Abstract
BACKGROUND
Obesity causes frailty in older adults; however, weight loss might accelerate age-related loss of muscle and bone mass and resultant sarcopenia and osteopenia.
METHODS
In this clinical trial involving 160 obese older adults, we evaluated the effectiveness of several exercise modes in reversing frailty and preventing reduction in muscle and bone mass induced by weight loss. Participants were randomly assigned to a weight-management program plus one of three exercise programs — aerobic training, resistance training, or combined aerobic and resistance training — or to a control group (no weight-management or exercise program). The primary outcome was the change in Physical Performance Test score from baseline to 6 months (scores range from 0 to 36 points; higher scores indicate better performance). Secondary outcomes included changes in other frailty measures, body composition, bone mineral density, and physical functions.
RESULTS
A total of 141 participants completed the study. The Physical Performance Test score increased more in the combination group than in the aerobic and resistance groups (27.9 to 33.4 points [21% increase] vs. 29.3 to 33.2 points [14% increase] and 28.8 to 32.7 points [14% increase], respectively; P=0.01 and P=0.02 after Bonferroni correction); the scores increased more in all exercise groups than in the control group (P<0.001 for between-group comparisons). Peak oxygen consumption (milliliters per kilogram of body weight per minute) increased more in the combination and aerobic groups (17.2 to 20.3 [17% increase] and 17.6 to 20.9 [18% increase], respectively) than in the resistance group (17.0 to 18.3 [8% increase]) (P<0.001 for both comparisons). Strength increased more in the combination and resistance groups (272 to 320 kg [18% increase] and 288 to 337 kg [19% increase], respectively) than in the aerobic group (265 to 270 kg [4% increase]) (P<0.001 for both comparisons). Body weight decreased by 9% in all exercise groups but did not change significantly in the control group. Lean mass decreased less in the combination and resistance groups than in the aerobic group (56.5 to 54.8 kg [3% decrease] and 58.1 to 57.1 kg [2% decrease], respectively, vs. 55.0 to 52.3 kg [5% decrease]), as did bone mineral density at the total hip (grams per square centimeter; 1.010 to 0.996 [1% decrease] and 1.047 to 1.041 [0.5% decrease], respectively, vs. 1.018 to 0.991 [3% decrease]) (P<0.05 for all comparisons). Exercise-related adverse events included musculoskeletal injuries.
CONCLUSIONS
Of the methods tested, weight loss plus combined aerobic and resistance exercise was the most effective in improving functional status of obese older adults. (Funded by the National Institutes of Health; LITOE ClinicalTrials.gov number, NCT01065636.)
More than a third of persons 65 years of age or older in the United States are obese,1 and this group constitutes a population vulnerable to adverse outcomes, because obesity exacerbates the age-related decline in physical function and causes frailty.2–5 However, appropriate management of obesity in older adults remains controversial, given the reported reduction in relative health risks associated with increasing body-mass index in this group.6 Moreover, an important concern is that weight loss could worsen frailty by accelerating the age-related decline in muscle and bone mass and resultant sarcopenia and osteopenia.7,8
Given the positive effects of exercise on physical function, healthy aging in obese older adults might require an intervention that involves regular exercise.9 We reported previously that exercise (combined aerobic and resistance training) in combination with weight loss was associated with greater improvement in physical function than weight loss alone or exercise alone.10 However, exercise attenuated but did not prevent loss in muscle and bone mass induced by weight loss and ameliorated but did not reverse frailty. The physiologic adaptations to aerobic and resistance exercise are distinctly different: aerobic exercise improves cardiovascular adaptations that increase peak oxygen consumption without significantly changing strength, whereas resistance exercise improves neuromuscular adaptations that increase strength without significantly changing peak oxygen consumption.11 These physiologic adaptations may interfere with each other when the two types of training are performed together.12–14 In the current clinical trial, we compared the effectiveness of aerobic exercise, resistance exercise, and combined exercise in reversing frailty and preserving muscle and bone mass during weight loss in obese older adults. We hypothesized that weight loss plus resistance exercise would improve physical function more than weight loss plus aerobic exercise or weight loss plus combined aerobic and resistance exercise.
METHODS
STUDY OVERSIGHT
We conducted the Lifestyle Intervention Trial in Obese Elderly (LITOE) from April 2010 through June 2015 at the University of New Mexico School of Medicine and New Mexico Veterans Affairs Health Care System. The study was approved by the institutional review board of the University of New Mexico School of Medicine and was monitored by an independent data and safety monitoring board; all participants provided written informed consent. All the authors had access to the data and vouch for the integrity, accuracy, and completeness of the data and analyses and for the fidelity of the study to the protocol. The first author wrote the first draft of the manuscript; all the authors participated in subsequent drafts and made the decision to submit the manuscript for publication. No commercial support was received.
PARTICIPANTS
Participants were recruited through advertisements and underwent comprehensive medical screening. Persons were eligible for inclusion if they were 65 years of age or older, were obese (body-mass index [the weight in kilograms divided by the square of the height in meters] ≥30), were sedentary (regular exercise <1 hour per week), and had had a stable body weight (loss or gain of no greater than 2 kg) and stable medication use for 6 months before enrollment. All participants had mild-to-moderate frailty, as defined by a score of 18 to 31 on the modified Physical Performance Test (scores range from 0 to 36 points, with higher scores indicating better performance).15 Persons who had severe cardiopulmonary disease (e.g., recent myocardial infarction or unstable angina), musculoskeletal or neuromuscular impairments that precluded exercise training, or cognitive impairments or who used drugs that affect bone metabolism were excluded.
STUDY OUTCOMES
The primary outcome was the change in score on the Physical Performance Test from baseline to 6 months. Secondary outcomes were changes in other frailty measures, body composition, bone mineral density, specific physical functions, and quality of life.
INTERVENTION
In this 26-week study, participants were randomly assigned, with stratification according to sex, to one of four groups — a control protocol that included neither a weight-management nor an exercise intervention, an aerobic group that participated in a weight-management program and aerobic exercise training, a resistance group that participated in a weight-management program and resistance exercise training, and a combination group that participated in a weight-management program and combined aerobic and resistance exercise training.
The control group was asked not to participate in external weight-loss or exercise programs. However, this group attended group educational sessions about a healthful diet during monthly visits.
The aerobic group participated in a weight-management program, in which the participants were prescribed a balanced diet that provided an energy deficit of 500 to 750 kcal per day and contained approximately 1 g of high-quality protein per kilogram of body weight per day.2 Participants met weekly with a dietitian for dietary adjustments and behavioral therapy (diet therapy). They were instructed to set weekly behavioral goals and attend weigh-in sessions. Food diaries were reviewed, and goals were set on the basis of diary reports. The goal was to achieve a weight loss of approximately 10% at 6 months. They also participated in aerobic exercise training sessions three times weekly. The sessions were approximately 60 minutes long and included 10 minutes of flexibility exercises, followed by 40 minutes of aerobic exercises and 10 minutes of balance exercises. The aerobic exercises consisted of treadmill walking, stationary cycling, and stair climbing. Participants exercised at approximately 65% of their peak heart rate, which was gradually increased to 70 to 85%.
The resistance group participated in the same weight-management program as the aerobic group, as well as resistance exercise training sessions three times weekly; the sessions were approximately 60 minutes long and included 10 minutes of flexibility exercises, followed by 40 minutes of resistance exercises and 10 minutes of balance exercises. The resistance training consisted of nine upper-body and lower-body exercises using weight-lifting machines. The initial sessions were 1 to 2 sets of 8 to 12 repetitions at 65% of the one-repetition maximum. This was increased progressively to 2 to 3 sets at approximately 85% of the one-repetition maximum.
The combination group participated in the same weight-management program as the other exercise groups, as well as combined aerobic and resistance exercise training sessions three times weekly. The sessions were 75 to 90 minutes long and included 10 minutes of flexibility exercises, followed by 30 to 40 minutes of aerobic exercises, 30 to 40 minutes of resistance exercises, and 10 minutes of balance exercises. To test the interference effect,12–14 aerobic and resistance training were balanced between groups: the longer duration of exercise in the combination group allowed the participants to perform an amount of aerobic exercise that was equivalent to that of the aerobic group and an amount of resistance exercise that was equivalent to that of the resistance group.
Exercise sessions were supervised by exercise trainers. Participants were advised to maintain their usual physical activity outside of exercise sessions. All participants received supplements to ensure an intake of approximately 1500 mg of calcium per day and approximately 1000 IU of vitamin D per day.2
BASELINE ASSESSMENTS
Physical Function
Frailty was assessed with the Physical Performance Test. The Physical Performance Test includes seven standardized tasks (walking 15.2 m [50 ft], putting on and removing a coat, picking up a penny, standing up from a chair, lifting a book, climbing one flight of stairs, and performing a progressive Romberg test) plus two additional tasks (going up and down four flights of stairs and making a 360-degree turn). The score for each task ranges from 0 to 4, with higher scores indicating better physical performance; a perfect score would be 36.15 Frailty was also assessed by measurement of peak oxygen consumption and by administration of the Functional Status Questionnaire. Peak oxygen consumption was assessed during graded treadmill walking, as described previously.3 Ability to perform activities of daily living was assessed with the use of the Functional Status Questionnaire (scores range from 0 to 36, with higher scores indicating better functional status).16 We also assessed strength, balance, gait speed, and one-repetition maximum (the maximum weight a participant can lift, in one attempt, in the biceps curl, bench press, seated row, knee extension, knee flexion, and leg press). We assessed static balance by measuring the time a participant could stand on a single leg3 and dynamic balance by measuring the time needed to complete an obstacle course.15 Fast gait speed was assessed by measurement of the time needed to walk 7.6 m (25 ft).
Body Composition and Bone Mineral Density
Fat mass, lean mass, and bone mineral density of the whole body and at the lumbar spine and total hip were measured with the use of dual-energy x-ray absorptiometry (Lunar DPX [General Electric] or Discovery A [Hologic] scanner), as described previously.3,17 For each participant, baseline and follow-up scans were obtained with the use of the same instrument. Thigh muscle and fat volumes were measured by magnetic resonance imaging (Magnetom Avanto [Siemens]), as described previously.18
Quality of Life
We used the physical and mental component subscales of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), version 2, to evaluate quality of life.19 Scores on the physical and mental component subscales of the SF-36 range from 0 to 100, with higher scores indicating better health status; the minimal clinically important difference is 2 points.20
FOLLOW-UP ASSESSMENTS
All baseline assessments were repeated at 6 months. The Physical Performance Test was also repeated at 3 months. Assessors were unaware of the study-group assignments.
STATISTICAL ANALYSIS
We estimated that a sample size of 40 participants per group would provide 80% power to detect a mean (±SD) clinically important difference between groups of 1.8±2.5 in the change in score on the Physical Performance Test, at an alpha level of 0.05. Intention-to-treat analyses were performed with SAS software, version 9.4 (SAS Institute). Baseline characteristics were compared with the use of analysis of variance or Fisher’s exact test. Longitudinal changes between groups were tested with the use of mixed-model repeated-measures analysis of variance, with adjustment for baseline values and sex. The primary focus of the analyses was the 6-month change in outcome in the four groups. When the overall P value for the interaction between group and time was less than 0.05, prespecified comparisons were used to test five hypotheses: that changes in the aerobic group would differ from those in the control group, that changes in the resistance group would differ from those in the control group, that changes in the aerobic group would differ from those in the resistance group, and that changes in the combination group would differ from those in the aerobic group and from those in the resistance group. For the Physical Performance Test score, Bonferroni correction was used to adjust for these comparisons. Within-group changes were analyzed with the use of repeated-measures analysis of variance. Secondary outcomes were grouped into five domains. In accordance with a gatekeeping strategy,21 a significant group-by-time interaction (P<0.01) and at least one significant difference between an exercise group and the control group and at least one significant difference among the exercise groups in the change in score on the Physical Performance Test were required to continue to testing of secondary outcomes; comparisons of the exercise groups with the control group were performed with Dunnett’s test and comparisons among the intervention groups were performed with the Fisher–Hayter test22 (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Sensitivity analyses that validated the statistical results included multiple imputation for missing fitness data. Data for change scores and percentage changes are presented as least-squares–adjusted means (±SE).
RESULTS
STUDY POPULATION
A total of 160 participants underwent randomization, and 141 participants (88%) completed the study (Fig. 1). Nineteen participants discontinued the intervention and were included in the intention-to-treat analyses (follow-up data were obtained for all 19 participants at 3 months but not at 6 months). There were no significant differences among the groups in baseline characteristics (Table 1).
Figure 1. Screening, Randomization, and Follow-up.
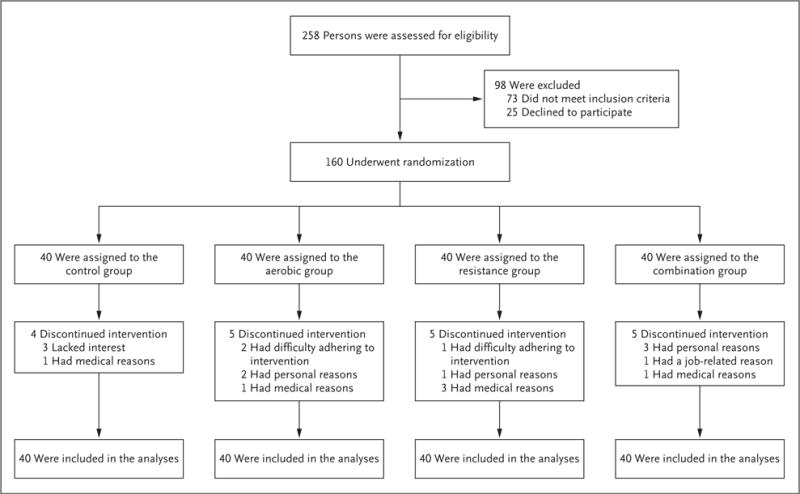
The study groups included a control group that received neither a weight-management nor an exercise intervention and three exercise groups: a group that received aerobic exercise training (aerobic group), a group that received resistance exercise training (resistance group), and a group that received combined aerobic and resistance exercise training (combination group); all three exercise groups also participated in a weight-management program.
Table 1.
Baseline Characteristics of the Participants.*
| Characteristic | Control (N = 40) |
Aerobic (N = 40) |
Resistance (N = 40) |
Combination (N = 40) |
|---|---|---|---|---|
| Age — yr | 70±5 | 70±4 | 70±5 | 70±5 |
| Sex — no. (%) | ||||
| Male | 12 (30) | 14 (35) | 15 (38) | 16 (40) |
| Female | 28 (70) | 26 (65) | 25 (62) | 24 (60) |
| Race — no. (%)† | ||||
| White | 36 (90) | 36 (90) | 33 (82) | 35 (88) |
| Black | 1 (2) | 0 | 3 (8) | 2 (5) |
| Other | 3 (8) | 4 (10) | 4 (10) | 3 (8) |
| Ethnic group — no. (%)† | ||||
| Hispanic or Latino | 13 (32) | 13 (32) | 12 (30) | 12 (30) |
| Not Hispanic or Latino | 27 (68) | 27 (68) | 27 (68) | 28 (70) |
| Unknown | 0 | 0 | 1 (2) | 0 |
| Education — no. (%) | ||||
| Less than a college degree | 14 (35) | 13 (32) | 15 (38) | 10 (25) |
| College degree | 20 (50) | 20 (50) | 12 (30) | 19 (48) |
| Graduate school | 6 (15) | 7 (18) | 13 (32) | 11 (28) |
| Marital status — no. (%) | ||||
| Single | 8 (20) | 4 (10) | 9 (22) | 5 (12) |
| Married | 15 (38) | 25 (62) | 22 (55) | 23 (58) |
| Divorced | 9 (22) | 7 (18) | 6 (15) | 8 (20) |
| Widowed | 8 (20) | 4 (10) | 3 (8) | 4 (10) |
| Body-mass index‡ | 36.7±5.0 | 35.9±4.4 | 36.7±5.8 | 35.8±4.5 |
| Chronic diseases — no.§ | 3.2±3.4 | 2.6±2.7 | 3.6±3.9 | 2.7±2.0 |
| Routine medications — no.¶ | 5.5±4.3 | 5.7±3.4 | 6.2±5.7 | 5.7±4.0 |
Plus-minus values are means ±SD. There were no significant between-group differences in baseline characteristics. The study groups included a control group that received neither a weight-management nor an exercise intervention and three exercise groups: a group that received aerobic exercise training (aerobic group), a group that received resistance exercise training (resistance group), and a group that received combined aerobic and resistance exercise training (combination group); all three exercise groups also participated in a weight-management program. Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the participants.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Chronic diseases included hypertension, diabetes, coronary artery disease, congestive heart failure, arthritis, and chronic lung disease.
Routine medications included antihypertensive, antidiabetic, antidyspeptic, antianginal, diuretic, antiarthritic, antilipidemic, and antidepressant medications.
Median attendance at the diet-therapy sessions was 96% (interquartile range, 87 to 100) in the aerobic group, 100% (interquartile range, 90 to 100) in the resistance group, and 97% (interquartile range, 89 to 100) in the combination group. Median attendance at exercise sessions was 96% (interquartile range, 84 to 100) in the aerobic group, 98% (interquartile range, 81 to 100) in the resistance group, and 93% (interquartile range, 83 to 100) in the combination group.
ADVERSE EVENTS
Exercise-related adverse events included falling, shoulder pain, and back pain in the aerobic group; atrial fibrillation, shoulder pain, and knee pain in the resistance group; and shoulder injury, knee pain, back pain, and hip pain in the combination group. There were no other differences in adverse events relative to the control group (Table S1 and S2 in the Supplementary Appendix).
PHYSICAL PERFORMANCE TEST AND OTHER FRAILTY MEASURES
The scores on the Physical Performance Test increased more in the combination group than in aerobic or resistance groups: 27.9 to 33.4 points (a change of 5.5±0.4 points [21% increase from the least-squares adjusted mean at baseline]) versus 29.3 to 33.2 points (a change of 3.9±0.4 points [14% increase]) and 28.8 to 32.7 points (a change of 3.9±0.4 points [14% increase]), respectively; scores in all three exercise groups increased more than scores in the control group (4% increase) (Table 2 and Fig. 2A). Peak oxygen consumption (measured as milliliters per kilogram of body weight per minute) increased more in the combination and aerobic groups than in the resistance group: 17.2 to 20.3 (a change of 3.1±0.3 [17% increase]) and 17.6 to 20.9 (a change of 3.3±0.3 [18% increase]), respectively, versus 17.0 to 18.3 (a change of 1.3±0.3 [8% increase]) (Table 2 and Fig. 2B). Functional Status Questionnaire scores increased more in the combination group than in the aerobic and resistance groups: 29.8 to 33.4 points (a change of 3.6±0.3 points [14% increase]) versus 30.1 to 32.1 points (a change of 2.0±0.3 points [7% increase]) and 29.3 to 31.6 points (a change of 2.3±0.3 points [8% increase]), respectively (Table 2 and Fig. 2C).
Table 2.
Effect of Specific Exercise Modes, Added to Diet-Induced Weight Loss, on Outcomes.*
|
Outcome |
Control (N =40) |
Aerobic (N = 40) |
Resistance (N = 40) |
Combination (N =40) |
P Value† | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group–Time Interaction |
Aerobic vs. Control |
Resistance vs. Control |
Aerobic vs. Resistance |
Combination vs. Aerobic |
Combination vs. Resistance |
|||||
| Primary outcome | ||||||||||
| PPT score | ||||||||||
| Baseline | 28.6±0.5 | 29.3±0.3 | 28.8±0.4 | 27.9±0.4 | ||||||
| Change at 3 mo | 0.4±0.3 | 2.9±0.4‡ | 3.2±0.4‡ | 4.0±0.4‡ | ||||||
| Change at 6 mo | 1.0±0.4 | 3.9±0.4‡ | 3.9±0.4‡ | 5.5±0.4‡ | <0.001 | <0.001 | <0.001 | 0.87 | 0.002 | 0.004 |
| Secondary outcomes | ||||||||||
| Other frailty measures | ||||||||||
| VO2peak (ml/kg/min) | ||||||||||
| Baseline | 17.0±0.5 | 17.6±0.5 | 17.0±0.6 | 17.2±0.6 | ||||||
| Change at 6 mo | 0.1±0.3 | 3.3±0.3‡ | 1.3±0.3‡ | 3.1±0.3‡ | <0.001 | <0.001 | 0.007 | <0.001 | 0.63 | 0.001 |
| FSQ score | ||||||||||
| Baseline | 29.8±0.5 | 30.1±0.5 | 29.3±0.6 | 29.8±0.6 | ||||||
| Change at 6 mo | 0.4±0.3 | 2.0±0.3‡ | 2.3±0.3‡ | 3.6±0.3‡ | <0.001 | 0.002 | <0.001 | 0.46 | 0.005 | 0.03 |
| Body composition | ||||||||||
| Body weight (kg) | ||||||||||
| Baseline | 97.9±2.9 | 96.9±2.3 | 101.8±2.9 | 99.0±2.9 | ||||||
| Change at 6 mo | −0.9±0.5 | −9.0±0.6‡ | −8.5±0.5‡ | −8.5±0.5‡ | <0.001 | <0.001 | <0.001 | 0.76§ | 0.72§ | 0.96§ |
| Lean mass (kg) | ||||||||||
| Baseline | 54.9±2.3 | 55.0±1.9 | 58.1±2.3 | 56.5±1.8 | ||||||
| Change at 6 mo | 0.0±0.2 | −2.7±0.3‡ | −1.0±0.3¶ | −1.7±0.3‡ | <0.001 | <0.001 | 0.03 | 0.001 | 0.047 | 0.20 |
| Fat mass (kg) | ||||||||||
| Baseline | 43.0±1.5 | 41.9±1.3 | 44.3±1.5 | 42.5±1.6 | ||||||
| Change at 6 mo | −0.9±0.4 | −6.3±0.5‡ | −7.3±0.4‡ | −7.0±0.5‡ | <0.001 | <0.001 | <0.001 | 0.18§ | 0.36§ | 0.67§ |
| Thigh muscle (cm3) | ||||||||||
| Baseline | 1302±63 | 1234±62 | 1190±48 | 1186±66 | ||||||
| Change at 6 mo | 10±7 | −77±7‡ | −23±7¶ | −40±7‡ | <0.001 | <0.001 | 0.008 | <0.001 | 0.005 | 0.21 |
| Thigh fat (cm3) | ||||||||||
| Baseline | 1774±132 | 1700±98 | 1848±108 | 1784±125 | ||||||
| Change at 6 mo | −2±36 | −260±35‡ | −280±35‡ | −288±35‡ | <0.001 | <0.001 | <0.001 | 0.64§ | 0.61§ | 0.97§ |
| BMD at total hip (g/cm2) | ||||||||||
| Baseline | 1.031±0.025 | 1.018±0.019 | 1.047±0.022 | 1.010±0.025 | ||||||
| Change at 6 mo | 0.004±0.004 | −0.027±0.004‡ | −0.006±0.004 | −0.014±0.004¶ | 0.001 | <.001 | 0.37 | 0.005 | 0.04 | 0.43 |
| Strength, balance, and gait | ||||||||||
| Total 1RM (kg)║ | ||||||||||
| Baseline | 269±21 | 265±20 | 288±30 | 272±16 | ||||||
| Change at 6 mo | 2±5 | 5.0±5 | 49±5‡ | 48±5‡ | <0.001 | 0.99 | <0.001 | <0.001 | <0.001 | 0.82 |
| Obstacle course (sec) | ||||||||||
| Baseline | 15.9±0.7 | 15.5±0.8 | 16.4±0.6 | 17.0±1.0 | ||||||
| Change at 6 mo | 0.0±0.3 | −1.5±0.4‡ | −2.2±0.3‡ | −2.9±0.3‡ | <0.001 | 0.02 | <0.001 | 0.20 | 0.01 | 0.20 |
| Gait speed (m/min) | ||||||||||
| Baseline | 75.0±2.6 | 74.6±2.0 | 74.3±2.3 | 68.8±2.2 | ||||||
| Change at 6 mo | −0.5±1.3 | 8.1±1.3‡ | 9.3±1.3‡ | 12.1±1.3‡ | <0.001 | <0.001 | <0.001 | 0.56 | 0.03 | 0.09 |
Plus-minus values for the change scores are the least-squares adjusted means ±SE from the repeated-measures analyses of variance; plus-minus values for the baseline values are the observed means ±SE. Scores on the Physical Performance Test (PPT) (primary outcome) range from 0 to 36, with higher scores indicating better physical function; the minimal clinically important difference is 1.8. Peak oxygen consumption (VO2peak) was assessed during graded treadmill walking. Scores on the Functional Status Questionnaire (FSQ) range from 0 to 36, with higher scores indicating better function. BMD denotes bone mineral density.
P values for the changes from baseline to 6 months in between-group comparisons were calculated with the use of mixed-model repeated-measures analyses of variance (with baseline values and sex as covariates) and are reported when the overall P value was lower than 0.05 for the interaction among the four groups over time. In a Bonferroni correction to adjust for the multiple comparisons in the PPT score (in which the P values were multiplied by 5 for the comparison with an alpha level of 0.05), the corrected P values were 0.01 for the combination group versus the aerobic group and 0.02 for the combination group versus the resistance group. In accordance with a gatekeeping strategy, a significant group-by-time interaction (P<0.01) and at least one significant difference between an exercise group and the control group and at least one significant difference among the exercise groups in the change in PPT score were required to continue to testing of the secondary outcomes; comparisons of the exercise groups with the control group were performed with Dunnett’s test and comparisons among the intervention groups were performed with the Fisher–Hayter test. Secondary analyses included a comparison between the combination group and the control group; all P values were less than 0.05.
P<0.001 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
The between-group differences were not significant in the last three columns because the group-by-time interaction for the three intervention groups (step 1 of the Fisher–Hayter test) was not significant; the family-wise error rate of 0.05 or less is maintained for the Fisher–Hayter multiple comparison procedure.
P<0.01 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
Total one-repetition maximum (1RM) is the total of the maximum weight a participant can lift, in one attempt, in the biceps curl, bench press, seated row, knee extension, knee flexion, and leg press.
Figure 2. (facing page). Mean Percentage Changes in Physical Function, Lean Mass, and BMD at the Total Hip during the Interventions.
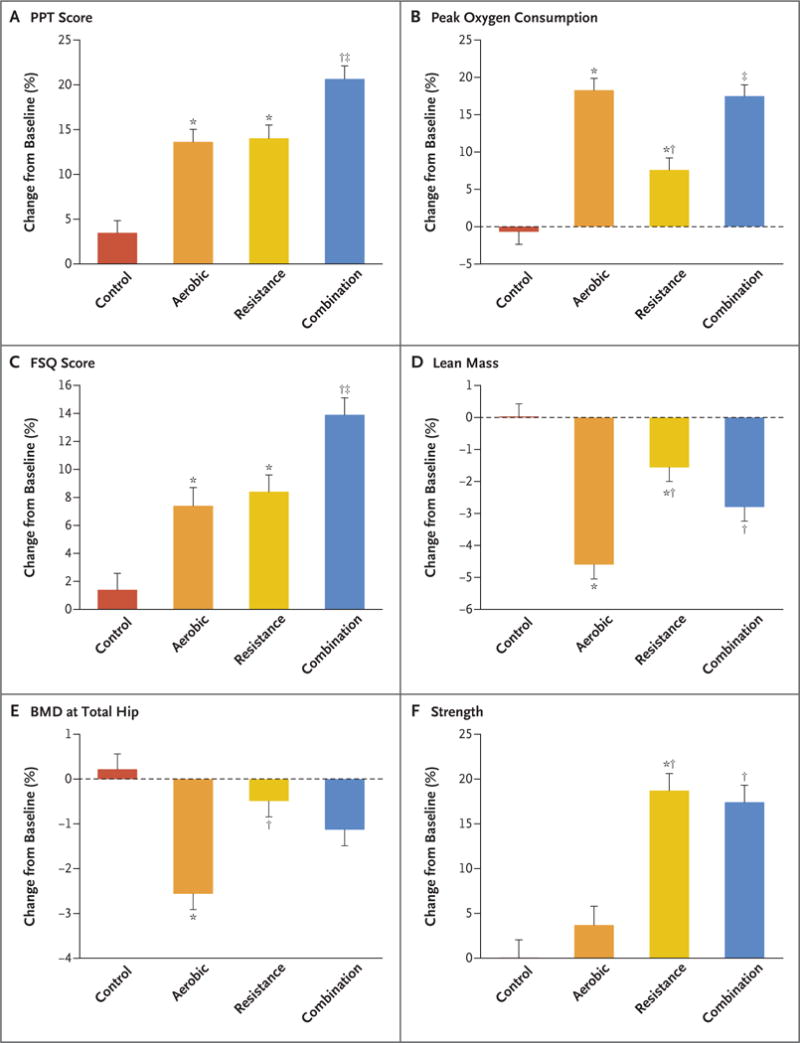
Measures of physical function included the Physical Performance Test (PPT; scores range from 0 to 36, with higher scores indicating better functional status), peak oxygen consumption, Functional Status Questionnaire (FSQ; scores range from 0 to 36, with higher scores indicating better functional status), and strength (measured as total one-repetition maximum [i.e., the total of the maximum weight a participant can lift, in one attempt, in the biceps curl, bench press, seated row, knee extension, knee flexion, and leg press]). Scores on the PPT were used as an objective measure of frailty (primary outcome), and scores on the FSQ were used as a subjective measure of frailty. The asterisk indicates P<0.05 for the comparison with the control group, the dagger P<0.05 for the comparison with the aerobic group, and the double dagger P<0.05 for the comparison with the resistance group. Percentage changes are presented as least-squares–adjusted means; T bars indicate standard errors. BMD denotes bone mineral density.
BODY COMPOSITION
Body weight decreased in the aerobic group (96.9 to 87.9 kg; a change of −9.0±0.6 kg, [9% decrease]), in the resistance group (101.8 to 93.3 kg; a change of −8.5±0.5 kg [9% decrease]), and in the combination group (99.0 to 90.5 kg; a change of −8.5±0.5 kg [9% decrease]), but there was no significant change in body weight in the control group (97.9 to 97.0 kg; a change of −0.9±0.5 kg [<1% decrease]) (Table 2). The time course of weight loss is shown in Figure 3. Lean mass decreased less in the combination group (56.5 kg to 54.8 kg; a change of −1.7±0.3 kg [3% decrease]) and in the resistance group (58.1 to 57.1 kg; a change of −1.0±0.3 kg [2% decrease]) than in the aerobic group (55.0 to 52.3 kg; a change of −2.7±0.3 kg [5% decrease]) (Table 2 and Fig. 2D). Fat mass decreased by −6.3±0.5 kg in the aerobic group (41.9 to 35.6 kg [16% decrease]), −7.3±0.4 kg in the resistance group (44.3 to 37.0 kg [17% decrease]), and −7.0±0.5 kg in the combination group (42.5 to 35.5 kg [17% decrease]). The changes in thigh muscle and thigh fat among the exercise groups were similar to those observed for lean mass and fat mass, respectively.
Figure 3. Mean Percent Changes in Body Weight during the Interventions.
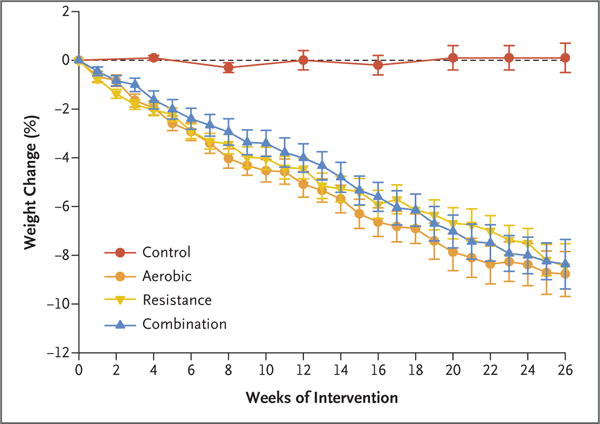
Percent changes are presented as least-squares–adjusted means; I bars indicate standard errors.
BONE MINERAL DENSITY
Bone mineral density at the total hip did not change significantly in the resistance group (1.047 to 1.041 g per square centimeter; a change of −0.006±0.004 g per square centimeter [<1% decrease]), whereas it decreased in the aerobic group (1.018 to 0.991 g per square centimeter; a change of −0.027±0.004 g per square centimeter [2.6% decrease]) and in the combination group (1.010 to 0.996 g per square centimeter; a change of −0.014±0.004 g per square centimeter [1.1% decrease]) (Table 2 and Fig. 2E). Bone mineral density of the whole body and at the lumbar spine did not change significantly in any of the study groups (Table S3 in the Supplementary Appendix).
STRENGTH, BALANCE, GAIT, AND QUALITY OF LIFE
Total one-repetition maximum strength increased in the resistance group (288 to 337 kg; a change of 49±5 kg [19% increase]) and in the combination group (272 to 320 kg; a change of 48±5 kg [18% increase]), whereas it was maintained in the aerobic group (265 to 270 kg; a change of 5±5 kg [4% increase]) (Table 2 and Fig. 2F). Time needed to complete the obstacle course decreased more in the combination group (17.0 to 14.1 seconds; a change of −2.9±0.3 seconds [13% decrease]) than in the aerobic group (15.5 to 14.0 seconds; a change of −1.5±0.4 seconds [7% decrease]). Gait speed increased more in the combination group (68.8 to 80.9 m per minute; a change of 12.1±1.3 m per minute [14% increase]) than in the aerobic group (74.6 to 82.7 m per minute; a change of 8.1±1.3 m per minute [9% increase]). The SF-36 physical-component score increased more in the combination group (45.9 to 55.4 points; a change of 9.5±0.7 points [24% increase]) than in the aerobic group (48.6 to 55.1 points; a change of 6.5±0.7 points [14% increase]) and the resistance group (51.0 to 58.4 points; a change of 7.4±0.8 points [17% increase]) (Table S3 in the Supplementary Appendix).
DISCUSSION
Our randomized, controlled trial involving obese adults 65 years of age or older indicated that weight loss plus a combination of aerobic and resistance exercise improved physical function and reduced frailty more than weight loss plus aerobic exercise or weight loss plus resistance exercise. Evidence-based data to guide treatment of older adults with obesity are limited5,23,24 and tend to rely on studies involving younger adults.2 Our study directly compared aerobic, resistance, and combined (aerobic and resistance) training during weight loss in obese older adults. The matched weight loss across groups facilitated the assessment of the independent and combined effects of aerobic and resistance training. Despite a negative energy balance, aerobic training improved cardiovascular fitness and resistance training improved strength. Contrary to our hypothesis, combined aerobic and resistance training improved cardiovascular fitness to the same extent as aerobic training alone and strength to the same extent as resistance training alone. Therefore, combined aerobic and resistance training resulted in additive effects that translated into the greatest improvement among the interventions in physical function and reduction of frailty. Both resistance training and combined resistance and aerobic training attenuated the loss of lean mass during aerobic training. Moreover, although only resistance training prevented the weight-loss–induced reduction in bone mineral density at the total hip, combined aerobic and resistance training nonetheless attenuated the loss of bone mineral density at the total hip during aerobic training. Our data suggest that obese older adults can adapt and respond to exercise training during an energy deficit and that combined aerobic and resistance training provides the greatest benefits with respect to physical function, with relative preservation of lean mass.
Our findings in obese older adults expand observations of the positive effects of exercise training without weight loss in nonobese older adults25,26 and support the results of previous studies that showed that exercise training was most beneficial in frail older adults in the earlier stages of frailty.27 Given the exercise goals for our frail and obese participants,28 we designed the aerobic and resistance training to be moderate to vigorous in intensity to induce exercise adaptations29 while keeping exercise volumes moderate.27 Using these exercise strategies, we found additive effects of aerobic and resistance training without interference effect from concurrent training.12–14 Adherence to exercise was high despite frailty, and adverse events were relatively few and consistent with coexisting medical conditions. Our findings suggest that the recommendation by the American Heart Association and American College of Sports Medicine to combine aerobic exercise with resistance exercise for overall health30 extends to obese older adults undertaking weight loss.
The improvements in objective measures of frailty in our participants may have important implications for preserving independent living. The Physical Performance Test assesses multiple domains of physical function15 and predicts disability, loss of independence, and death.31,32 The peak oxygen consumption relative to body weight is the best indicator of cardiovascular endurance33 and is important for performing daily tasks with increased body weight.3,34 Improvements in the objective Physical Performance Test score and peak oxygen consumption were consistent with improvements in Functional Status Questionnaire and SF-36 scores, which indicate subjective improvements in functional ability.
Although combined aerobic and resistance training improved physical function the most among the interventions, the reductions in lean mass and bone mineral density that were attenuated but not prevented might represent an adverse effect in that these reductions further diminish tissue reserves superimposed on age-related losses. However, resistance training improved strength despite muscle loss induced by weight loss. Conversely, whether improved physical function lowers the risks of falls and fractures despite the decline in bone mineral density is currently unclear. In future studies, additional strategies to preserve lean mass might include improving the efficiency of vitamin D and protein intake, increasing weight-bearing exercises, and perhaps administering anabolic hormone therapy.35,36 Another adverse effect was exercise-related musculoskeletal injuries, which could be minimized through individualized exercises.
Strengths of our study include the randomized, controlled trial design, the comprehensive lifestyle programs, the high rate of adherence to the trial interventions, the similar weight-loss management that allowed for unbiased group comparisons, and the use of objective and subjective measures of physical function. Because this was an efficacy study, the 6-month duration was appropriate to determine which exercise was most efficacious in improving physical function during weight loss. Data from long-term studies that show whether weight loss plus combined aerobic and resistance training prolongs physical independence in obese older adults are currently lacking. The findings from our study may have pragmatic implications, because Medicare currently covers behavioral therapy for weight loss,37 and a growing number of Medicare plans now offer gym memberships.38 Data from a previous trial show that older adults may be more successful in achieving long-term weight loss than younger adults.39
Our study has limitations. First, in accordance with the exclusion criteria, the participants in our study were physically able to participate in a lifestyle program and thus may not be fully representative of the general obese older adult population. Second, our sample was not large enough to analyze differences according to sex. Finally, most of the participants were women, white, and well educated, which limits broader generalization.
In conclusion, our study showed that weight loss plus resistance training or aerobic training improved physical function and ameliorated frailty; however, weight loss plus combined aerobic and resistance training provided greater improvement in physical function and reduction of frailty than either intervention alone and was associated with relative preservation of lean mass.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the Department of Veterans Affairs (VA).
Supported by grants from the National Institutes of Health (RO1-AG031176, UL1-TR000041, and P30-DK020579).
We thank the participants for their cooperation, Kenneth Fowler for study coordination, Brandy Martinez and Erik Faria for exercise training, and Ronni Farris and Reed Vawter for weight-loss training. We also thank the members of the Alkek Foundation for their support. The findings reported in this article are the result of work supported with resources and the use of facilities at the New Mexico VA Health Care System and Michael E. DeBakey VA Medical Center.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 3.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 4.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–34. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 5.Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J Am Med Dir Assoc. 2014;15:240–50. doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol. 2013;48:1054–61. doi: 10.1016/j.exger.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 8.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–91. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 9.Evans WJ. Exercise as the standard of care for elderly people. J Gerontol A Biol Sci Med Sci. 2002;57:M260–M261. doi: 10.1093/gerona/57.5.m260. [DOI] [PubMed] [Google Scholar]
- 10.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert CP, Evans WJ. Adaptations to aerobic and resistance exercise in the elderly. Rev Endocr Metab Disord. 2005;6:137–43. doi: 10.1007/s11154-005-6726-5. [DOI] [PubMed] [Google Scholar]
- 12.Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45:255–63. doi: 10.1007/BF00421333. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JM, Marin PJ, Rhea MR, Wilson SM, Loenneke JP, Anderson JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. 2012;26:2293–307. doi: 10.1519/JSC.0b013e31823a3e2d. [DOI] [PubMed] [Google Scholar]
- 14.Cadore EL, Pinto RS, Lhullier FL, et al. Physiological effects of concurrent training in elderly men. Int J Sports Med. 2010;31:689–97. doi: 10.1055/s-0030-1261895. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 16.Jette AM, Cleary PD. Functional disability assessment. Phys Ther. 1987;67:1854–9. doi: 10.1093/ptj/67.12.1854. [DOI] [PubMed] [Google Scholar]
- 17.Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–10. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 18.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985) 2007;102:634–40. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons RA, Perry HM, Littlepage BN. Evidence for the validity of the Short-form 36 Questionnaire (SF-36) in an elderly population. Age Ageing. 1994;23:182–4. doi: 10.1093/ageing/23.3.182. [DOI] [PubMed] [Google Scholar]
- 20.Maruish ME, editor. User’s manual for the SF-36v2 health survey. 3rd. Lincoln, RI: QualityMetric; 2011. [Google Scholar]
- 21.Dmitrienko A, Tamhane AC. Mixtures of multiple testing procedures for gatekeeping applications in clinical trials. Stat Med. 2011;30:1473–88. doi: 10.1002/sim.4008. [DOI] [PubMed] [Google Scholar]
- 22.Hayter AJ. The maximum familywise error rate of Fisher’s least significant difference test. J Am Stat Assoc. 1986;12:1000–4. [Google Scholar]
- 23.Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: do benefits exceed potential risks? Exp Gerontol. 2016;86:4–13. doi: 10.1016/j.exger.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batsis JA, Gill LE, Masutani RK, et al. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc. 2017;65:257–68. doi: 10.1111/jgs.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 27.Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguirre LE, Villareal DT. Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser. 2015;83:83–92. doi: 10.1159/000382065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine Position Stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 30.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 31.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients: the Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–12. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 33.Binder EF, Birge SJ, Spina R, et al. Peak aerobic power is an important component of physical performance in older women. J Gerontol A Biol Sci Med Sci. 1999;54:M353–M356. doi: 10.1093/gerona/54.7.m353. [DOI] [PubMed] [Google Scholar]
- 34.Shah K, Wingkun NJ, Lambert CP, Villareal DT. Weight-loss therapy improves endurance capacity in obese older adults. J Am Geriatr Soc. 2008;56:1157–9. doi: 10.1111/j.1532-5415.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20:412–9. doi: 10.1097/01.med.0000433071.11466.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N) Baltimore: Centers for Medicare & Medicaid Services; Nov 21, 2011. [Google Scholar]
- 38.Nguyen HQ, Maciejewski ML, Gao S, Lin E, Williams B, Logerfo JP. Health care use and costs associated with use of a health club membership benefit in older adults with diabetes. Diabetes Care. 2008;31:1562–7. doi: 10.2337/dc08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svetkey LP, Clark JM, Funk K, et al. Greater weight loss with increasing age in the weight loss maintenance trial. Obesity (Silver Spring) 2014;22:39–44. doi: 10.1002/oby.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


