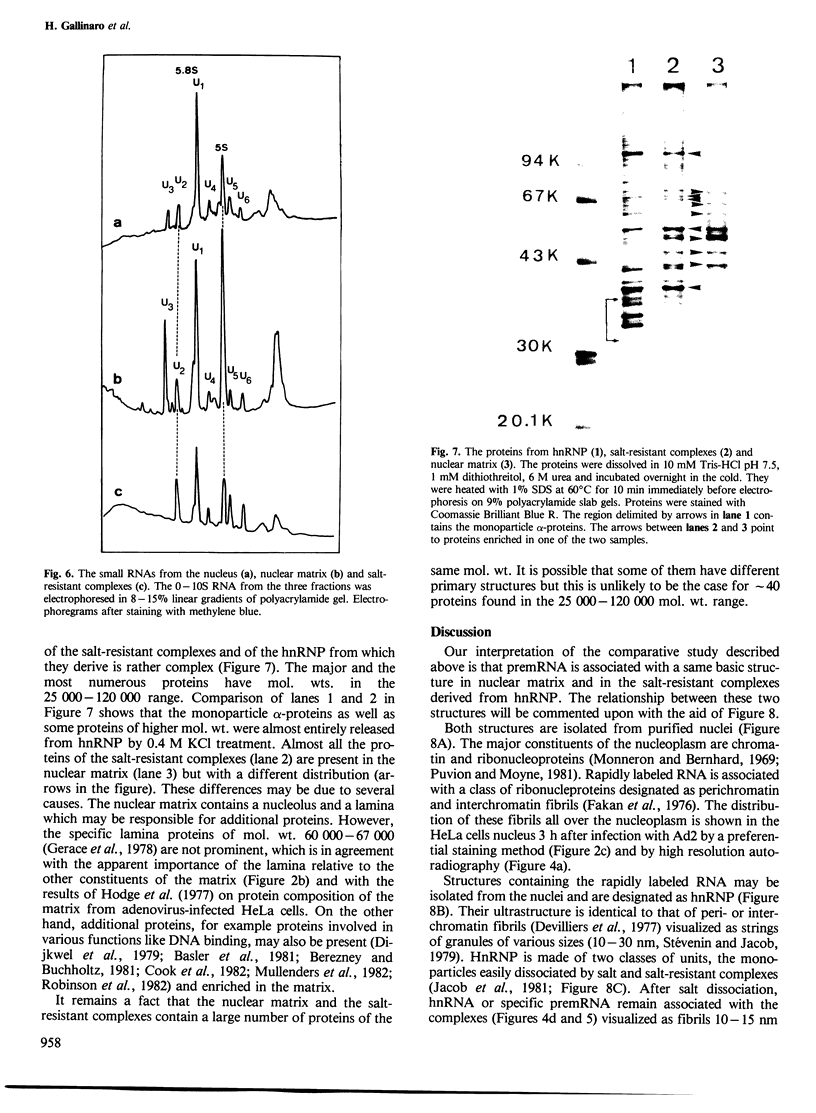
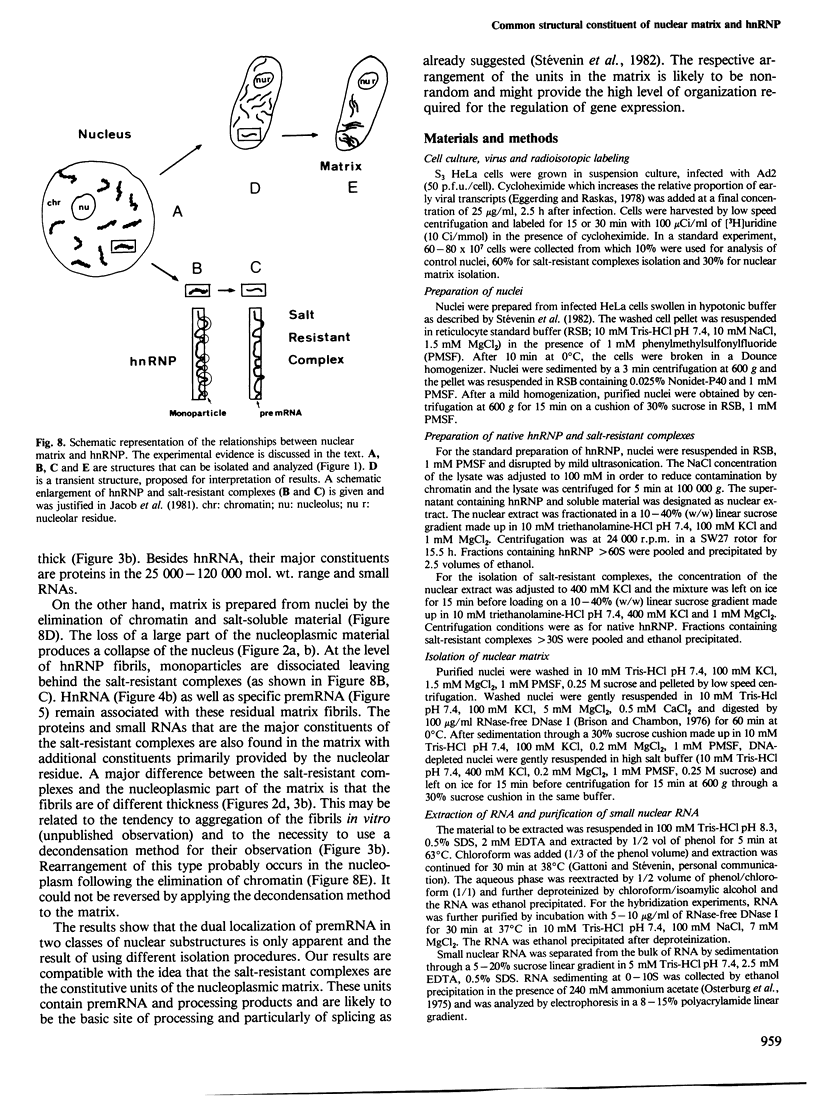
Nuclear matrix and heterogeneous nuclear ribonucleoprotein (hnRNP) were compared to establish whether premessenger RNA (premRNA) was associated with a same constituent in both structures. The isolation of nuclear matrix included the removal of chromatin and of 0.4 M KCl-soluble material. HnRNP, isolated by a standard method was also treated by 0.4 M KCl. Both isolation procedures caused the removal of DNA, histones, a fraction of small nuclear RNA and of nonhistone proteins including the hnRNP proteins in the 30 000-40 000 mol. wt. range. High resolution autoradiography showed that hnRNA remained associated with the residual fibrils in both structures. They both contained the same premRNA and maturation products as shown by the analysis of the transcripts of the early region 3 of adenovirus 2. In addition, the small nuclear RNA and protein of the salt-resistant complexes were also present in the matrix. The results are compatible with the idea that the salt-resistant complexes from hnRNP constitute the fibrils associated with premRNA in the nucleoplasmic matrix. The fibrils may be the basic unit of splicing and their organization in matrix might provide the spatial configuration necessary for regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler J., Hastie N. D., Pietras D., Matsui S. I., Sandberg A. A., Berezney R. Hybridization of nuclear matrix attached deoxyribonucleic acid fragments. Biochemistry. 1981 Nov 24;20(24):6921–6929. doi: 10.1021/bi00527a027. [DOI] [PubMed] [Google Scholar]
- Berezney R., Buchholtz L. A. Dynamic association of replicating DNA fragments with the nuclear matrix of regenerating liver. Exp Cell Res. 1981 Mar;132(1):1–13. doi: 10.1016/0014-4827(81)90076-8. [DOI] [PubMed] [Google Scholar]
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. doi: 10.1016/s0022-5320(69)80016-x. [DOI] [PubMed] [Google Scholar]
- Brasch K. Fine structure and localization of the nuclear matrix in situ. Exp Cell Res. 1982 Jul;140(1):161–171. doi: 10.1016/0014-4827(82)90167-7. [DOI] [PubMed] [Google Scholar]
- Brison O., Cambon P. A simple and efficient method to remove ribonuclease contamination from pancreatic deoxyribonuclease preparations. Anal Biochem. 1976 Oct;75(2):402–409. doi: 10.1016/0003-2697(76)90094-4. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Mullenders L. H., Wanka F. Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res. 1979 Jan;6(1):219–230. doi: 10.1093/nar/6.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerding F., Raskas H. J. Effect of protein synthesis inhibitors on viral mRNA's synthesized early in adenovirus type 2 infection. J Virol. 1978 Jan;25(1):453–458. doi: 10.1128/jvi.25.1.453-458.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Puvion E., Sphor G. Localization and characterization of newly synthesized nuclear RNA in isolate rat hepatocytes. Exp Cell Res. 1976 Apr;99(1):155–164. doi: 10.1016/0014-4827(76)90690-x. [DOI] [PubMed] [Google Scholar]
- Fuchs J. P., Jacob M. Fractionation of constituents of ribonucleoproteins containing heterogeneous nuclear ribonucleic acid. Biochemistry. 1979 Sep 18;18(19):4202–4208. doi: 10.1021/bi00586a026. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gallinaro-Matringe H., Stevenin J., Jacob M. Salt dissociation of nuclear particles containing DNA-like RNA. Distribution of phosphorylated and nonphosphorylated species. Biochemistry. 1975 Jun 3;14(11):2547–2554. doi: 10.1021/bi00682a039. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. The status of small nuclear RNA in the ribonucleoprotein fibrils containing heterogeneous nuclear RNA. Biochim Biophys Acta. 1981 Jan 29;652(1):109–120. doi: 10.1016/0005-2787(81)90214-8. [DOI] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Devilliers G., Jacob M. Size heterogeneity of monoparticles from nuclear ribonucleoproteins containing premessenger RNA. FEBS Lett. 1978 Jun 15;90(2):318–323. doi: 10.1016/0014-5793(78)80395-0. [DOI] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Jacob M. Comparison of the nuclear ribonucleoproteins containing the transcripts of adenovirus-2 and HeLa cell dna. Eur J Biochem. 1980;108(1):203–211. doi: 10.1111/j.1432-1033.1980.tb04713.x. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge L. D., Mancini P., Davis F. M., Heywood P. Nuclear matrix of HeLa S3 cells. Polypeptide composition during adenovirus infection and in phases of the cell cycle. J Cell Biol. 1977 Jan;72(1):194–208. doi: 10.1083/jcb.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Kucherer C., Marty L., Blanchard J. M. Presence of the pre-mRNA for the 72k DNA binding protein in hnRNP from early adenovirus-2 infected HeLa cells. Biochem Biophys Res Commun. 1982 Mar 30;105(2):603–609. doi: 10.1016/0006-291x(82)91477-2. [DOI] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Maxwell E. S., Puvion E., Scherrer K. The nuclear matrix of duck erythroblasts is associated with globin mRNA coding sequences but not with the major proteins of 40S nuclear RNP. Exp Cell Res. 1981 Dec;136(2):435–445. doi: 10.1016/0014-4827(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and small molecular weight RNA species. J Cell Biol. 1978 Mar;76(3):692–704. doi: 10.1083/jcb.76.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneron A., Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res. 1969 May;27(3):266–288. doi: 10.1016/s0022-5320(69)80017-1. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., Eygensteyn J., Broen A., Wanka F. Composition and DNA-binding properties of the nuclear matrix proteins from mammalian cell nuclei. Biochim Biophys Acta. 1982 Jul 30;698(1):70–77. doi: 10.1016/0167-4781(82)90186-5. [DOI] [PubMed] [Google Scholar]
- Osterburg H. H., Allen J. K., Finch C. E. The use of ammonium acetate in the precipitation of ribonucleic acid. Biochem J. 1975 May;147(2):367–368. doi: 10.1042/bj1470367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Davis N. G. Messenger RNA processing and nuclear structure: isolation of nuclear ribonucleoprotein particles containing beta-globin messenger RNA precursors. J Cell Biol. 1980 Oct;87(1):47–54. doi: 10.1083/jcb.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko A. W., Tonato M., Lewis B. C., Busch H. Heterogeneity of nucleolar U3 ribonucleic acid of the Novikoff hepatoma. J Biol Chem. 1971 Jan 10;246(1):182–187. [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Puvion E. New aspects of intranuclear structures following partial decondensation of chromatin: a cytochemical and high-resolution autoradiographical study. J Cell Sci. 1980 Apr;42:305–321. doi: 10.1242/jcs.42.1.305. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Jacob M. Structure of premRNP. Models and pitfalls. Mol Biol Rep. 1979 May 31;5(1-2):29–35. doi: 10.1007/BF00777485. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Keohavong P., Jacob M. Mild nuclease treatment as a probe for a non-random distribution of adenovirus-specific RNA sequences and of cellular RNA in nuclear ribonucleoprotein fibrils. J Mol Biol. 1982 Mar 5;155(3):185–205. doi: 10.1016/0022-2836(82)90001-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Shepherd J. H., Mulvihill E. R., Palmiter R. D. Isolation of a nuclear ribonucleoprotein fraction from chick oviduct containing ovalbumin messenger RNA sequences. J Mol Biol. 1981 Aug 5;150(2):143–160. [PubMed] [Google Scholar]
- Zieve G., Penman S. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell. 1976 May;8(1):19–31. doi: 10.1016/0092-8674(76)90181-1. [DOI] [PubMed] [Google Scholar]