Abstract
Adult tuberculosis (TB) is the main cause of TB epidemic and death. The infection results mainly by endogenous reactivation of latent TB infection and secondarily transmitted by exogenous infection. There is no vaccine for adult TB. To this end, we first chose antigens from a potential antigenic reservoir. The antigens strongly recognized T cells from latent and active TB infections that responded to antigens expressed by Mycobacterium tuberculosis cultured under different metabolic states. Fusions of single-stage polyprotein CTT3H, two-stage polyprotein A1D4, and multistage CMFO were constructed. C57BL/6 mice vaccinated with DMT adjuvant ed CMFO (CMFO-DMT) were protected more significantly than by CTT3H-DMT, and efficacy was similar to that of the only licensed vaccine, Bacillus Calmette–Guérin (BCG) and A1D4-DMT in the M. tuberculosis primary infection model. In the setting of BCG priming and latent TB infection, M. tuberculosis in the lung and spleen was eliminated more effectively in mice boosted with CMFO-DMT rather than with BCG, A1D4-DMT, or CTT3H-DMT. In particular, sterile immunity was only conferred by CMFO-DMT, which was associated with expedited homing of interferon-gamma+ CD4+ TEM and interleukin-2+ TCM cells from the spleen to the infected lung. CMFO-DMT represents a promising candidate to prevent the occurrence of adult TB through both prophylactic and therapeutic methods, and warrants assessment in preclinical and clinical trials.
Keywords: Tuberculosis, Subunit vaccine, CMFO-DMT, Primary infection, Latent infection, Reactivation
Abbreviations: TB, tuberculosis; LTBI, latent tuberculosis infection; ATB, active TB patient; BCG, Mycobacterium bovis Bacillus Calmette-Guerin; CTT3H, CFP10-TB10.4-TB8.4-Rv3615-HBHA; A1D4, Rv1813-Rv2660c-Ag85B-Rv2623-HspX; CMFO, Rv2875-Rv3044-Rv2073c-Rv0577; DMT, DDA-MPLA-TDB; TEM, Effector memory T; TCM, Centery memory T
Highlights
-
•
CMFO-DMT provides the comparable protection against primary infection with M. tuberculosis as BCG vaccine does.
-
•
CMFO-DMT boosts an effective protection of BCG primed mice to eliminate latent infection and thwart reactivation.
-
•
CMFO-DMT is a promising vaccine candidate for the prevention of adult TB disease.
Adult pulmonary TB is the main clinical form of the disease and the main component of TB epidemics. There is no effective vaccine to protect adults from primary and secondary TB. Vaccine candidates were constructed using combinations of one-, two- or multi-stage antigens of M. tuberculosis representing different stages of the infection. The antigen combinations directed at different stages of TB may help control adult TB.
1. Introduction
As the only vaccine for tuberculosis (TB), Mycobacterium bovis Bacillus Calmette-Guerin (BCG) has a high annual coverage in up to 90% infants worldwide. While providing effective protection for children against tuberculous meningitis and miliary TB, BCG protection persists for only about 15 years (Mangtani et al., 2014) and is insufficient to thwart the reactivation of latent tuberculosis infection (LTBI), which is often the cause of adult pulmonary TB (Andersen and Doherty, 2005). The World Health Organization (WHO) estimated that one-third of the world's population harbors LTBI, and that 10% these individuals will progress to active pulmonary TB resulting from the endogenous reactivation of the latent Mycobacterium tuberculosis persisting (WHO, 2015). This is especially true in people co-infected with M. tuberculosis and human immunodeficiency virus (WHO, 2015, WHO, 2016). Approximately 10 million new cases of TB occur annually, mostly in adults (WHO, 2016). Therefore, there is an urgent need to develop an efficacious vaccine for better control of LTBI and adult TB.
TB subunit vaccines have defined compositions and are safe, and so have attracted extensive attention compared to attenuated M. tuberculosis strains, recombinant BCG strains, DNA, and viral vectors (Kaufmann, 2014). Novel candidates have been evaluated in clinical trials designed to assess their prophylactic potential use. The candidates were constructed conventionally based on antigens secreted by logarithmically growing M. tuberculosis, such as the antigen85 complex, ESAT-6 protein, and TB10.4 protein (Kaufmann, 2014). In particular, the heat shock protein X (HspX), which is characteristically expressed by M. tuberculosis growing under low O2 condition, was demonstrated to enhance BCG-induced protection against primary TB infection in a mouse model (Shi et al., 2010). Subsequently, the construction of two-stage subunits ID93 (Rv3619-Rv1813-Rv3620-Rv2608; Bertholet et al., 2010) or H56 (Ag85B-ESAT-6-Rv2660c; Aagaard et al., 2011) was accomplished by fusing secreted and latent antigens of M. tuberculosis. Recently, the co-existence of both actively growing and latent M. tuberculosis strains in LTBIs and patients with active TB (ATB) infections were reported (Wang et al., 2015, Ma et al., 2016). A multistage subunit designated WH121 contains fusion polypeptide comprised of antigens Rv3407, PhoY2, Ag85A, Rv2626c, and RpfB. These antigens are expressed in different stages of TB infection. The use of WH121 in an adjuvant preparation of DMT (DDA-MPLA-TDB) reportedly provides protection against primary TB infection that is comparable to that provided by the BCG vaccine, and also enhances the protection of BCG-primed mice against the post-exposure infection (Ma et al., 2016). However, adult TB is mainly caused by endogenous reactivation of LTBI (WHO, 2015) and the secondary transmission route is exogenous infection (Verver et al., 2005). All the TB candidate vaccines could elicit antigen-specific Th1-type immune responses and confer significant protection against primary infection in different animal models, including mice (Ma et al., 2016), monkeys (Reed et al., 2009, Lin et al., 2012, Coler et al., 2013) and humans (Luabeya et al., 2015). However, no TB vaccine devised so far can achieve sterile immunity and thus thwart the reactivation of LTBI.
Antigen Rv0577 is a secretory antigen and a virulence factor associated with the replication of M. tuberculosis. The antigen is an agonist of Toll-like receptor 2 (TLR2) and can promote maturation of dendritic cells (Byun et al., 2012). Rv2875 encodes a cell membrane-associated antigen, MPT70, of M. tuberculosis that has strong immunogenicity (Windish et al., 2011). Rv3044 is a gene that is present in the genomes of M. tuberculosis complex, including BCG and its substrains. The gene product functions in the uptake and transport of Fe2 + in the phagosome (Wagner et al., 2005). Rv2073c is located in the RD9 region that is unique to the genome of virulent M. tuberculosis in the genus Mycobacteria (Behr et al., 1999, Tan et al., 2015).
With the exception of Rv0577, the aforementioned three genes are characteristically expressed by M. tuberculosis in hypoxic environments in vivo (Sassetti and Rubin, 2003). With the goal of developing an efficient vaccine for adult TB, these four antigens were screened using a recombinant CFP21-MPT64-whole blood interferon-gamma (IFN-γ) release assay (rCM-WBIA) (Fu et al., 2009). The antigens were strongly recognized by T cells from LTBIs and ATBs. A multistage fusion protein subunit, CMFO (Rv2875-Rv3044-Rv2073c-Rv0577), based on these four antigens was constructed. DMT was used as the adjuvant because of its ability as a strong inducer of Th1-type immunity (Teng et al., 2015). The protective efficacy of DMT adjuvant and CMFO was compared with that of the subunits of single-stage CTT3H (CFP10-TB10.4-TB8.4-Rv3615c-HBHA) (Teng et al., 2015) and two-stage A1D4 (Rv1813-Rv2660c-Ag85B-Rv2623-HspX) (Wang et al., 2015) in immunized mice against primary infection, latent infection, and reactivation, respectively. The immunological mechanisms associated with the protection elicited by CMFO-DMT were characterized.
2. Materials and Methods
2.1. Ethics Statement
The protocols for human experiments were reviewed and approved by the Ethics Committee of Tongji Medical College (Wuhan, China), and all subjects recruited in the current study provided written informed consent. Animal experiments were performed in accordance with the guidelines of the Chinese Council on Animal Care. Research protocols were approved by Tongji School Committees on Biosafety and Animal Experimental Ethics, and Wuhan University ABSL-3 laboratory.
2.2. Preparation of Recombinant Antigens
The synthesized gene sequence encoding CMFO, based on a tandem-linked fusion of Rv2875, Rv3044, Rv2073c, and Rv0577 with a c-terminal His tag, was used to construct the recombinant prokaryotic expression plasmid pET30b-CMFO. The plasmid was used to transform Escherichia coli BL21 strains. Other recombinant plasmids expressing single multistage antigens were also constructed (Supplementary Table 1). The expressions of the multistage protein CMFO and single antigens were induced with 1 mM isopropyl thio-β-d-galactoside (IPTG; Cat#367931; Sigma, St. Louis, MO, USA) for 4 h, and nitrilotriacetic acid-metal ion affinity chromatography (Cat#29058803; GE Healthcare, Piscataway, NJ, USA) was performed to purify the recombinant proteins, as previously described (Wang et al., 2015). After dialysis against sterile phosphate-buffered saline (PBS), each recombinant protein was lyophilized and diluted in PBS, using pyrogen-free reagents. Residual endotoxin contamination in the solution was verified to be < 0.1 Endotoxin Unit/mL. Finally, the protein concentration was detected using a BCA Protein Assay Kit (Cat#P0009; Beyotime, Shanghai, China). The purified proteins were identified by 12% SDS-PAGE.
2.3. T Cells From Different Human Populations Responding to CMFO and Multistage Antigens
Patients with ATBs and LTBIs (Xinxiang TB Prevention and Control Institute, Xinxiang, China), and non-infected healthy volunteers (HCs; Tongji Medical College, Wuhan, China) were recruited according to the default diagnostic criteria (Supplementary Table 2). rCM-WBIA was performed as we described previously (Fu et al., 2009). In brief, 0.5 mL of freshly heparinized whole blood was incubated with 20 μL of each antigen (10 μg/mL) in each well of 24-well plates for 24 h at 37 °C. Phytohemagglutinin (PHA, 20 μg/mL; Cat#5662-75-9; Sigma) served as a positive control and saline solution was the negative control. The supernatant from each well was collected and a human IFN-γ ELISA Kit (Cat#70EK180HS96, Multi Sciences Ltd., Hangzhou, China) was used to detect the concentration of IFN-γ in the supernatant of each sample. The cut-off value for a positive reaction against each antigen was set as three times the mean of saline-WBIA of HCs.
2.4. Vaccine Preparation and Immunization of Mice
Each dose of the vaccine containing 20 μg/100 μL of protein emulsified in 100 μL of the DMT adjuvant was prepared as previously described (Teng et al., 2015). Specific-pathogen-free, 6-week-old, female C57BL/6 mice (Huafukang Biological Science, Beijing, China) were maintained in a Biosafety level 3 laboratory and fed standard laboratory chow. The mice were immunized subcutaneously (s.c.) twice with 0.2 mL of each antigen-DMT mixture at a 3-week interval. Control mice were vaccinated with 200 μL of either PBS or DMT. BCG China at 1 × 106 colony forming units (CFU) was vaccinated s.c. as a positive control. All experiments were repeated twice.
2.5. Evaluation of Protection Against Primary Infection With M. tuberculosis
Nine weeks after immunization, mice were challenged intranasally (i.n.) with virulent M. tuberculosis strain H37Rv. The next day, three mice in the PBS control group were sacrificed and the whole lung was removed aseptically from each mouse to determine the actual infectious dose by enumeration of bacterial load (Ma et al., 2015). Four weeks post-challenge, differential protective efficacy between three subunit vaccines and BCG was determined by comparing the bacterial load in the spleen and lung (n = 6), and by observing the lung histopathological changes as previously described (n = 3) (Wang et al., 2015).
2.6. Evaluation of Protection Against Latent Infection and Reactivation
To establish the models of M. tuberculosis latent infection and reactivation, BCG was first used to vaccinate mice once prior to i.n. challenge with virulent M. tuberculosis strain H37Rv 4 weeks later. At week 9, the infected mice (n = 12 per group) were randomly assigned to receive a boost vaccination with each antigen-DMT mixture. BCG repeat vaccination was used as positive control. The negative control mice were treated with DMT or PBS. At week 15, protective efficacy between groups (n = 6) was compared as previously described (Ma et al., 2015). The remaining mice in each group were injected s.c. with dexamethasone (DXM; Cat#50022; Sigma) at 6 mg per kg body weight every 2 days, for a total of three injections (Lowrie et al., 1999). Eight weeks after the first injection, the reactivation of M. tuberculosis in each group (n = 6) was further evaluated by comparison of lung bacterial load.
2.7. CMFO-specific IgG Antibody Titers
To evaluate immunological mechanisms of CMFO-DMT, the CMFO-specific antibodies IgG, IgG1, and IgG2c (Cat#151276, 133045, 157720; Abcam, Cambridge, MA, USA) in the sera of mice vaccinated with PBS, DMT, BCG, or CMFO-DMT were determined by ELISA at week 10 after immunization. The results are expressed as the mean ± standard error of the mean (SEM) log10 endpoint titers per group (n = 6), as previously described (Wang et al., 2015).
2.8. CMFO-specific Th1 Cytokines Secreted by Splenocytes
Splenocytes (2.5 × 106) from each mouse were seeded in triplicate in a 24-well plate at 37 °C in an atmosphere of 5% CO2. The cells were re-stimulated with 10 μg/mL CMFO for 24 h for the detection of IL-2 and for 72 h for the detection of and IFN-γ and tumor necrosis factor-alpha (TNF-α). Culture supernatant was collected for analysis using double-sandwich ELISA kits (Cat#70EK2022/2, 70EK2802/2, 70EK2822/2, Multi Science Ltd.), as described previously (Wang et al., 2015). The results were expressed as the mean ± standard derivation (SD) (pg/mL) for each group (n = 6).
2.9. CMFO-specific Memory T Cells in the Spleen and Lung
Intracellular flow cytometry was performed as previously described (Liang et al., 2015, Ma et al., 2015). In brief, splenocytes from each mouse or T cells from the whole mouse lung were prepared, counted, and then seeded in triplicate at 5 × 106 cells/well in a 24-well plate, which were incubated with CMFO (10 μg/mL) and anti-CD28/CD49d (1 μg/mL, Cat#13028982, 11049282; eBioscience, Franklin Lakes, NJ, USA) for 20 h at 37 °C in an atmosphere of 5% CO2. RPMI1640 medium was used as negative control. Brefeldin A (3 μg/mL) and monensin solution (2 μM, Cat#00450651, 00450551; eBioscience) were added for a further 4 h. A cell stimulation cocktail (1 μg/mL, Cat#00-4970; eBioscience) was used to monitor cell responses. Cells were stained with the following monoclonal antibodies (mAbs): anti-CD4-PE-Cy7 (clone GK1.5, Cat#25004182; eBioscience), anti-CD8α-PE (clone53-6.7, Cat#12008182; eBioscience), anti-CD62L-FITC (clone MEL-14, Cat#561917; BD Pharmingen, San Jose, CA, USA), and anti-CD44-APC-Cy7 (clone IM7, Cat#565480; BD Pharmingen). After permeabilization using a Cytofix/Cytoperm kit (Cat#554714; BD Pharmingen), cells were stained with the following mAbs: anti-IFN-PerCP-Cy5.5 (clone XMG1.2, Cat#45731182; eBioscience), and anti-IL-2-allophycocyanin (clone JES6-5H4, Cat#17702182; eBioscience). One million cells were collected from each sample and examined by multicolor flow cytometry using a LSRII device (BD Biosciences San Jose, CA, USA). FlowJo software was used to determine the absolute number of IFN-γ or IL-2 positive T cell subsets, including CD4+, CD8+, TCM (central memory T cells, CD62LhiCD44hi), and TEM (effector memory T cells, CD62LloCD44hi) cells (Supplementary Fig. 1). A medium control was subtracted from the CMFO-stimulated response for each sample. The results are presented as mean ± SD per group (n = 6).
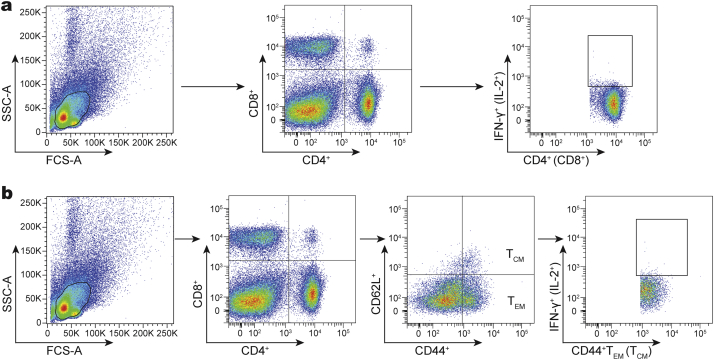
Supplementary Fig. 1.
Flow cytometry gating strategies. (a) Gating strategy for the identification of IFN-γ+ or IL-2+ CD4+ and CD8+ T cells per spleen or lung from different vaccinated mice. (b) Gating strategy for the identification of memory T cells per spleen or lung from different vaccinated mice.
2.10. Statistical Analyses
A single factor analysis of variance (one-way ANOVA test) was performed to compare the parameters among different experiment groups of mice. When applicable for further pairwise comparison, a post hoc Fisher's least significant difference (LSD) test or Student's test was performed. Statistical analysis was performed with SPSS 17.0 software, and the difference was considered significant when the p-value was < 0.05.
3. Results
3.1. Better Recognition of CMFO and Multistage Antigens by T Cells From LTBIs and ATBs Than by Those From HCs
The recombinant prokaryotic expression plasmid pET30b-CMFO was constructed successfully (Fig. 1A). Each multistage antigen (Rv0577, Rv2875, Rv2073c, and Rv3044) of M. tuberculosis was expressed from the recombinant E. coli strains and purified. The respective molecular weights were 36, 22, 26, and 44 kDa (Fig. 1B). The molecular mass of these recombinant proteins was slightly larger than each native form or theoretical prediction value, because of possible protein folding (Huard et al., 2003). The recombinant CMFO was expressed as the fusion form of four antigens with a molecular weight of 128 kDa and was purified from pET30b-CMFO-transformed E. coli strain and confirmed by 12% SDS-PAGE (Fig. 1B).
Fig. 1.
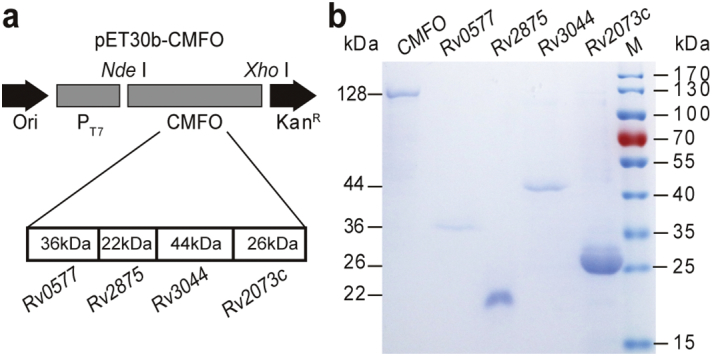
Expression and identification of CMFO and multistage antigens. (a) The structural diagram of the recombinant prokaryotic expression plasmid pET30b-CMFO. (b) The identification of purified CMFO and each multistage antigens Rv0577, Rv2875, Rv3044 and Rv2073c by 12% SDS-PAGE.
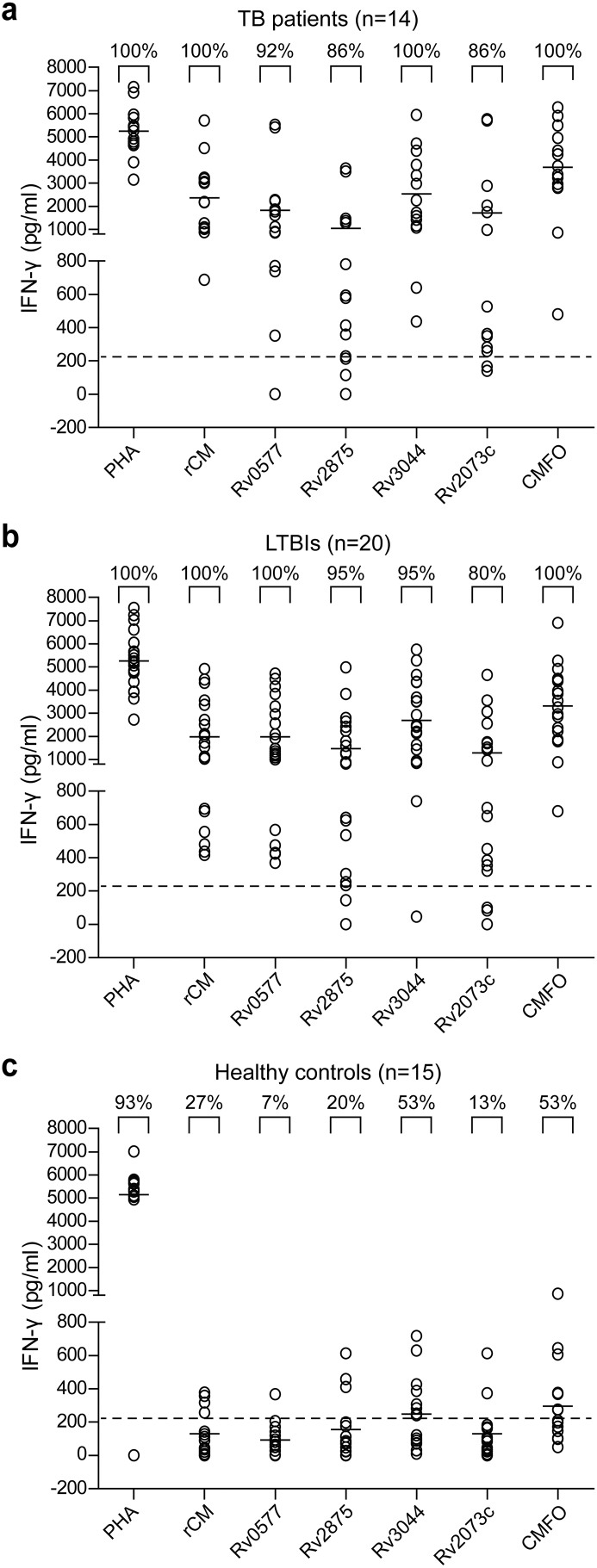
WBIA based on CMFO, single multistage antigens, PHA, and saline, was performed in 14 cases of ATB, 20 cases of LTBI, and 15 HCs. The levels of IFN-γ detected in positive PHA controls between the different populations had no statistical difference. As expected, higher levels of IFN-γ response to multistage antigens Rv0577, Rv2875, Rv3044, Rv2073c, and CMFO were observed in LTBIs or ATBs, compared to that in HCs (Fig. 2). The number of responders to single multistage antigens was low in HCs, ranging from 7% to 53%. In contrast, approximately 80–100% of ATP or LTBI patients responded well. In particular, CMFO had stronger antigenicity than the single multistage antigens, as evidenced by the levels of IFN-γ. All LTBIs and ATBs reacted against CMFO, and the frequency recognized by T cells in the HCs was only 53% (Fig. 2).
Fig. 2.
Comparison of the antigenicity of CMFO and multistage antigens. Whole blood samples were obtained from each donors of ATBs (n = 14), LTBIs (n = 20), and HCs (n = 15) and stimulated with 20 μL of either CMFO, Rv0577, Rv2875, Rv3044, and Rv2073c (each 10 μg), or saline for 18 to 24 h. ELISA was used to detect the concentration of IFN-γ in the supernatants and each spot represents the antigen-specific concentration of IFN-γ in a sample. The dotted line represents the cut-off value for a positive response to each multistage antigen and the median values are indicated by horizontal lines.
3.2. Better Protection Against Primary Infection is Conferred by CMFO-DMT
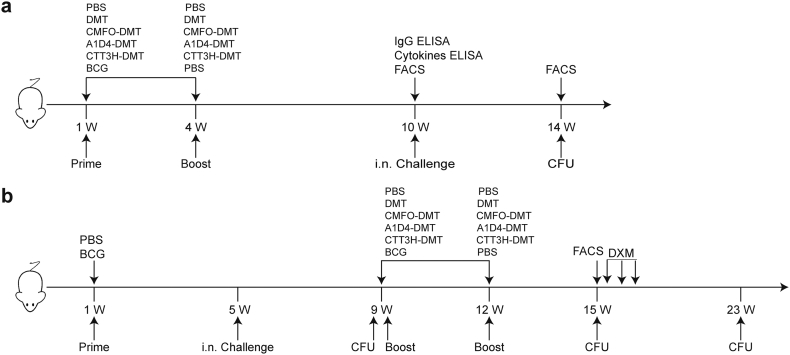
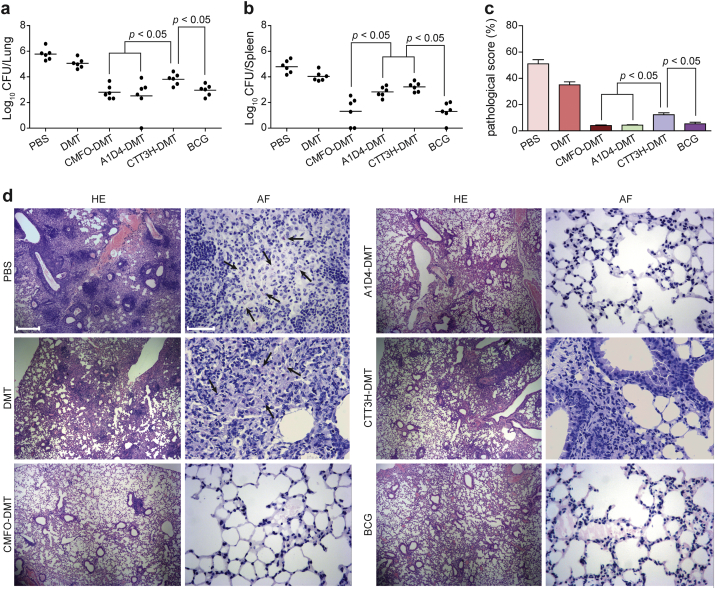
To compare the protective efficacy of the DMT adjuvant subunits of multistage CMFO, two-stage A1D4, and single-stage CTT3H, the vaccination schedules for primary infection, latent infection, and reactivation are detailed in Fig. 3. Vaccinated mice were challenged i.n. with 80 CFU of M. tuberculosis strain H37Rv at week 10 after immunization. Four weeks post-challenge, the bacterial load in the lung and spleen and the lung pathology in different groups were compared for the protection against primary infection. PBS control mice and positive control BCG-vaccinated mice had the highest and the lowest bacterial load of M. tuberculosis in the lung and spleen, respectively (p < 0.05, Fig. 4). Consistent with our previous observation, significant inhibition of M. tuberculosis growth in vivo was noted in mice treated with adjuvant DMT alone compared to that in the PBS group (Fig. 4). Although single-stage subunit CTT3H-DMT vaccination also conferred stronger protection than the DMT or PBS controls, a significantly higher bacterial load in the lung and spleen remained detectable in this group compared to that in the CMFO-DMT or A1D4-DMT groups (Fig. 4). Much lower bacterial load was found in the spleen from CMFO-DMT-vaccinated mice than in the A1D4-DMT group. Therefore, multistage subunit CMFO-DMT conferred the strongest protection against primary infection among the three subunit groups. More importantly, there was no statistical difference in the bacterial loads in both the lung and spleen between the mice vaccinated with CMFO-DMT and BCG vaccine.
Fig. 3.
The detail vaccination schedules. (a) Mouse model of primary infection. (b) Mouse models of latent infection and reactivation.
Fig. 4.
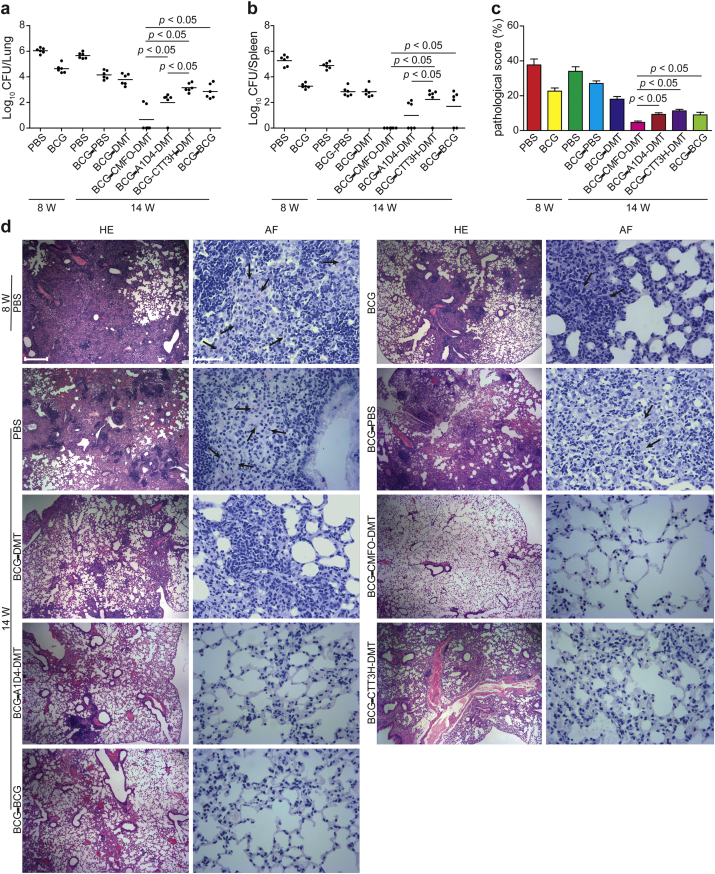
Protective efficacy of CMFO-DMT vaccinated mice against primary infection. C57BL/6 mice were vaccinated with CMFO-DMT, A1D4-DMT, CTT3H-DMT, BCG, DMT, or PBS. 9 weeks later, vaccinated C57BL/6 mice were challenged i.n. with approximately 80 CFU virulent M. tuberculosis strain H37Rv. Four weeks post-challenge, spleens and lungs were obtained and the CFU numbers per organ were enumerated. The bacterial load in the lung (a) and spleen (b) of the different groups were shown as mean ± SEM log10 CFU/organ (n = 6). Lung pathological scores (c) and representative lung pathological changes from the different groups (HE, scale bar = 400 μm) are also shown (d). AF staining (scale bar = 50 μm) of lung tissue section also supported the hierarchy of lung bacterial load in the different groups (d). Arrowheads indicate AF positive bacteria.
In accordance with the lung bacterial load results, hematoxylin-eosin and acid-fast stained lung sections also showed apparent hierarchy (Fig. 4). PBS or DMT control mice had the most severe lung pathological scores, and AFB was present throughout the tissue section. Compared with that in the control groups, less obvious interstitial pneumonia was observed in the lungs from mice vaccinated with CMFO-DMT, A1D4-DMT, or BCG, and almost no acid-fast bacilli were found in lung tissues in these groups (Fig. 4). Taken together, the lung pathological scores demonstrated that CMFO-DMT, A1D4-DMT, and BCG vaccine could achieve equivalent protection in the lung against primary infection.
3.3. CMFO-DMT Confers Stronger Ability to Protect Against Latent Infection
To compare the protective efficacy of DMT adjuvant subunit vaccines against latent infection and reactivation, BCG-vaccinated mice were challenged i.n. with 80 CFU of M. tuberculosis 4 weeks after immunization. Eight weeks later and after BCG vaccination, mice were successfully infected with M. tuberculosis. At least 103 CFU of bacteria colonized both the lung and spleen, confirming the establishment of the latent infection model. Fourteen weeks later, PBS control mice still had the highest bacterial load in the lung and spleen (p < 0.05, Fig. 5). Of all BCG-primed mice, the DMT-alone and PBS-boosted controls had the highest bacterial loads in both organs (p < 0.05, Fig. 5). Much lower bacterial load was persistently evident in mice that had been repeatedly vaccinated using BCG than in BCG-primed and PBS-boosted controls (p < 0.05, Fig. 5). Interestingly, a clear difference in protection was conferred by the three subunit vaccines. CTT3H-DMT provided protection comparable to that in the BCG-boosted group, with no statistical difference in bacterial loads between both groups. A lower number of M. tuberculosis in both lung and spleen was obtained from A1D4-DMT-vaccinated mice than from those vaccinated with CTT3H-DMT (p < 0.05, Fig. 5). Notably, the strongest protection was observed in the CMFO-DMT group, in which few M. tuberculosis colonized persistently in both organs. In particular, 4/6 and 6/6 of CMFO-DMT-vaccinated mice did not have M. tuberculosis detectable in the lung and spleen, respectively (Fig. 5).
Fig. 5.
Protective efficacy of CMFO vaccinated mice against latent infection. Mice were first immunized s.c. with BCG or PBS, and then challenged i.n. with about 80 CFU of M. tuberculosis strain H37Rv at the fifth week after immunization. Eight weeks later, the bacterial load of both lung and spleen, and lung pathology in PBS controls and BCG vaccinated mice were compared to confirm the establishment of latent infection. The infected mice were further immunized with CMFO-DMT, A1D4-DMT, CTT3H-DMT, BCG or PBS. Fourteen weeks later, lungs and spleens of mice were harvested, and the CFU numbers per organ were counted. The results are shown as the mean of log10 CFU ± SEM per lung (a) and spleen (b) for each group (n = 6). (c) The lung pathological scores of different vaccinated mice. (d) The representative lung pathology of different vaccinated C57BL/6 mice after infection. The left lung lobes from different vaccinated mice were fixed and embedded for HE staining (scar bar, 400 μm) and acid-fast (AF) staining (scar bar, 50 μm). Arrows indicate AF-positive bacteria.
Lung pathology was consistent with the results of bacterial enumeration. PBS control mice had the most severe lung pathology and a few acid-fast bacilli were persistently found in lung sections throughout the experimental period. Mice primed only with BCG showed moderate alterations in lung pathology in all groups (Figs. 5c and d). Little pathological change was detected in the lungs of mice who received the different subunit vaccines and the BCG-boost group, and few acid-fast bacilli were visible in the lung sections. In particular, no apparent change in lung pathology was found in the CMFO-DMT group, which appeared to be normal (Figs. 5c and d).
3.4. CMFO-DMT Displays Stronger Ability to Thwart Reactivation
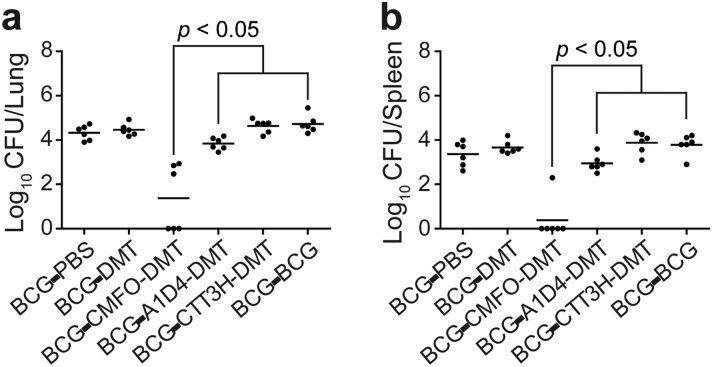
To confirm the effects of the different vaccines on the reactivation, infected mice were further treated with immunosuppressive glucocorticoids, and residual M. tuberculosis in organs was compared. In BCG-primed mice boosted using the subunit vaccines, the lowest number of M. tuberculosis in the lung and spleen was observed in the CMFO/DMT group (Fig. 6). M. tuberculosis was completely eradicated in the lung and spleen from 3/6 and 5/6 CMFO/DMT-vaccinated mice, respectively. In contrast, residual M. tuberculosis was detected in all the mice in the other groups, with no statistical difference in bacterial load among these groups (Fig. 6).
Fig. 6.
Reactivation of M. tb in BCG prime-subunit boosted mice. Mice were first immunized s.c. with BCG or PBS, and then challenged i.n. with about 80 CFU of M. tb strain H37Rv at the fifth week after immunization. At the ninth week, the infected mice were further boosted with CMFO-DMT, A1D4-DMT, CTT3H-DMT, BCG or PBS, respectively. At the fifteenth week, mice were injected s.c. with DXM. Eight weeks after treatment, spleens and lungs were obtained and the CFU numbers per organ were enumerated. The bacterial load in the lung (a) and spleen (b) of the different groups were shown as mean ± SEM log10 CFU/organ (n = 6).
3.5. CMFO-DMT Elicits Th1-type Biased Responses
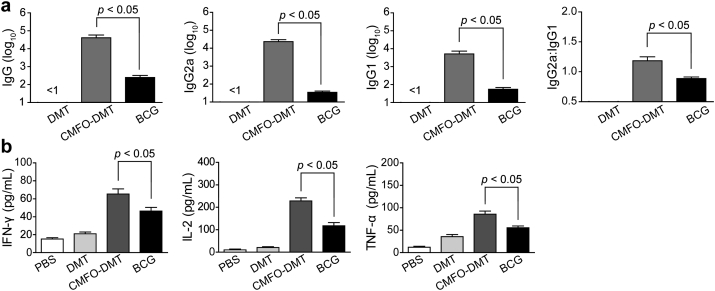
To elucidate the protective mechanism of CMFO-DMT, the immunogenicity of CMFO in C57BL/6 mice with DMT adjuvant was evaluated and titers of CMFO-specific antibodies, including IgG, IgG1, and IgG2a, were determined by ELISA at week 10 after immunization (Fig. 7a). As expected, these antibodies were not detected in the control mice. Higher levels of CMFO-specific IgG, IgG1, and IgG2a antibodies were measured in mice vaccinated with CMFO-DMT than in the BCG group (p < 0.05). In addition, the ratio of IgG2a/IgG1 in the CMFO-DMT group was much higher than that in the BCG group (Fig. 7a).
Fig. 7.
Th1-biased immune responses to CMFO in immunized mice (n = 6). 9 weeks after immunization, sera were collected from each C57BL/6 mouse vaccinated with CMFO-DMT, BCG, DMT, or PBS. (a) The IgG, IgG1, and IgG2a antibodies against CMFO in the immunized mice were detected by ELISA. The results are shown as the mean (± SEM) log10 endpoint titer and the ratio of IgG2a:IgG1 in the differently vaccinated mice. (b) CMFO-specific IFN-γ, IL-2 or TNF-α levels secreted by the splenocytes of different vaccinated mice. The IFN-γ concentrations in the suspension were detected with a commercial ELISA kit. The results are shown as the mean ± SD (pg/mL).
The CMFO-specific Th1-type cytokines from splenocytes, including IFN-γ, IL-2, and TNF-α, were also compared (Fig. 7b). Again, the PBS control mice had the lowest levels of CMFO-specific cytokines among all groups (p < 0.05). BCG-vaccinated mice displayed higher levels of these cytokines than the PBS control mice, whereas higher levels of IFN-γ, IL-2, and TNF-α response to CMFO were induced in the CMFO-DMT group than in the BCG-vaccinated mice (p < 0.001, Fig. 7b).
3.6. CMFO-DMT Induces Higher Levels of Effector and Central Memory T Cells in the Spleen Than BCG
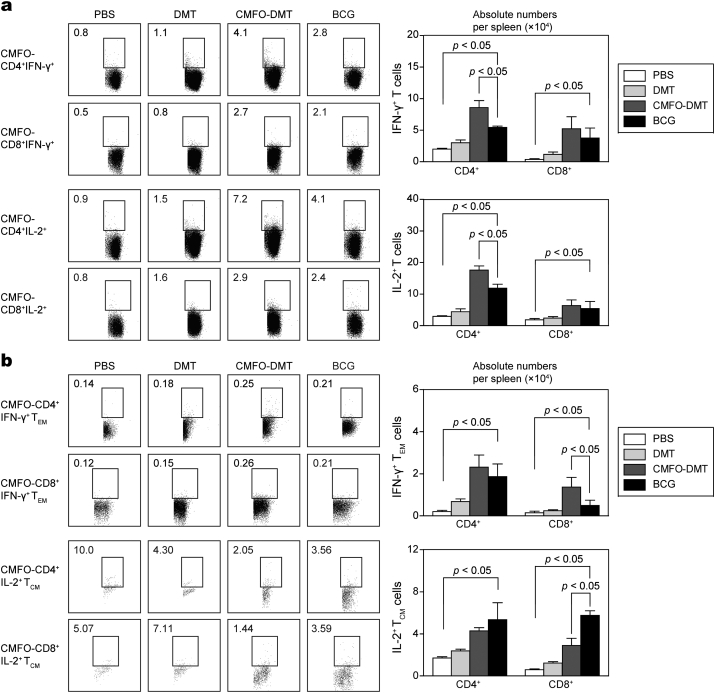
The vaccine-induced early and persistent protection against primary infection is respectively associated with the number of IFN-γ+ TEM cells and IL-2+ TCM cells in the spleen (Ma et al., 2016). At week 10 after immunization, CMFO-specific IFN-γ- or IL-2 secreting T cells (Fig. 8a), IL-2-expressing TCM (CD62LhiCD44hi) cells, and IFN-γ-positive TEM (CD62LloCD44hi) cells (Fig. 8b) in splenocytes between different vaccinated mice were also determined. IL-2+ TCM cells predominated in BCG-vaccinated and CMFO-DMT-vaccinated mice (p < 0.05, Fig. 8b). In particular, more IL-2+ CD8+ TCM cells were detected in the BCG group than in the CMFO-DMT-vaccinated mice (p < 0.05, Fig. 8b). In contrast, more IFN-γ+ CD4+ T cells, IL-2+ CD4+ T cells, and IFN-γ+ CD8+ TEM cells were present in the CMFO-DMT group than in the BCG group (p < 0.05). The levels of other T cells, including IFN-γ+ or IL-2+ CD8+ T cells, IFN-γ+ CD4+ TEM cells, and IL-2+ CD4+ TCM in the CMFO-DMT group were comparable to those in the BCG group. The PBS and DMT groups had the lowest numbers of these T cells (p < 0.05, Fig. 8).
Fig. 8.
CMFO-specific effector and central memory T cells in the spleen of immunized mice (n = 6). 9 weeks after immunization, splenocytes were prepared from each C57BL/6 mouse vaccinated with CMFO-DMT, BCG, DMT, or PBS, respectively. The subsets of CMFO-specific effector and central memory T cells were identified by intracellular cytokines staining (ICS) and multicolor flow cytometer. (a) The absolute numbers of IFN-γ+ or IL-2+ CD4+ and CD8+ T cells, or (b) TCM cells secreting IL-2 and TEM cells secreting IFN-γ are showed as the mean ± SD.
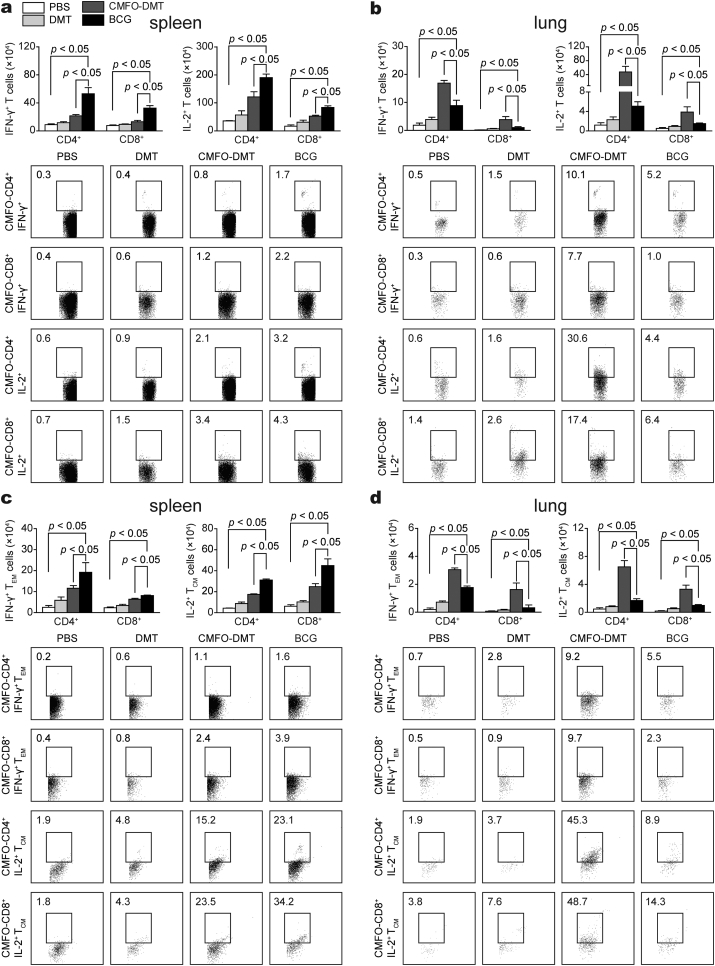
3.7. CMFO-DMT Induces Higher Levels of Effector and Central Memory T Cells in the Lung Against Primary Infection
To explore vaccine-induced protective mechanisms against latent infection, vaccinated mice were challenged with M. tuberculosis 9 weeks after immunization, and T cells were detected in the spleen and lung by fluorescence-activated cell sorting (FACS) 4 weeks later. Although IL-2+ T cells, IL-2+ TCM cells, and especially CD4+ T cells, were dominant in the spleen of both groups, many more IFN-γ+ or IL-2+ T cells, IFN-γ+ TEM, and IL-2+ TCM cells were present in the BCG group than in the CMFO-DMT-vaccinated mice (Fig. 9). In contrast, CMFO-DMT elicited higher levels of all of these T cells in the lung than BCG, although the number of these T cells in the lung was much lower than that in the spleen of each group. In addition, IFN-γ+ CD4+ T cells and IFN-γ+ CD4+ TEM were miainly produced in the lungs of mice in the BCG group, while IL-2+ CD4+ T cells and IL-2+ CD4+ TCM cells were elicited more efficaciously in the lungs of mice in the CMFO-DMT group.
Fig. 9.
CMFO-specific effector and central memory T cells in the spleen and lung of different vaccinated mice against primary infection (n = 6). 9 weeks after immunization, mice were challenged i.n. with about 80 CFU of M. tb strain H37Rv. Four weeks later, splenocytes and lung cells were prepared from each C57BL/6 mouse vaccinated with CMFO-DMT, BCG, DMT, or PBS, respectively. The subsets of CMFO-specific effector and central memory T cells were identified by intracellular cytokines staining (ICS) and multicolor flow cytometer. (a, b) The absolute numbers of IFN-γ+ or IL-2+ CD4+ and CD8+ T cells, or (c, d) TCM cells secreting IL-2 and TEM cells secreting IFN-γ are showed as the mean ± SD.
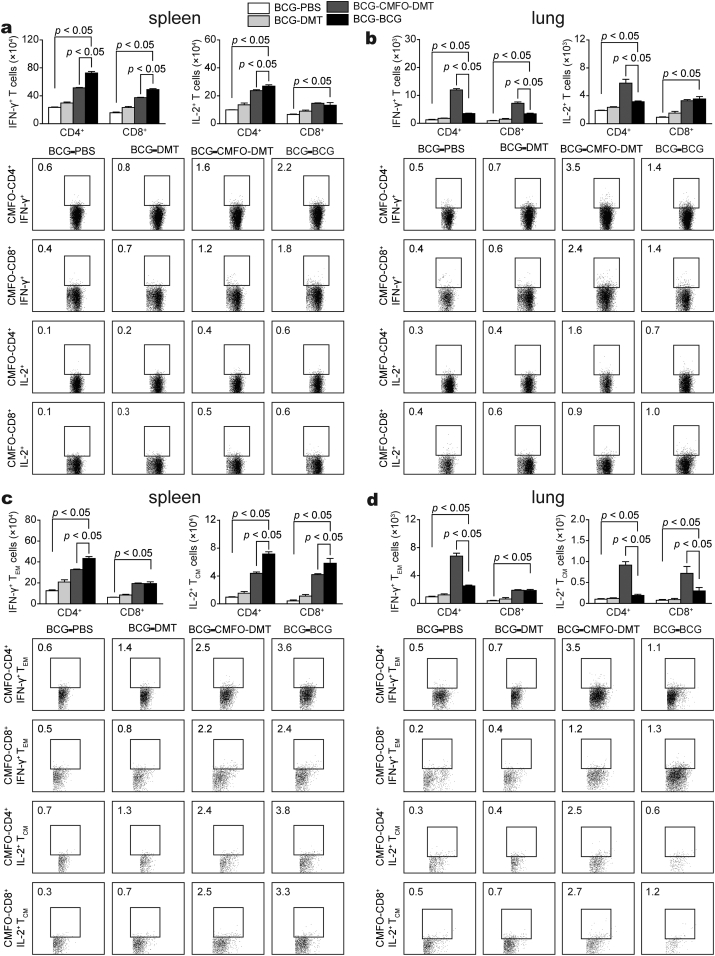
3.8. CMFO-DMT Enhances Protection Against Latent Infection and Reactivation in BCG-primed Mice by Driving Effector and Central Memory T Cells Homing to the Lung
To confirm the vaccine-induced protective mechanisms against latent infection, BCG-primed mice were challenged with M. tuberculosis 5 weeks after immunization, and then boosted with CMFO-DMT. T cells were detected in the spleen and lung by FACS 14 weeks later. Many more IFN-γ+ T cells, IL-2+ CD4+ T cells, IFN-γ+ CD4+ TEM, and IL-2+ TCM cells were induced in the spleen of the BCG repeat-vaccinated group than that in the CMFO-DMT boost group (Fig. 10). Although there was no statistical difference in terms of the numbers of IL-2+ CD8+ T cells and IFN-γ+ CD8+ TEM cells between the spleen and lung of both groups, more CD4+ T cells than CD8+ T cells were induced in the spleen of both groups. In the lung, CMFO-DMT induced higher numbers of IFN-γ+ T cells, IL-2+ CD4+ T cells, IFN-γ+ CD4+ TEM, and IL-2+ TCM cells than those in the BCG repeat-vaccinated mice against latent infection, although the number of these T cells in the lung was much lower than the cell number in the spleen of both groups.
Fig. 10.
CMFO-specific effector and central memory T cells in BCG prime and different boosters vaccinated mice against latent infection (n = 6). Mice were first immunized s.c. with BCG, and then challenged i.n. with about 80 CFU of M. tb strain H37Rv at the fifth week after immunization. Four weeks later, mice were further boosted with CMFO-DMT, BCG, DMT, or PBS. Six weeks after boost vaccination, lungs and spleens of mice were harvested. The subsets of CMFO-specific effector and central memory T cells were identified by intracellular cytokines staining (ICS) and multicolor flow cytometer. (a, b) The absolute numbers of IFN-γ+ or IL-2+ CD4+ and CD8+ T cells, or (c, d) TCM cells secreting IL-2 and TEM cells secreting IFN-γ are showed as the mean ± SD.
4. Discussion
Although BCG is used globally, the lack of effective vaccines to prevent adults against TB is a significant obstacle toward the WHO goal of global eradication of TB by 2050. The development of an efficient vaccine against adult TB is the key strategy for successful control of TB and remains a global priority. However, a rational strategy to develop an efficient vaccine for adult TB remains elusive. In the current study, the protective efficacy of DMT adjuvant subunits of multistage CMFO, two-stage A1D4, and single-stage CTT3H was compared in mice models of primary infection, latent infection, and reactivation. Our results demonstrate that only the multistage CMFO-DMT vaccine produces effective protection in BCG-primed mice to eliminate latent infection and prevent reactivation. In addition, CMFO-DMT provides protection against primary infection with M. tuberculosis that is comparable to the effect of BCG vaccine, while each multistage antigen from CMFO confers a lower level of protection against aerosol infection with M. tuberculosis when compared with that in BCG-vaccinated mice (Bertholet et al., 2008).
M. tuberculosis expresses different antigens when adapting to conditions at different stages during infection, which has crucial role in escaping the pre-established immunity in the infected host (Ernst, 2012). The genome of M. tuberculosis contains 4018 open reading frames and about 450 antigens are secreted during the early acute infection (de Souza et al., 2008). Forty-eight genes controlled by DosR in the genome of M. tuberculosis were demonstrated to be expressed during hypoxia in vitro, by mimicking the microenvironment in the host during latent infection (Park et al., 2003). At least 230 genes were found to be characteristically up-regulated during hypoxia in vitro (Rustad et al., 2008). In particular, genes of M. tuberculosis persistently expressed in infected mice were also identified (Sassetti and Rubin, 2003).
We selected vaccine targets from this potential antigenic reservoir, based on the high recognition frequencies of T cells from LTBIs and ATBs. CTT3H was constructed based on low molecular-weight proteins like CFP-10 and TB10.4, which are the main species secreted by M. tuberculosis and presented in the early culture supernatant in vitro (Teng et al., 2015). Currently, one-stage vaccine candidates, such as the fusion subunits Ag85B-ESAT-6 and Ag85B-TB10.4, are being evaluated in clinical trials (Kaufmann, 2014, Reither et al., 2014). Among our three subunit vaccines, CTT3H-DMT conferred the worst protection against primary infection. Our results indicate that the strategy of subunit vaccine that is based solely on the early secretory antigens of M. tuberculosis might be not very effective for the prevention of primary infection, as evidenced by the co-existence of actively growing and latent M. tuberculosis after infection. Although we previously reported that A1D4 in the adjuvant of MTO provided inferior protection against primary infection with BCG (Wang et al., 2015), DMT adjuvanted A1D4 was as efficacious in the lung as BCG and CMFO-DMT, and the results were consistent with previously reported subunit vaccines of two-stage H56 and ID93, and multistage WH121. Because the protection against primary infection induced by repeat BCG vaccination was inferior to that induced by BCG vaccination alone (Cruz et al., 2010), two- or multistage subunit vaccines might be a suitable replacement of BCG to prevent against primary infection in adults.
BCG has been integrated into the Expanded Immunization Program of WHO since 1974 and neonatal vaccination with BCG is still used to prevent TB in countries with a high burden of TB (Marais et al., 2016). Correspondingly, adolescents and adults with LTBI always occur epidemiologically following inoculation with BCG after birth. To mimic the occurrence of LTBI, mice were vaccinated with BCG and then infected with M. tuberculosis to create the mouse model of LTBI (Nuermberger, 2008, Zhang et al., 2009, Ma et al., 2016), characterized by at least 1000 CFU of M. tuberculosis and the pre-establishment of cell-mediated immunity in the lung. In this study, BCG as a booster resulted in a lower bacterial load in both lung and spleen than in the PBS-control mouse LTBI model. It is surprising that repeat BCG vaccination benefits the control of LTBI, while the regimen cannot block the reactivation of dormant M. tuberculosis in mice. Although WHO does not recommend adult vaccination with BCG, some countries in Eastern European still practice the strategy of BCG repeat vaccination in adults. Moreover, the commercial WBIA technologies, such as T-SPOT TB, may allow the screening and diagnosis of LTBI. Therefore, clinical evaluation of the preventive effect elicited by BCG repeat vaccination is needed to prevent latent infection.
Importantly, mice boosted with A1D4-DMT also displayed stronger efficacy against latent infection than those with CTT3H-DMT, while the strongest protection was conferred by the CMFO-DMT subunit vaccine. More importantly, only CMFO-DMT could control the reactivation of the latent infection in mice, which was evaluated by enumeration of persisting M. tuberculosis in the lung and spleen after immunosuppressive treatment. Although vaccine-induced mechanisms to eliminate LTBI are still not fully understood, the pathogenesis of M. tuberculosis largely depends on the ability to delay the time of the antigen-specific CD4+ Th1 cells homing to the infected lung (Wolf et al., 2008, Griffiths et al., 2016), which benefits the replication of M. tuberculosis in the lung during early 3 weeks after primary infection and promotes the establishment of a latent infection (Ernst, 2012). Moreover, reactivation of LTBI that progresses to active TB is more likely in persons co-infected with human immunodeficiency virus. Therefore, CD4+ Th1 type responses can be thought of as key in the control of TB (Cooper, 2009) and also play important roles in the containment of the LTBI. Rv2073c is absent from the genome of BCG (Behr et al., 1999). Rv2875 (Charlet et al., 2005) and Rv0577 (Huard et al., 2003) were presently expressed at a lower level in BCG than in M. tuberculosis. Although BCG also elicited CMFO-specific CD4+ Th1 type immune responses and induced CD8+ T cells in the spleen of vaccinated mice, more IFN-γ+ or IL-2+ CD4+ T cells and IFN-γ+ CD8+ TEM cells against CMFO in the spleen were induced by CMFO-DMT compared to that induced by BCG, which were associated with the protection induced by CMFO-DMT against primary infection. The stronger protection conferred by CMFO-DMT than that conferred by A1D4-DMT and CTT3H-DMT might be the result of more comprehensive immunity elicited by each multistage antigen of CMFO-DMT against different status of M. tuberculosis in vivo. In particular, systemic antigen-specific IFN-γ + CD4+ TEM and IL-2+ TCM cells induced by CMFO-DMT could more easily home to the infected lung, which is also closely related with stronger protection against latent infection and more significant ability of CMFO-DMT as a booster to thwart the reactivation of LTBI than BCG repeat vaccination. Therefore, promoting the homing of T cells to the lung after vaccination might be an important vaccine development strategy. As previously reported in the treatment of TB with ID93 (Coler et al., 2013), infected animals that received a boost with subunit vaccine or BCG did not show any adverse effects during the entire experimental period. No Koch-like pathological changes were evident in the lungs of mice. Still, the safety of CMFO-DMT needs to be comprehensively evaluated.
In conclusion, multistage subunit vaccine has the potential to block two pathways active in adult TB through prophylactic and therapeutic effects. CMFO-DMT is a promising vaccine candidate for adult TB disease. It should be evaluated further in preclinical and clinical trials.
The following are the supplementary data related to this article.
Supplementary table 1
Supplementary table 2
Funding Sources
This work was supported by the National Mega-projects of Science Research for the 12th Five-year Plan of China grant no. 2012ZX10003008-005, the National High Technology Research and Development of China grant no. 2012AA02A401, Wuhan Bureau of Science and Technology grant no. 2015060101010029, and the Fundamental Research Funds for the Central Universities grants HUST no. 2015MS098 and no. 2015ZZGH012. The funders had no role in study design, data collection, data analysis, interpretation, and writing of the report.
Conflicts of Interest
The authors declare no conflicts of interests.
Patent Application
Xionglin Fan is the inventor of the reported technologies, which was applied for the invention patents of China (no. CN201410836669.4 and no. CN201510523087.5).
Author Contributions
XF designed the experiments, interpreted the data and wrote the manuscript. JM and XT performed the experiments with animal models. XW prepared the expression strains of multistage antigens and CMFO. MT, ZZ, YW, and LL performed the human experiments.
Acknowledgements
We thank Zhihui Liang (Department of Immunology, Huazhong University of Science Technology) for technical assistance with flow cytometry and analysis.
References
- Aagaard C., Hoang T., Dietrich J., Cardona P.J., Izzo A., Dolganov G., Schoolnik G.K., Cassidy J.P., Billeskov R., Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 2011;17(2):189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- Andersen P., Doherty T.M. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3(8):656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- Behr M.A., Wilson M.A., Gill W.P., Salamon H., Schoolnik G.K., Rane S., Small P.M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284(5419):1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- Bertholet S., Ireton G.C., Kahn M., Guderian J., Mohamath R., Stride N., Laughlin E.M., Baldwin S.L., Vedvick T.S., Coler R.N., Reed S.G. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 2008;181(11):7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet S., Ireton G.C., Ordway D.J., Windish H.P., Pine S.O., Kahn M., Phan T., Orme I.M., Vedvick T.S., Baldwin S.L., Coler R.N., Reed S.G. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci. Transl. Med. 2010;2(53):53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun E.H., Kim W.S., Kim J.S., Jung I.D., Park Y.M., Kim H.J., Cho S.N., Shin S.J. Mycobacterium tuberculosis Rv0577, a novel TLR2 agonist, induces maturation of dendritic cells and drives Th1 immune response. FASEB J. 2012;26(6):2695–2711. doi: 10.1096/fj.11-199588. [DOI] [PubMed] [Google Scholar]
- Charlet D., Mostowy S., Alexander D., Sit L., Wiker H.G., Behr M.A. Reduced expression of antigenic proteins MPB70 and MPB83 in Mycobacterium bovis BCG strains due to a start codon mutation in sigK. Mol. Microbiol. 2005;56(5):1302–1313. doi: 10.1111/j.1365-2958.2005.04618.x. [DOI] [PubMed] [Google Scholar]
- Coler R.N., Bertholet S., Pine S.O., Orr M.T., Reese V., Windish H.P., Davis C., Kahn M., Baldwin S.L., Reed S.G. Therapeutic immunization against Mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis. 2013;207(8):1242–1252. doi: 10.1093/infdis/jis425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Fraga A.G., Fountain J.J., Rangel-Moreno J., Torrado E., Saraiva M., Pereira D.R., Randall T.D., Pedrosa J., Cooper A.M., Castro A.G. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 2010;207(8):1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J.D. The immunological life cycle of tuberculosis. Nat. Rev. Immunol. 2012;12(8):581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- Fu R., Wang C., Shi C., Lu M., Fang Z., Lu J., Wang F., Fan X. An improved whole-blood gamma interferon assay based on the CFP21-MPT64 fusion protein. Clin. Vaccine Immunol. 2009;16(5):686–691. doi: 10.1128/CVI.00486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths K.L., Ahmed M., Das S., Gopal R., Horne W., Connell T.D., Moynihan K.D., Kolls J.K., Irvine D.J., Artyomov M.N., Rangel-Moreno J., Khader S.A. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat. Commun. 2016;7:13894. doi: 10.1038/ncomms13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard R.C., Chitale S., Leung M., Lazzarini L.C., Zhu H., Shashkina E., Laal S., Conde M.B., Kritski A.L., Belisle J.T., Kreiswirth B.N., Lapa e Silva J.R., Ho J.L. The Mycobacterium tuberculosis complex-restricted gene cfp32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect. Immun. 2003;71(12):6871–6883. doi: 10.1128/IAI.71.12.6871-6883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S.H. Tuberculosis vaccine development at a divide. Curr. Opin. Pulm. Med. 2014;20(3):294–300. doi: 10.1097/MCP.0000000000000041. [DOI] [PubMed] [Google Scholar]
- Liang J., Teng X., Yuan X., Zhang Y., Shi C., Yue T., Zhou L., Li J., Fan X. Enhanced and durable protective immune responses induced by a cocktail of recombinant BCG strains expressing antigens of multistage of Mycobacterium tuberculosis. Mol. Immunol. 2015;66:392–401. doi: 10.1016/j.molimm.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Lin P.L., Dietrich J., Tan E., Abalos R.M., Burgos J., Bigbee C., Bigbee M., Milk L., Gideon H.P., Rodgers M., Cochran C., Guinn K.M., Sherman D.R., Klein E., Janssen C., Flynn J.L., Andersen P. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J. Clin. Invest. 2012;122(1):303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D.B., Tascon R.E., Bonato, Lima V.M., Faccioli L.H., Stavropoulos E., Colston M.J., Hewinson R.G., Moelling K., Silva C.L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400(6741):269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- Luabeya A.K., Kagina B.M., Tameris M.D., Geldenhuys H., Hoff S.T., Shi Z., Kromann I., Hatherill M., Mahomed H., Hanekom W.A., Andersen P., Scriba T.J., H56-032 Trial Study Group, Schoeman E., Krohn C., Day C.L., Africa H., Makhethe L., Smit E., Brown Y., Suliman S., Hughes E.J., Bang P., Snowden M.A., McClain B., Hussey G.D. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine. 2015;33(33):4130–4140. doi: 10.1016/j.vaccine.2015.06.051. [DOI] [PubMed] [Google Scholar]
- Ma J., Lu J., Huang H., Teng X., Tian M., Yu Q., Yuan X., Jing Y., Shi C., Li J., Fan X. Inhalation of recombinant adenovirus expressing granulysin protects mice infected with Mycobacterium tuberculosis. Gene Ther. 2015;22(12):968–976. doi: 10.1038/gt.2015.73. [DOI] [PubMed] [Google Scholar]
- Ma J., Tian M., Fan X., Yu Q., Jing Y., Wang W., Li L., Zhou Z. Mycobacterium tuberculosis multistage antigens confer comprehensive protection against pre- and post-exposure infections by driving Th1-type T cell immunity. Oncotarget. 2016;7(39):63804–63815. doi: 10.18632/oncotarget.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E., Rodrigues L.C., Smith P.G., Lipman M., Whiting P.F., Sterne J.A. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 2014;58(4):470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- Marais B.J., Seddon J.A., Detjen A.K., van der Werf M.J., Grzemska M., Hesseling A.C., Curtis N., Graham S.M. Interrupted BCG vaccination is a major threat to global child health. Lancet Respir. Med. 2016;4(4):251–253. doi: 10.1016/S2213-2600(16)00099-0. [DOI] [PubMed] [Google Scholar]
- Nuermberger E. Using animal models to develop new treatments for tuberculosis. Semin. Respir. Crit. Care. Med. 2008;29(5):542–551. doi: 10.1055/s-0028-1085705. [DOI] [PubMed] [Google Scholar]
- Park H.D., Guinn K.M., Harrell M.I., Liao R., Voskuil M.I., Tompa M., Schoolnik G.K., Sherman D.R. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003;48(3):833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.G., Coler R.N., Dalemans W., Tan E.V., DeLa Cruz E.C., Basaraba R.J., Orme I.M., Skeiky Y.A., Alderson M.R., Cowgill K.D., Prieels J.P., Abalos R.M., Dubois M.C., Cohen J., Mettens P., Lobet Y. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc. Natl. Acad. Sci. U. S. A. 2009;106(7):2301–2306. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reither K., Katsoulis L., Beattie T., Gardiner N., Lenz N., Said K., Mfinanga E., Pohl C., Fielding K.L., Jeffery H., Kagina B.M., Hughes E.J., Scriba T.J., Hanekom W.A., Hoff S.T., Bang P., Kromann I., Daubenberger C., Andersen P., Churchyard G.J. Safety and immunogenicity of H1/IC31®, an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PLoS One. 2014;9(12):e114602. doi: 10.1371/journal.pone.0114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad T.R., Harrell M.I., Liao R., Sherman D.R. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One. 2008;3(1):e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C.M., Rubin E.J. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. U. S. A. 2003;100(22):12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Chen L., Chen Z., Zhang Y., Zhou Z., Lu J., Fu R., Wang C., Fang Z., Fan X. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine. 2010;28(32):5237–5244. doi: 10.1016/j.vaccine.2010.05.063. [DOI] [PubMed] [Google Scholar]
- de Souza G.A., Målen H., Søfteland T., Saelensminde G., Prasad S., Jonassen I., Wiker H.G. High accuracy mass spectrometry analysis as a tool to verify and improve gene annotation using Mycobacterium tuberculosis as an example. BMC Genomics. 2008;9:316. doi: 10.1186/1471-2164-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K., Zhang J., Teng X., Liang J., Wang X., Yuan X., Tian M., Fan X. High level of IFN-gamma released from whole blood of human tuberculosis infections following stimulation with Rv2073c of Mycobacterium tuberculosis. J. Microbiol. Methods. 2015;114:57–61. doi: 10.1016/j.mimet.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Teng X., Tian M., Li J., Tan S., Yuan X., Yu Q., Jing Y., Zhang Z., Yue T., Zhou L., Fan X. Immunogenicity and protective efficacy of DMT liposome-adjuvanted tuberculosis subunit CTT3H vaccine. Hum. Vaccin. Immunother. 2015;11(6):1456–1464. doi: 10.1080/21645515.2015.1037057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver S., Warren R.M., Beyers N., Richardson M., van der Spuy G.D., Borgdorff M.W., Enarson D.A., Behr M.A., van Helden P.D. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am. J. Respir. Crit. Care Med. 2005;171(12):1430–1435. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- Wagner D., Sangari F.J., Parker A., Bermudez L.E. fecB, a gene potentially involved in iron transport in Mycobacterium avium, is not induced within macrophages. 2005. fecB, a gene potentially involved in iron transport in Mycobacterium avium, is not induced within macrophages. FEMS. Microbiol. Lett. 2005;247(2):185–191. doi: 10.1016/j.femsle.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang J., Liang J., Zhang Y., Teng X., Yuan X., Fan X. Protection against Mycobacterium tuberculosis infection offered by a new multistage subunit vaccine correlates with increased number of IFN-gamma+ IL-2+ CD4+ and IFN-gamma+ CD8+ T cells. PLoS One. 2015;10(3):e0122560. doi: 10.1371/journal.pone.0122560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2015. Guidelines on the Management of Latent Tuberculosis Infection. (Geneva: Switzerland) [PubMed] [Google Scholar]
- WHO . 2016. Global Tuberculosis Report. (Geneva: Switzerland) [Google Scholar]
- Windish H.P., Duthie M.S., Misquith A., Ireton G., Lucas E., Laurance J.D., Bailor R.H., Coler R.N., Reed S.G. Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate. Vaccine. 2011;29(44):7842–7848. doi: 10.1016/j.vaccine.2011.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A.J., Desvignes L., Linas B., Banaiee N., Tamura T., Takatsu K., Ernst J.D. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008;205(1):105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhang M., Rosenthal I.M., Grosset J.H., Nuermberger E.L. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 2009;180(11):1151–1157. doi: 10.1164/rccm.200905-0795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1
Supplementary table 2