Abstract
Severe influenza diseases with high mortality have been frequently reported, especially in those patients infected with avian influenza A (H5N1, H7N9 or H10N8) or during a pandemic. Respiratory distress, which is attributed to alveolar damage associated with immunopathological lesions, is the most common cause of death. There is a wealth of information on pathogenesis or treatment options. In this study, we showed that high levels of C-reactive protein (CRP) were induced and correlated with complement activation in patients infected with severe influenza A (H5N1, H7N9 or H10N8), and higher levels were induced in fatal patients than in survivors. CRP treatment enhanced the phagocytosis of monocytes THP-1 to H5N1 virus as well as the expression of proinflammatory cytokines or apoptosis-associated genes in THP-1 cells or pneumocytes A-549 respectively. CRP may link to proinflammatory mediators contributing to activation of complement and boosting inflammatory response in severe influenza infections. Compound 1,6-bis(phosphocholine)-hexane improved the severity and mortality of mice infected with lethal influenza virus significantly. These observations showed that CRP is involved in deterioration of severe influenza diseases, and indicated a substantial candidate molecule for immunotherapy of severe influenza diseases.
Keywords: C-reactive protein, Complement response, Immunotherapy, Severe influenza infection
Graphical Abstract
Highlights
-
•
CRP induces exacerbated immunoresponse toward overt pulmonary inflammation in severe influenza infections.
-
•
CRP may link to proinflammatory mediators contributing to activation of complement and boosting inflammatory response.
-
•
CRP stabilizer can alleviate the immunopathological lesions and mortality in mice infected with lethal influenza virus.
Severe influenza diseases with high mortality have been frequently reported, especially in those patients infected with avian influenza A (H5N1, H7N9, or H10N8) or during a pandemic. Respiratory distress associated with immunopathological lesions is the most common cause of death in patients infected by these viruses. In this study, we found that CRP may be linked to exacerbated immunoresponse toward overt pulmonary inflammation, which led to alveolar damage and respiratory failure in severe influenza infection. Our data identified that CRP stabilizer can be used to alleviate the immunopathological lesions and mortality in mice infected with lethal influenza virus.
1. Introduction
Influenza comprises a substantial portion of morbidity and mortality caused by acute respiratory infections, which are the fourth leading cause of death and the second highest cause of years of life lost (Organization, 2014, Radin et al., 2012, Nair et al., 2011). Severe influenza diseases with high mortality have been frequently reported, especially in those patients infected with avian influenza (AI) A (H5N1, H5N6, H7N9 or H10N8) viruses or during a pandemic (Organization, 2015, Ortiz et al., 2013, Chen et al., 2014). Severe AI or pandemic patients present with rapidly progressive pneumonia, leading to acute respiratory distress syndrome or the complication of multiple organ failure (Gao et al., 2013a, Pan et al., 2016, Chen et al., 2014, Investigators et al., 2009). Respiratory distress is the most common cause of death in patients infected by these viruses (Liem et al., 2009, Gao et al., 2013a). Autopsy or postmortem biopsy studies showed that extensive diffuse alveolar damage is the most consistent finding, and the virus predominantly infected lung parenchyma in H5N1, H5N6, and H7N9 patients or fatal cases infected by influenza pandemic viruses (Guo et al., 2014, Gao et al., 2016, Shieh et al., 2010).
In terms of therapy, other than lung protective ventilation for support for oxygenation, antiviral treatment is an important component to manage those patients infected with these viruses (Gao et al., 2013a, Yu et al., 2008, Zhang et al., 2009). However, influenza viruses mutate readily, and produce drug-resistant strains or unmanageable stains using vaccine (Govorkova et al., 2013, Hu et al., 2013, Shore et al., 2013). In addition, in case of lethal influenza infection, antiviral treatment is needed early to block virus replication and prevent triggering dysregulation of immune response, thereby abrogating the immunopathology (Wong et al., 2011). Once triggered, the immune-mediated tissue damage possibly presents limited sensitivity to antiviral agents (Yen et al., 2005, Govorkova et al., 2009).
C-reactive protein (CRP), a pentraxin, is a marker of inflammation that has been extensively used in clinical practice. CRP is involved in the innate immune response by attaching to microorganisms and damaged cellular components via phosphocholine. This leads to complement activation and phagocytosis. Excessive host immune response including complement activation has been shown to play a critical role on pathogenesis of severe influenza infections (Us, 2008, Gao et al., 2013b). Activation of the complement system has been implicated in the development of acute lung diseases induced by highly pathogenic influenza viruses (Garcia et al., 2013, Sun et al., 2013, Sun et al., 2015). Recent studies have brought up the idea of CRP to be not only a systemic marker of inflammation but also a mediator in inflammation faci (Thiele et al., 2015), and indicated that high levels of CRP is a high risk factor of fatality in H7N9 patients (Cheng et al., 2015, Wu et al., 2016). However, its exact physiological roles remain largely unknown, especially in severe influenza diseases. In this study, we characterized the role of CRP on pathogenesis of severe infections by influenza A (H5N1, H7N9 or H10N8) viruses, and indicated a candidate molecule for immunotherapy of severe influenza infection.
2. Materials and Methods
2.1. Influenza Patients and the Assessment of Patient Sera
Archived serum samples were obtained from local Centers for Diseases Control and Prevention (CDC) participating in the National Influenza Surveillance Network. H5N1, H7N9, H10N8, or H1N1 pdm09 infection was confirmed by reverse transcription polymerase chain reaction (RT-PCR) or viral isolation according Chinese influenza surveillance guideline. No samples were obtained from patients with presumptive (clinical diagnosis). Control samples were set from 10 normal donors from poultry workers. The sera CH50 levels were detected by MicroVue CH40 Eq EIA kit (Quidel, USA) per the kit's protocol. The sera CRP and C3 levels were tested by Bejing CIC clinical laboratory using turbidimetric inhibition immuno assay.
2.2. Viral Titration
The influenza viruses used in this study were titrated by a TCID50 (50% tissue culture infectious dose) in MDCK cells. Briefly, 100 μL/well of MDCK cells (3 × 104 cells/mL) were seeded one day before infection in 96-well microtiter plates. Serial semi-logarithmic dilutions of each virus or samples were made with Dulbecco modified Eagle medium containing 1% bovine serum albumin and 2 μg/mL TPCK-treated trypsin from 10− 2 to 10− 7. Dilutions of each virus or sample were added to MDCK cells (4 wells for each dilution, 100 μL/well). The cells were incubated for 48 h at 37 °C. The contents of each well were tested for hemagglutination by incubating 50 μL of the tissue culture supernatant with 0.5% turkey erythrocytes. The TCID50 was calculated according to the Reed and Muench method. For mouse lung tissue processing, in brief, whole lung tissues from each mouse were homogenized in 2 mL of phosphate buffered saline (PBS). The supernatant was sampled after centrifugation at 3000 rpm for 15 min at 4 °C.
2.3. Viral Infection In Vitro
The infection was performed by seeding the paired A-549 and THP-1 cell at a 1:1 cell ratio (100,000 cells each) onto a Transwell insert co-culture system (SI Appendix Fig. S4). A-549 were seeded onto the lower chamber of a 12-well plate and THP-1 onto Millicell hanging cell culture inserts (0.4 μm pore size; Costar, USA) in 0.5 mL of MEM (supplemented with 1% BSA and 1% antibiotic-antimycotic solution) with or without CRP (20 mg/L, BiosPacific, USA) at 37 °C. Another group was set as that CRP receptor blockers (100-fold diluted monoclonal antibodies against FcyR-I and FcyR-II; R&D, USA) were added into CRP-treated cells. The A-549 cells were infected with 0.1 multiplicity of infection (MOI) of A/Guangxi/1/2010(H5N1) virus. Four duplicates were set for each infection or MOCK group. The A-54ss9 or THP-1 cells were harvested for quantitative RT-PCR or immunochemistry detections at 6 h or 24 h post of infection, respectively. All steps were performed in biosafety level - 3 containment laboratory.
2.4. Mouse Infection
All animal studies were performed according to guidelines approved by the Investigational Animal Care and Use Committee of the National Institute for Viral Diseases Control and Prevention of the China CDC. We performed viral challenge by i.n. inoculation of 105 TCID50 of A/PR/8/34 (10-fold 50% lethal dose) to anesthetized 6- to 8-week adult female C57 BL/6 mice in 50 μL PBS. Intraperitoneal administration of 1,6-bis PC or/and peramivir (Abmole Bioscience, USA) was started 48 h after virus infection. A total of 4 types of treatment regimens (0.1 mM 1,6-bis PC, 0.02 mM 1,6-bis PC, 3 mg peramivir, and 3 mg peramivir plus 0.1 mM 1,6-bis PC per kg each day) were administered until day 7 postinfection. Compound 1,6-bis PC was synthesized in SKS Chem according to a previous report (Pepys et al., 2006). All compounds were diluted in TC (0.01 M Tris plus 0.14 M NaCl and 0.002 M CaCl2, pH 8.0) buffer. If the mice lost over 25% of their initial body weight, they were humanely euthanized and necropsied.
2.5. Immunochemistry Assay
An immunochemistry test for detection of influenza A antigen was performed on the slides with a THP-1 smear by using a polymer-based colorimetric indirect peroxidase method (ZSBio, China). The THP-1 cells were fixed using 80% acetone. A mouse monoclonal antibody against the nucleoprotein of influenza A was used (Serotec, UK). For controls, we used an antibody against nucleoprotein of influenza B virus in place of the primary antibody.
2.6. RNA Extraction and Quantitative RT-PCR
RNA was extracted from THP-1 or A549 cells using automated RNA extraction system QIAsymphony RGQ (Qiagen, Germany) with QIAsymphony RNA extraction kits as per the kit's protocol. To quantify the influenza viral load, proinflammation cytokine (IL-6 and IP-10), apoptosis-associated (TRAIL and FAS) gene, C5a receptor (C5aR1 and C5aR2) and CRP receptor (FCGR2 and FCGR3) mRNA levels, a quantitative real-time RT-PCR was performed on a real-time PCR detection system (Agilent Technologies Inc., Santa Clara, CA). The primer and probe sets were described in our previous report (Gao et al., 2013b) or from commercial products (Hs02375669, Hs01906226, Hs04206243, Hs04211858; Life Science Technologies, USA).
2.7. Histopathology and Immunohistochemistry
Routine hematoxylin and eosin staining was used for histopathology evaluation. For immunohistochemistry, 4 μm deparaffinized formalin fixed paraffin embedded sections were stained with monoclonal antibody against CRP (Abcam, USA), CD20cy (Dako Denmark), CD8 (Dako Denmark) and complement C5a (Abcam, USA) by using a polymer-based colorimetric indirect peroxidase method (ZSBio, China).
2.8. Mouse CRP, Lactate Dehydrogenase B, and Creatine Kinase-MB Assay
Mouse CRP, lactate dehydrogenase B (LDHB) and creatine kinase-MB (CK-MB) levels in mice sera were determined using enzyme-linked immunosorbent assay according to the manufacturer's instructions (CRP detection kit from R&D system, USA; LDHB and CK-MB detection kits from Dldevelop, China).
2.9. Statistical Analysis
Statistical analysis was performed using Instat software (Version 5.0, GraphPad Prism). Statistical significance was determined by Mann-Whitney U test or Kruskal-Wallis, Pearson's correlation or nonparametric t-test. Differences were considered significant at p < 0.05 with two-tailed testing.
3. Results
3.1. CRP Levels may be Linked to the Severity and Lethality of Avian Influenza Infection
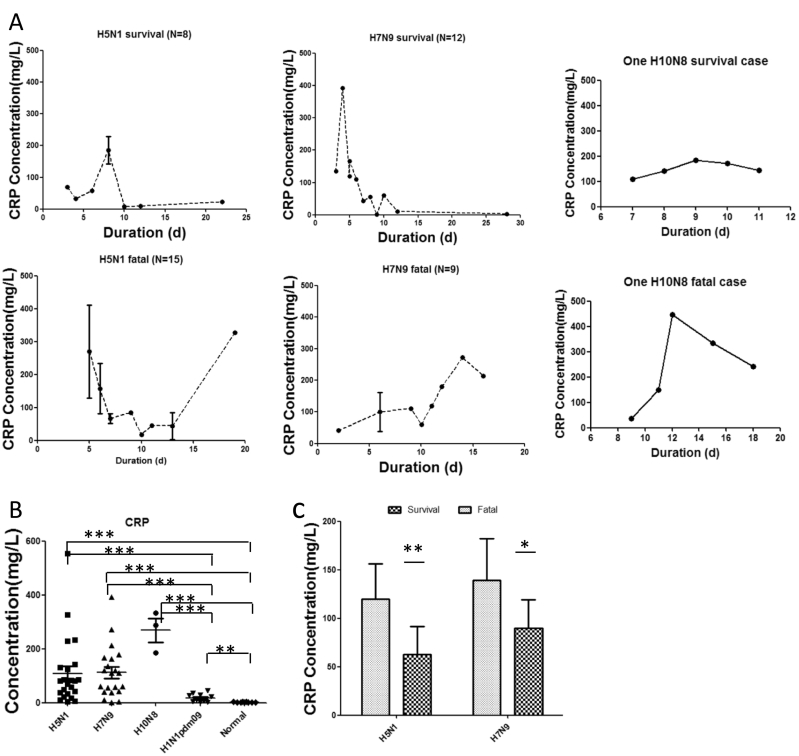
Serum samples were collected from total of 47 severe patients infected with H5N1 (n = 23), H7N9 (n = 21) or H10N8 (n = 3) avian influenza viruses. Twenty-six subjects died: 14 of H5N1, 10 of H7N9, and 2 of H10N8 cases. The other 21 survivors required intensive care. Median age of patients was 21 years (range, 2–62) in H5N1, 55.5 years (range, 24–67) in H7N9, and 64 years (range, 55–75) in H10N8. In addition, sera of mild influenza patients were involved from 10 adult outpatients infected with H1N1 pdm09. Control sera were set from 9 normal healthy adult volunteers (SI Appendix Table S1). With the development of illness duration, the kinetics of CRP showed that CRP levels might be linked to the severity and lethality of avian influenza infection in H5N1, H7N9 or H10N8 patients (Fig. 1A). Compared to H1N1 pdm09 patients with mild illness or healthy controls, remarkably increased serum CRP levels were detected in those patients with avian influenza (Fig. 1B). In addition, except for no case number for comparable analysis in H10N8 patients, CRP levels were much higher in H5N1 or H7N9 deceased patients than in survivors (Fig. 1C).
Fig. 1.
Serum levels of CRP in patients with severe infection with avian influenza A viruses. (A) The graphs depict the kinetics of serum CRP in H5N1, H7N9 or H10N8 (Sequent samples were collected from one dead H10N8 patients or one survivor with H10N8 infection, respectively) survivors or fatal cases over different durations. (B) CRP levels in H5N1, H7N9, H10N8 (The three samples were collected at day 7, 9, or 15, respectively), mild H1N1 pdm09 patients or healthy volunteers. (C) CRP levels in fatal cases or survivors infected with H5N1 or H7N9 during the acute stage (< 14 days after illness onset). Error bars represent mean with SEM. Mann-Whitney U test was performed to assess statistical significance, *p < 0.05, **p < 0.01 and ***p < 0.001.
3.2. Complement was Activated with Highly Levels in Avian Influenza Infections
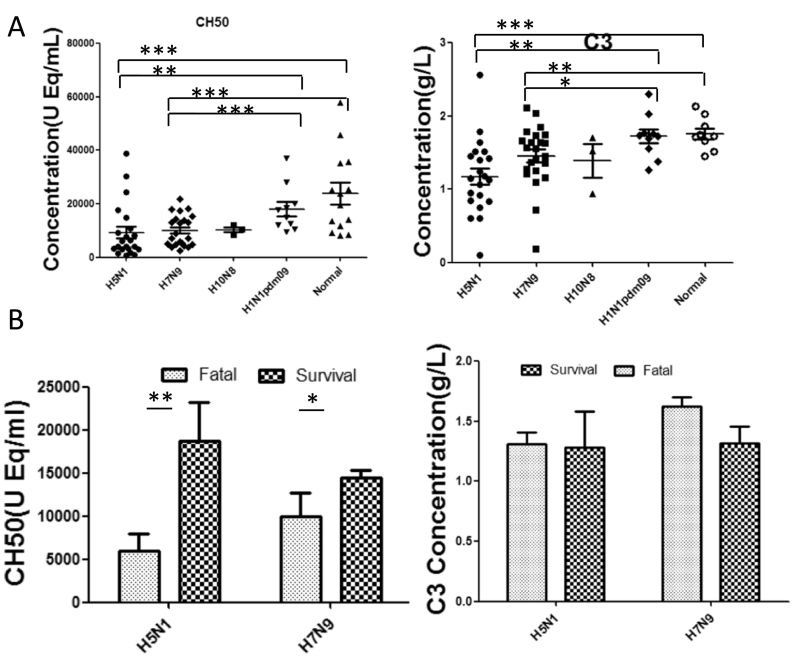
Complement activation present in avian influenza A (H5N1, H7N9, H10N8) infections. Serum CH50 levels were significantly decreased in those avian influenza patients when compared to patients infected with H1N1 pdm09 or healthy volunteers (Fig. 2A). In addition, CH50 levels were much lower in fatal cases than in survivors with H5N1 or H7N9 (p < 0.05) (Fig. 2B). C3, whose activation is part of the classic pathway of complement (Muller-Eberhard, 1986), was significantly decreased in those H5N1 or H7N9 patients when compared to healthy controls (Fig. 2C), indicating activation of complement system with high levels in these infections. The levels of C3 exhibited no significant difference between fatal cases and survivals of those patients with avian influenza (p > 0.05) (Fig. 2D), suggesting that other alterable factors may be involved in the process.
Fig. 2.
Complements CH50 and C3 levels in sera of patients with severe infection with avian influenza A viruses. (A) CH50 or C3 levels in H5N1, H7N9, H10N8, mild H1N1 pdm09 patients, or healthy volunteers. (B) CH50 or C3 levels in fatal cases or survivors infected with H5N1 or H7N9 during acute stage (< 14 days after illness onset). Error bars represent mean with standard error of the mean (SEM). Mann-Whitney U test was performed to assess statistical significance, *p < 0.05, **p < 0.01 and ***p < 0.001 (two-tailed test).
3.3. CRP Levels Correlated With Complement Activation in Avian Influenza Infections
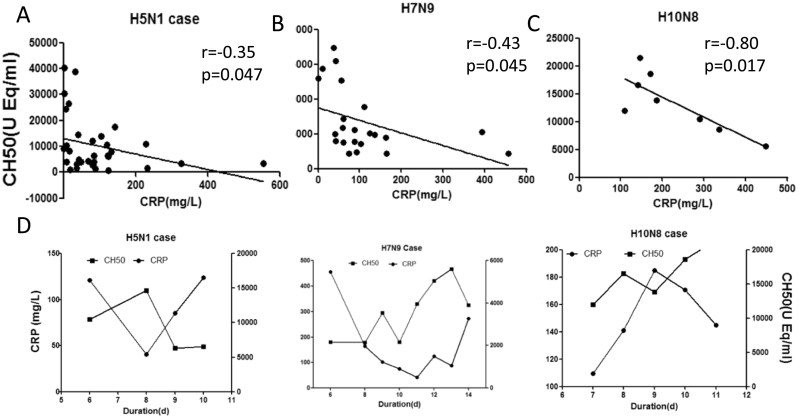
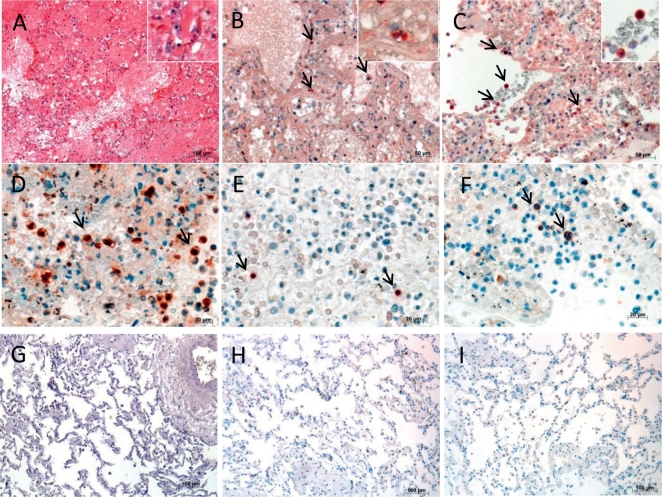
As shown in Fig. 3, the CRP levels presented slightly negative correlation with CH50 levels in sera of H5N1or H7N9 avian influenza patients, or remarkably negative correlation with CH50 levels in sera of H10N8 avian influenza patients during acute stage of illness (< day 14 after illness onset). The kinetic of CRP and CH50 presented relatively opposite way in sequential samples of H5N1, H7N9 or H10N8 patients (Fig. 3D). In addition, to determine possible immunopathology associated with CRP, we performed several tests on autopsy lung tissues of H5N1 fatal cases. As previous reports have shown, lung sections of fatal cases with H5N1 infection showed intra-alveolar edema, interstitial hemorrhages, necrosis of alveolar line cells, focal desquamation of pneumocytes in alveolar spaces, and mononuclear inflammatory cell infiltrates (Fig. 4A). Immunohistochemistry staining showed that extensive CRP + cells were frequently seen in lung sections of H5N1 or H5N6 fatal cases (Fig. 4B,C). In addition, complement C5a deposition, and both B lymphocytes (CD20cy +) and cytotoxic T lymphocytes (CD8 +) were scatted in alveolar of H5N1 (Fig. 4D–F) or H5N6 fatal case as reported previously(Gao et al., 2016).
Fig. 3.
Correlations between CRP and CH50 in avian influenza infections. Correlations between serum CRP and CH50 levels in patients infected with H5N1 (A), H7N9 (B) or H10N8 (C) during the acute stage (< 14 days after illness onset). The Pearson correlation coefficients (r) and p values are provided in each graph. (D) Kinetics of CRP and CH50 serum levels in sequential samples from patients infected with H5N1, H7N9, or H10N8.
Fig. 4.
Histology and immunopathology of lung sections from H5N1 or H5N6 fatal cases. (A) Pulmonary histopathology in representative lung sections of H5N1 fatal patient (H&E). Immunohistochemistry for CRP + pulmonary cells (black arrows) in representative lung sections of H5N1 (B) and H5N6 (C) fatal cases. (D) C5a + pulmonary cells (black arrows), (E) CD20cy + lymphocytes (black arrows), (F) CD8 + (black arrows) lymphocytes in representative lung sections of patients infected with H5N1. A normal lung tissue as negative control for CRP (G), C5a (H), and CD20cy or CD8 staining (I). Original magnification: × 10 (A, G, H, I), × 20 (B, C), × 40 (D–F).
3.4. CRP Enhanced Inflammatory Response of Immune Cells and Apoptosis of Infected Cells
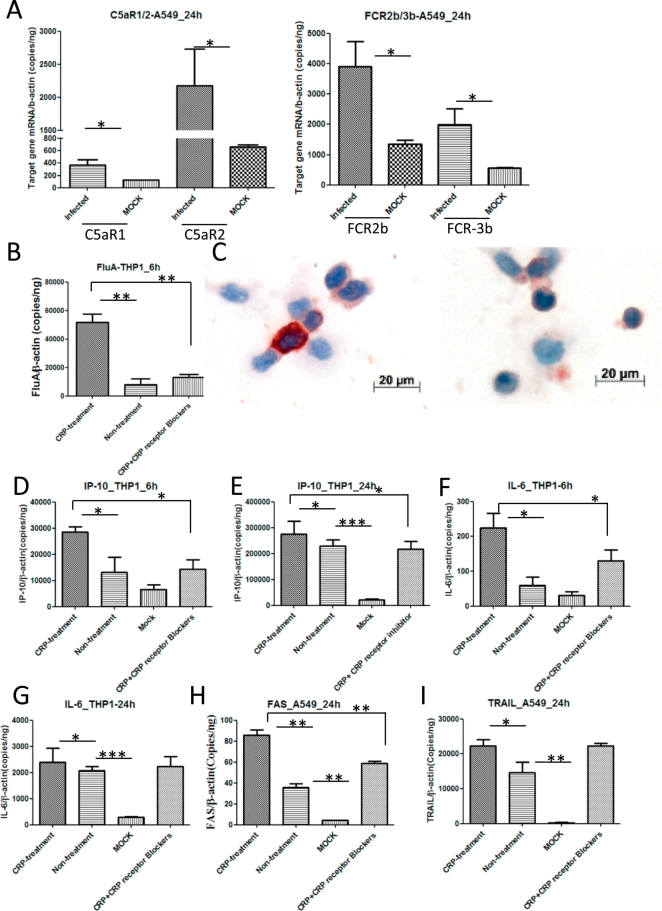
To define the effect of CRP in the progress of avian influenza virus infection, we used the Trans-well insert co-culture system with monocytes THP-1 and pneumocytes A-549. A-549 cells were infected with H5N1 virus in the system while treated with or without CRP and/or CRP receptors blockers. The results showed that H5N1 infection boosted expression of CRP receptors (FcyR2 and FcyR3) as well as C5a receptors (C5aR1 and C5aR2) significantly in A-549 cells (Fig. 5A). CRP treatment increased the viral load in THP-1 cells (Fig. 5B). Consistently, immunochemistry staining showed much more influenza virus phagocytosed in THP-1cells with CRP treatment than without CRP treatment (Fig. 5 C). Correspondingly, CRP treatment increased the mRNA expression of proinflammatory factors IP-10 and IL-6 in THP-1cells at 6 h or/and 24 h post infection (Fig. 5D–G), and elevated mRNA levels of apoptosis genes (FAS and TRAIL) in infected A-549 cells at 24 h post infection (Fig. 5H, I). In contrast, these effects can be interrupted by CRP blocker (Fig. 5B–H).
Fig. 5.
CRP and C5a receptors expression, viral load and host response in H5N1 infected cells. (A) Elevated CRP or C5a receptors mRNA expression in infected A-549 cells. B) Elevated viral load in infected-THP cells with CRP treatment. (C) Original magnification: × 50. More influenza nucleoprotein staining (in brown) in infected-THP cells with CRP treatment (left panel) than without CRP treatment (right panel). Elevated proinflammatory factors IP-10 and IL-6 mRNA levels in THP-1 cells with CRP treatment 6 h postinfection (D, E) or 24 h postinfection (F, G). Apoptosis-associated gene FAS and TRAIL mRNA levels showed elevated in A549 cells with CRP treatment 24 h postinfection (H, I). Unpaired t-test was performed to assess statistical significance, *p < 0.05, **p < 0.01, and ***p < 0.001 with a two-tailed test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Compound 1,6-Bis PC Improved the Severity and Mortality of Infection With Lethal Influenza Virus in Mice
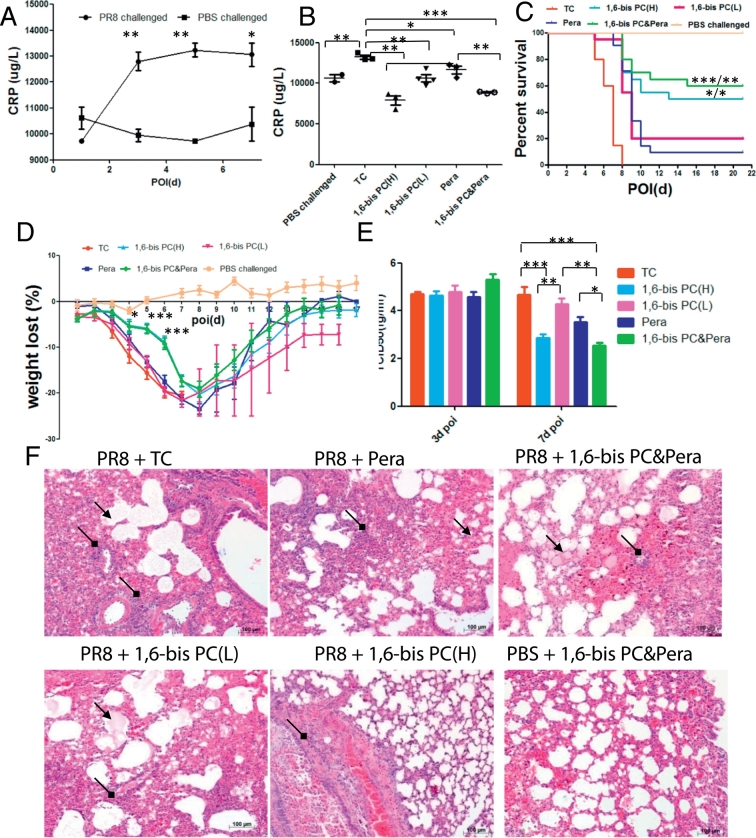
Monomeric CRP (mCRP) is considered to be the inflammatory derivative of circulating pentameric CRP (pCRP). The stabilization of pCRP with 1,6-bis(phosphocholine)-hexane (1,6-bis PC) abolished mCRP formation and deposition in vivo (Thiele et al., 2014). CRP levels were increased significantly in sera of mice infected with PR8 virus at day 3 of infection. The high CRP levels were sustained at day 5 or 7 of infection (Fig. 6A). To determine the role of CRP in progression of severe influenza disease, compound 1,6-bis PC was used to treat the C57 bl/6 mice infected with lethal influenza virus PR8. TC (0.01 M Tris plus 0.14 M NaCl and 0.002 M CaCl2, pH 8.0) buffer or neuraminidase inhibitor peramivir treatment for placebo or antiviral therapy group was set on respectively. Compared to TC treatment, sera CRP levels were remarkably inhibited in infected mice with treatment of 1,6-bis PC, peramivir, or 1,6-bis PC plus peramivir. CRP levels presented lower in high dose of 1,6-bis PC (1,6-bis PC(H)) group than in low doses of the 1,6-bis PC (1,6-bis PC(L)) group, or in the 1,6-bis PC plus peramivir group than in the peramivir group at day 5 of infection(Fig. 6B). Compared to the TC-treated group, the mice mortality rate was significantly improved in 1,6-bis PC(H), 1,6-bis PC(L), peramivir, or 1,6-bis PC plus peramivir treatment group (p < 0.001) (Fig. 6C). The improved mortality rate was better with 1,6-bis PC(H) or 1,6-bis PC plus peramivir treatment than with peramivir or 1,6-bis PC(L) treatment. In addition, weight loss or weight recovery presented milder or earlier in the 1,6-bis PC(H) or 1,6-bis PC plus peramivir group than in other groups (Fig. 6D).
Fig. 6.
1,6-bis PC reduce the severity and mortality of lethal influenza infection in mice. C57 bl/6 mice were injected with 1,6-bis PC or/and peramivir, or TC buffer as a vehicle control 48 h after i.n. inoculation of phosphate buffered saline (PBS) or A/PR/8/34 live virus (105 TCID50). (A) Sera CRP kinetics in TC buffer treated mice. n = 3 to 4 mice per time point (mean ± SEM). *p < 0.05, **p < 0.01 (two-tailed t-test) when comparing 1 day postinfection. (B) Sera CRP levels were assessed 5 days postinfection. n = 3 to 4 mice per group (mean ± SEM). *P < 0.05, **P < 0.01 (two-tailed t-test). (C) Kaplan-Meier survival curves were recorded. n = 5 mice for PBS challenged group, n = 20 for every other group. *p < 0.05, ** p < 0.01, ***p < 0.001 (log-rank test) when comparing the peramivir group/1,6-bis PC(L) group. (D) Weight loss was recorded for all survived mice until 21 days postinfection. (E) Representative lung histopathology of PR8 or PBS challenged mice with or without 1,6-bis PC or/and peramivir treatment at day 7 post of infection. The infiltration of inflammatory cells (black square arrow) and hyaline membrane formation (black arrow) were presented in lung sections. Original magnification: × 10. Each experiment was repeated at least three times.
Virus shedding was detected in lung tissues of inoculated mice at day 3 or 7 postinfection. The results showed that the viral titers had no significant difference in lungs at day 3 postinfection among those different groups. However, the viral titers were decreased in the1,6-bis PC(H) or 1,6-bis PC plus peramivir treated group compared to TC or the 1,6-bis PC(L) group at day 7, and presented lower levers in the 1,6-bis PC plus peramivir group than in the single peramivir treatment group (Fig. 6E). In microscopic pathology, lethal influenza virus PR8 infections resulted into pneumonia, pulmonary bronchitis, and diffuse alveolar damage in lungs of infected mice (Fig. 6F). Compound 1,6-bis PC (H) treatment improved the pathological lesions remarkably. The inflammatory damages present as localization in 1,6-bis PC (H) treated mice instead of appearing diffuse in TC treated mice. In comparison, peramivir or 1,6-bis PC(L) treatment slightly improved the pathological damages. Compared to the TC group, the infiltration of inflammatory cells presented decreased, and edema changes were slightly alleviated. The pathological lesions showed moderate improvement in lungs of peramivir plus 1,6-bis PC treated mice. No histologic significant differences were found in heart tissue among infected-mice or PBS-challenged-mice (data not shown).
Compared to the PBS-challenged group, the levels of CK-MB isoenzyme have not significant differences in sera of infected mice with different treatment regimens including the TC control group (SI Appendix Fig. S1). However, LDHB levels presented significantly decreased in TC treated mice when comparing PBS challenged mice (SI Appendix Fig. S2A). Compounds 1,6-bis PC or peramivir treatment restored the LDHB levels of infected mice (SI Appendix Fig. S2B). LDHB levels were higher in the 1,6-bis PC(H) group than in other groups at 5 days postinfection although no statistical difference was seen among the different treatment groups. Except for the 1,6-bis PC(H) group, significantly decreased levels of LDHB were present in other treatment groups when comparing PBS-challenged group (SI Appendix Fig. S2B). LDHB levels were significantly negatively correlated with CRP levels (SI Appendix Fig. S2C). Further analysis showed that LDHB levels were significantly negative correlated with the weight changes of mice until 5 days postinfection in the TC group (SI Appendix Fig. S3A). In compound treatment groups, LDHB levels also presented significant correlation with the weight loss of mice at 5 or 7 days postinfection but not at 3 days postinfection (SI Appendix Fig. S3B–D).
4. Discussion
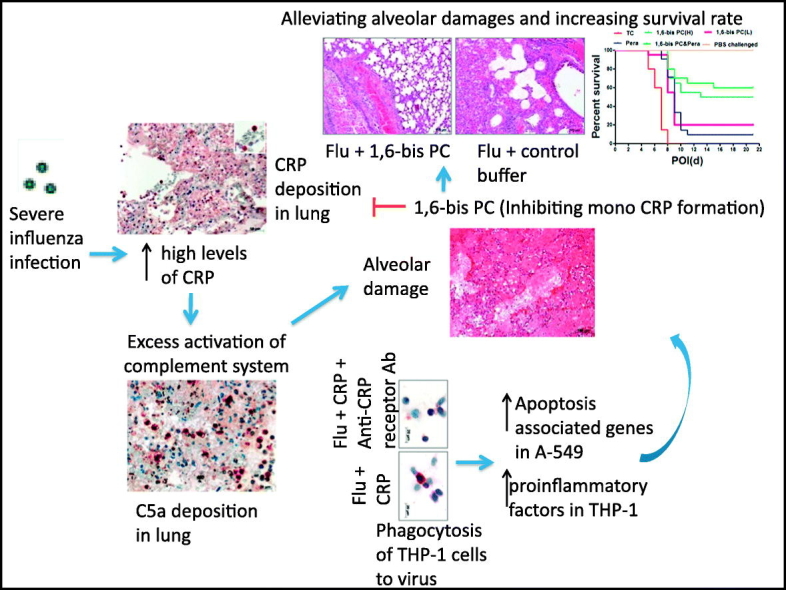
Our data established an important role of CRP in the pathogenesis of avian influenza A (H5N1, H7N9 or H10N8) infections. Although highly pathogenic avian influenza virus infections have a different epidemiology, the most common cause of death is respiratory distress caused by severe alveolar damage in infected patients. Innate host response plays a key role in the pathogenesis of severe avian influenza diseases (Liu et al., 2016, Darwish et al., 2011, Us, 2008). Previous studies suggested that anaphylatoxin C5a has been implicated in the pathogenesis of ARDS by mediating neutrophil attraction, aggregation, activation, and subsequently pulmonary endothelia damage (Wang et al., 2015, Tate and Repine, 1983, Sacks et al., 1978). Animal experiments showed that C5a inhibition can partially alleviate the pathogenicity of H5N1 or H7N9 infection (Sun et al., 2015, Sun et al., 2013). Our study showed that CRP correlated with complement activation in patients with severe avian influenza (H5N1, H7N9 or H10N8). The C5a deposition was extensive in lung tissues of fatal H5N1 cases. Globally, the biological role of CRP after binding to ligands is to trigger the complement pathway (Pepys and Hirschfield, 2003, Lelubre et al., 2013). CRP is recognized by complement protein C1q and potently activates the classic complement pathway, engaging C3, and the main molecule of adhesion of the complement system (Lelubre et al., 2013, Agrawal et al., 2001). CRP located upstream of immune mediation would be a better immune mediator than C5a when designing a candidate molecule for immunotherapy for severe avian influenza diseases.
Our data indicated that CRP may be associated with tissue lesions. In addition to hepatic synthesis (Pfafflin and Schleicher, 2009), CRP can be synthesized by lung cells (e.g. epithelial cells, lymphocytes) (Ramage et al., 2004, Marc et al., 2004). However, local production in extrahepatic synthesis of CRP may be a process of localized inflammation and a marker for local cellular damage (Juma et al., 2011). Tissue studies have shown that extensive diffuse alveolar damage is the most notably histological feature in severe fatal cases with avian influenza infection. The alveolar damage was accompanied by extensive diffusion, although the distribution of virus was scattered or rare in lung tissue of fatal cases (Nakajima et al., 2013). Excessive host response including virus-induced cytokine dysregulation contributed to the pathogenesis of severe influenza disease (Lee et al., 2009, Gao et al., 2013b, Narasaraju et al., 2011). In this study, CRP + cells presented extensive distribution in lung tissues of H5N1 or H5N6 fatal cases in addition to higher CRP levels in fatal cases. In addition, CRP enhanced induction of proinflammatory factors in THP-1 cells, and increased the expression of apoptosis gene in H5N1-infected A-549 cells in the Transwell insert co-culture system.
Inflammation is a time-dependent process, usually starting locally, and is recognized centrally via blood-borne mediators (Pfafflin and Schleicher, 2009). Generally, proinflammatory cytokines appear within 1 h after the start of infection, and synthesis of CRP starts 6 to 8 h after onset (Pfafflin and Schleicher, 2009). CRP is produced in response to stimulation by proinflammatory factor (e.g. IL-6, tumor necrosis factor alpha, interferon gamma, or IL-1) (Ramage et al., 2004, Lelubre et al., 2013). Previous reports have shown that both IL-6 and IP-10 have significantly higher levels in H5N1 or H7N9 patients compared with healthy volunteers (Zhou et al., 2013, Deng et al., 2008), and may represent a candidate molecule for immunotherapy in the setting of severe respiratory tract infection (Mok et al., 2014, Zeng et al., 2005). The levels of elevated cytokines were positively related to viral load in patients during acute duration (De Jong et al., 2006, Sirinonthanawech et al., 2011). In this study, CRP enhanced phagocytosis of THP-1 cells to infected virus in a co-culture system with H5N1 infection, and elevated the expression of the detected IL-6 and IP-10 in THP-1 cells. Hence, CRP may relate to deteriorated inflammation as well as tissue lesions in severe influenza infection when a high viral load is produced in individuals with lethal influenza infection.
Additionally, we found that enhanced CRP levels were correlated with downregulated expression of LDHB in sera of infected mice. The function of LDHB, which is consistent with an increased glycolytic phenotype, is required to maintain cell growth (Drent et al., 1996, Mccleland et al., 2013). In normal tissues, LDHB is expressed in the liver, red blood cells, kidney, and heart. Its inhibition may have deleterious consequences including correlating with unfavorable survival diseases (Dawson et al., 1964, Markert et al., 1975, Chen et al., 2015). In this study, high doses of compound 1,6-bis PC treatment slightly restored the levels of LDHB in sera of infected mice whereas PR8 infection decreased LDHB level in mice. In addition, down-regulated LDHB levels correlated with increased weight loss of infected mice. Therefore, CRP-mediated immune response involved multiple sites, worsening the severity of lethal influenza infection.
Animal experiments showed that 1,6-bis PC can improve the severity of illness and mortality of mice infected with lethal influenza virus in this study. Compound 1,6-bis PC, a derivative of CRP-ligand phosphocholine, can prevent dissociation of pCRP, and subsequently inhibit the generation and proinflammatory activity of mCRP. A previous study has showed that 1,6-bis PC can inhibit mCRP deposition and inflammation in rat myocardial infarction (Thiele et al., 2014). In the current study, intraperitoneal 1,6-bis PC treatment lightened the inflammatory lesions in lung tissues of mice infected with lethal influenza virus while reducing the production of CRP. In contrast, 1,6-bis PC treatment alleviated the mortality of infected mice significantly, and improve weight loss and recovery in mice. Therefore, CRP may be a target for management of severe influenza diseases.
Neuraminidase inhibitors (NAI) are the most frequently used antiviral drug for management of influenza infection. Early NAI treatment was associated with shorter illness duration, and is most beneficial when treatment started within 2 days of illness onset (Lee et al., 2015). However, except for induction of resistant strains, NAI treatment can not reverse the immune mediated tissue damage triggered by infection. Peramivir used in this study is an effective NAI against a variety of influenza A and B subtypes. The results suggested that both 1,6-bis PC and peramivir can improve mortality and illness severity in mice infected with lethal influenza infection. However, high-dose treatment of 1,6-bis PC had better efficiency than peramivir; combinated treatment with 1,6-bis PC and peramivir yield even better efficiency. Previous studies suggested that lethal dissemination of influenza virus is associated with dysregulation of inflammation (Cilloniz et al., 2010), and immune mediated tissue damage may present limited sensitivity to antiviral agents after a robust host innate immune response was triggered (Sidwell et al., 1999, Yen et al., 2005, Govorkova et al., 2009). Our results indicated that CRP may link to proinflammatory mediators contributing to activation of complement and boosting inflammatory response in severe influenza infections.
Although additional mechanistic studies are needed, our data shows that virus initiates the expression of proinflammatory cytokines-stimulated CRP after influenza infection begins. Then, CRP enhances phagocytosis of immune cells, and activates a series of innate immunoresponses, including complement activation, aggravation of inflammation responses, and apoptosis induction. That may be why high concentration of CRP induces exacerbated immunoresponse toward overt pulmonary inflammation, which leads to alveolar damage and respiratory failure. Importantly, our data identify CRP stabilizer as a potential mode to alleviate mortality in individuals infected with lethal influenza viruses. These observations provide a biological mechanism for immunopathological lesions of severe avian influenza diseases, and indicated a candidate molecule for immunotherapy.
Funding Sources
This study was supported by the Young Scholar Scientific Researcher Foundation of China CDC (no. 2015A 101 to Dr. Rongbao Gao), National Natural Science Foundation of China (81373107 to Dr. Rongbao Gao), and the National Mega-Projects for Infectious Diseases (2017ZX10304402-001-019 to Dr. Rongbao Gao, and 2014ZX10004002 to Dr. Yuelong Shu.).
Conflicts of Interest
No conflicts of interest declared.
Author Contributions
R.B.G. conceived and designed research; R.B.G., L.J.W., H.B., T.B. and Y.Z. performed research; R.B.G. and L.J.W. analyzed the data; and R.B.G. wrote the manuscript. Y.L.S contributed to the management of the laboratory and influenza surveillance network.
Acknowledgements
The authors would like to thank the Chinese National Influenza Surveillance Network for the collection of serum samples. The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of China CDC and other organizations.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.07.010.
Contributor Information
Rongbao Gao, Email: gaorongbao@cnic.org.cn.
Yuelong Shu, Email: yshu@cnic.org.cn.
Appendix A. Supplementary data
Supplementary material
References
- Agrawal A., Shrive A.K., Greenhough T.J., Volanakis J.E. Topology and structure of the C1q-binding site on C-reactive protein. J. Immunol. 2001;166:3998–4004. doi: 10.4049/jimmunol.166.6.3998. [DOI] [PubMed] [Google Scholar]
- Chen H., Yuan H., Gao R., Zhang J., Wang D., Xiong Y., Fan G., Yang F., Li X., Zhou J., Zou S., Yang L., Chen T., Dong L., Bo H., Zhao X., Zhang Y., Lan Y., Bai T., Dong J., Li Q., Wang S., Zhang Y., Li H., Gong T., Shi Y., Ni X., Li J., Zhou J., Fan J., Wu J., Zhou X., Hu M., Wan J., Yang W., Li D., Wu G., Feng Z., Gao G.F., Wang Y., Jin Q., Liu M., Shu Y. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- Chen R., Zhou X., Yu Z., Liu J., Huang G. Low expression of LDHB correlates with unfavorable survival in hepatocellular carcinoma: strobe-compliant article. Medicine (Baltimore) 2015;94:e1583. doi: 10.1097/MD.0000000000001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q.L., Ding H., Sun Z., Kao Q.J., Yang X.H., Huang R.J., Wen Y.Y., Wang J., Xie L. Retrospective study of risk factors for mortality in human avian influenza A (H7N9) cases in Zhejiang Province, China, March 2013 to June 2014. Int. J. Infect. Dis. 2015;39:95–101. doi: 10.1016/j.ijid.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Cilloniz C., Pantin-Jackwood M.J., Ni C., Goodman A.G., Peng X., Proll S.C., Carter V.S., Rosenzweig E.R., Szretter K.J., Katz J.M., Korth M.J., Swayne D.E., Tumpey T.M., Katze M.G. Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection. J. Virol. 2010;84:7613–7624. doi: 10.1128/JVI.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish I., Mubareka S., Liles W.C. Immunomodulatory therapy for severe influenza. Expert Rev. Anti-Infect. Ther. 2011;9:807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- Dawson D.M., Goodfriend T.L., Kaplan N.O. Lactic dehydrogenases: functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation. Science. 1964;143:929–933. doi: 10.1126/science.143.3609.929. [DOI] [PubMed] [Google Scholar]
- De Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N., Hoang D.M., Chau N.V., Khanh T.H., Dong V.C., Qui P.T., Cam B.V., Ha Do Q., Guan Y., Peiris J.S., Chinh N.T., Hien T.T., Farrar J. Fatal outcome of human influenza a (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R., Lu M., Korteweg C., Gao Z., Mcnutt M.A., Ye J., Zhang T., Gu J. Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J. Pathol. 2008;216:328–336. doi: 10.1002/path.2417. [DOI] [PubMed] [Google Scholar]
- Drent M., Cobben N.A., Henderson R.F., Wouters E.F., Van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir. J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- Gao H.N., Lu H.Z., Cao B., Du B., Shang H., Gan J.H., Lu S.H., Yang Y.D., Fang Q., Shen Y.Z., Xi X.M., Gu Q., Zhou X.M., Qu H.P., Yan Z., Li F.M., Zhao W., Gao Z.C., Wang G.F., Ruan L.X., Wang W.H., Ye J., Cao H.F., Li X.W., Zhang W.H., Fang X.C., He J., Liang W.F., Xie J., Zeng M., Wu X.Z., Li J., Xia Q., Jin Z.C., Chen Q., Tang C., Zhang Z.Y., Hou B.M., Feng Z.X., Sheng J.F., Zhong N.S., Li L.J. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 2013;368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- Gao R., Bhatnagar J., Blau D.M., Greer P., Rollin D.C., Denison A.M., Deleon-Carnes M., Shieh W.J., Sambhara S., Tumpey T.M., Patel M., Liu L., Paddock C., Drew C., Shu Y., Katz J.M., Zaki S.R. Cytokine and chemokine profiles in lung tissues from fatal cases of 2009 pandemic influenza A (H1N1): role of the host immune response in pathogenesis. Am. J. Pathol. 2013;183:1258–1268. doi: 10.1016/j.ajpath.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Pan M., Li X., Zou X., Zhao X., Li T., Yang H., Zou S., Bo H., Xu J., Li S., Zhang M., Li Z., Wang D., Zaki S.R., Shu Y. Post-mortem findings in a patient with avian influenza A (H5N6) virus infection. Clin. Microbiol. Infect. 2016;22(574):e1–5. doi: 10.1016/j.cmi.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Garcia C.C., Weston-Davies W., Russo R.C., Tavares L.P., Rachid M.A., Alves-Filho J.C., Machado A.V., Ryffel B., Nunn M.A., Teixeira M.M. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One. 2013;8:e64443. doi: 10.1371/journal.pone.0064443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova E.A., Baranovich T., Seiler P., Armstrong J., Burnham A., Guan Y., Peiris M., Webby R.J., Webster R.G. Antiviral resistance among highly pathogenic influenza A (H5N1) viruses isolated worldwide in 2002–2012 shows need for continued monitoring. Antivir. Res. 2013;98:297–304. doi: 10.1016/j.antiviral.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova E.A., Ilyushina N.A., Mcclaren J.L., Naipospos T.S., Douangngeun B., Webster R.G. Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model. Antimicrob. Agents Chemother. 2009;53:3088–3096. doi: 10.1128/AAC.01667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Huang J.A., Zhao D., Jin J., Liu S., Fraidenburg D.R. Pathological changes in a patient with acute respiratory distress syndrome and H7N9 influenza virus infection. Crit. Care. 2014;18:666. doi: 10.1186/s13054-014-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Lu S., Song Z., Wang W., Hao P., Li J., Zhang X., Yen H.L., Shi B., Li T., Guan W., Xu L., Liu Y., Wang S., Zhang X., Tian D., Zhu Z., He J., Huang K., Chen H., Zheng L., Li X., Ping J., Kang B., Xi X., Zha L., Li Y., Zhang Z., Peiris M., Yuan Z. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381:2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- Investigators A.I., Webb S.A., Pettila V., Seppelt I., Bellomo R., Bailey M., Cooper D.J., Cretikos M., Davies A.R., Finfer S., Harrigan P.W., Hart G.K., Howe B., Iredell J.R., Mcarthur C., Mitchell I., Morrison S., Nichol A.D., Paterson D.L., Peake S., Richards B., Stephens D., Turner A., Yung M. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- Juma W.M., Lira A., Marzuk A., Marzuk Z., Hakim A.M., Thompson C.S. C-reactive protein expression in a rodent model of chronic cerebral hypoperfusion. Brain Res. 2011;1414:85–93. doi: 10.1016/j.brainres.2011.07.047. [DOI] [PubMed] [Google Scholar]
- Lee N., Leo Y.S., Cao B., Chan P.K., Kyaw W.M., Uyeki T.M., Tam W.W., Cheung C.S., Yung I.M., Li H., Gu L., Liu Y., Liu Z., Qu J., Hui D.S. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur. Respir. J. 2015;45:1642–1652. doi: 10.1183/09031936.00169714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., Gardy J.L., Cheung C.Y., Cheung T.K., Hui K.P., Ip N.Y., Guan Y., Hancock R.E., Peiris J.S. Systems-level comparison of host-responses elicited by avian H5N1 and seasonal H1N1 influenza viruses in primary human macrophages. PLoS One. 2009;4:e8072. doi: 10.1371/journal.pone.0008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelubre C., Anselin S., Zouaoui Boudjeltia K., Biston P., Piagnerelli M. Interpretation of C-reactive protein concentrations in critically ill patients. Biomed. Res. Int. 2013;2013:124021. doi: 10.1155/2013/124021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem N.T., Tung C.V., Hien N.D., Hien T.T., Chau N.Q., Long H.T., Hien N.T., Mai Le Q., Taylor W.R., Wertheim H., Farrar J., Khang D.D., Horby P. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin. Infect. Dis. 2009;48:1639–1646. doi: 10.1086/599031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhou Y.H., Yang Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc M.M., Korosec P., Kosnik M., Kern I., Flezar M., Suskovic S., Sorli J. Complement factors c3a, c4a, and c5a in chronic obstructive pulmonary disease and asthma. Am. J. Respir. Cell Mol. Biol. 2004;31:216–219. doi: 10.1165/rcmb.2003-0394OC. [DOI] [PubMed] [Google Scholar]
- Markert C.L., Shaklee J.B., Whitt G.S. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 1975;189:102–114. doi: 10.1126/science.1138367. [DOI] [PubMed] [Google Scholar]
- Mccleland M.L., Adler A.S., Deming L., Cosino E., Lee L., Blackwood E.M., Solon M., Tao J., Li L., Shames D., Jackson E., Forrest W.F., Firestein R. Lactate dehydrogenase B is required for the growth of KRAS-dependent lung adenocarcinomas. Clin. Cancer Res. 2013;19:773–784. doi: 10.1158/1078-0432.CCR-12-2638. [DOI] [PubMed] [Google Scholar]
- Mok C.K., Kang S.S., Chan R.W., Yue P.Y., Mak N.K., Poon L.L., Wong R.N., Peiris J.S., Chan M.C. Anti-inflammatory and antiviral effects of indirubin derivatives in influenza A (H5N1) virus infected primary human peripheral blood-derived macrophages and alveolar epithelial cells. Antivir. Res. 2014;106:95–104. doi: 10.1016/j.antiviral.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard H.J. The membrane attack complex of complement. Annu. Rev. Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A., Simmerman J.M., Gordon A., Sato M., Howie S., Krishnan A., Ope M., Lindblade K.A., Carosone-Link P., Lucero M., Ochieng W., Kamimoto L., Dueger E., Bhat N., Vong S., Theodoratou E., Chittaganpitch M., Chimah O., Balmaseda A., Buchy P., Harris E., Evans V., Katayose M., Gaur B., O'callaghan-Gordo C., Goswami D., Arvelo W., Venter M., Briese T., Tokarz R., Widdowson M.A., Mounts A.W., Breiman R.F., Feikin D.R., Klugman K.P., Olsen S.J., Gessner B.D., Wright P.F., Rudan I., Broor S., Simoes E.A., Campbell H. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Van Tin N., Sato Y., Thach H.N., Katano H., Diep P.H., Kumasaka T., Thuy N.T., Hasegawa H., San L.T., Kawachi S., Liem N.T., Suzuki K., Sata T. Pathological study of archival lung tissues from five fatal cases of avian H5N1 influenza in Vietnam. Mod. Pathol. 2013;26:357–369. doi: 10.1038/modpathol.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., Phoon M.C., Van Rooijen N., Chow V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. Human Cases of Influenza at the Human-Animal Interface, January 2014–April. 2015. http://www.who.int/wer/2015/wer9028.pdf?ua=1 Available: [PubMed]
- Organization W.H. World Health Statistics. 2014. http://apps.who.int/iris/bitstream/10665/112738/1/9789240692671_eng.pdf?ua=1 Available: (Accessed 5 March 2015)
- Ortiz J.R., Rudd K.E., Clark D.V., Jacob S.T., West T.E. Clinical research during a public health emergency: a systematic review of severe pandemic influenza management. Crit. Care Med. 2013;41:1345–1352. doi: 10.1097/CCM.0b013e3182771386. [DOI] [PubMed] [Google Scholar]
- Pan M., Gao R., Lv Q., Huang S., Zhou Z., Yang L., Li X., Zhao X., Zou X., Tong W., Mao S., Zou S., Bo H., Zhu X., Liu L., Yuan H., Zhang M., Wang D., Li Z., Zhao W., Ma M., Li Y., Li T., Yang H., Xu J., Zhou L., Zhou X., Tang W., Song Y., Chen T., Bai T., Zhou J., Wang D., Wu G., Li D., Feng Z., Gao G.F., Wang Y., He S., Shu Y. Human infection with a novel highly pathogenic avian influenza A (H5N6) virus: virological and clinical findings. J. Infect. 2016;72:52–59. doi: 10.1016/j.jinf.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M.B., Hirschfield G.M., Tennent G.A., Gallimore J.R., Kahan M.C., Bellotti V., Hawkins P.N., Myers R.M., Smith M.D., Polara A., Cobb A.J., Ley S.V., Aquilina J.A., Robinson C.V., Sharif I., Gray G.A., Sabin C.A., Jenvey M.C., Kolstoe S.E., Thompson D., Wood S.P. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- Pfafflin A., Schleicher E. Inflammation markers in point-of-care testing (POCT) Anal. Bioanal. Chem. 2009;393:1473–1480. doi: 10.1007/s00216-008-2561-3. [DOI] [PubMed] [Google Scholar]
- Radin J.M., Katz M.A., Tempia S., Talla Nzussouo N., Davis R., Duque J., Adedeji A., Adjabeng M.J., Ampofo W.K., Ayele W., Bakamutumaho B., Barakat A., Cohen A.L., Cohen C., Dalhatu I.T., Daouda C., Dueger E., Francisco M., Heraud J.M., Jima D., Kabanda A., Kadjo H., Kandeel A., Bi Shamamba S.K., Kasolo F., Kronmann K.C., Mazaba Liwewe M.L., Lutwama J.J., Matonya M., Mmbaga V., Mott J.A., Muhimpundu M.A., Muthoka P., Njuguna H., Randrianasolo L., Refaey S., Sanders C., Talaat M., Theo A., Valente F., Venter M., Woodfill C., Bresee J., Moen A., Widdowson M.A. Influenza surveillance in 15 countries in Africa, 2006-2010. J Infect Dis. 2012;206(Suppl. 1):S14–21. doi: 10.1093/infdis/jis606. [DOI] [PubMed] [Google Scholar]
- Ramage L., Proudfoot L., Guy K. Expression of C-reactive protein in human lung epithelial cells and upregulation by cytokines and carbon particles. Inhal. Toxicol. 2004;16:607–613. doi: 10.1080/08958370490464599. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C.F., Craddock P.R., Bowers T.K., Jacob H.S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J. Clin. Invest. 1978;61:1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W.J., Blau D.M., Denison A.M., Deleon-Carnes M., Adem P., Bhatnagar J., Sumner J., Liu L., Patel M., Batten B., Greer P., Jones T., Smith C., Bartlett J., Montague J., White E., Rollin D., Gao R., Seales C., Jost H., Metcalfe M., Goldsmith C.S., Humphrey C., Schmitz A., Drew C., Paddock C., Uyeki T.M., Zaki S.R. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am. J. Pathol. 2010;177:166–175. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D.A., Yang H., Balish A.L., Shepard S.S., Carney P.J., Chang J.C., Davis C.T., Donis R.O., Villanueva J.M., Klimov A.I., Stevens J. Structural and antigenic variation among diverse clade 2 H5N1 viruses. PLoS One. 2013;8:e75209. doi: 10.1371/journal.pone.0075209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R.W., Bailey K.W., Bemis P.A., Wong M.H., Eisenberg E.J., Huffman J.H. Influence of treatment schedule and viral challenge dose on the in vivo influenza virus-inhibitory effects of the orally administered neuraminidase inhibitor GS 4104. Antivir. Chem. Chemother. 1999;10:187–193. doi: 10.1177/095632029901000403. [DOI] [PubMed] [Google Scholar]
- Sirinonthanawech N., Uiprasertkul M., Suptawiwat O., Auewarakul P. Viral load of the highly pathogenic avian influenza H5N1 virus in infected human tissues. J. Med. Virol. 2011;83:1418–1423. doi: 10.1002/jmv.22146. [DOI] [PubMed] [Google Scholar]
- Sun S., Zhao G., Liu C., Fan W., Zhou X., Zeng L., Guo Y., Kou Z., Yu H., Li J., Wang R., Li Y., Schneider C., Habel M., Riedemann N.C., Du L., Jiang S., Guo R., Zhou Y. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin. Infect. Dis. 2015;60:586–595. doi: 10.1093/cid/ciu887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Zhao G., Liu C., Wu X., Guo Y., Yu H., Song H., Du L., Jiang S., Guo R., Tomlinson S., Zhou Y. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am. J. Respir. Cell Mol. Biol. 2013;49:221–230. doi: 10.1165/rcmb.2012-0428OC. [DOI] [PubMed] [Google Scholar]
- Tate R.M., Repine J.E. Neutrophils and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Thiele J.R., Habersberger J., Braig D., Schmidt Y., Goerendt K., Maurer V., Bannasch H., Scheichl A., Woollard K.J., Von Dobschutz E., Kolodgie F., Virmani R., Stark G.B., Peter K., Eisenhardt S.U. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130:35–50. doi: 10.1161/CIRCULATIONAHA.113.007124. [DOI] [PubMed] [Google Scholar]
- Thiele J.R., Zeller J., Bannasch H., Stark G.B., Peter K., Eisenhardt S.U. Targeting C-reactive protein in inflammatory disease by preventing conformational changes. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/372432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Us D. Cytokine storm in avian influenza. Mikrobiyol. Bul. 2008;42:365–380. [PubMed] [Google Scholar]
- Wang R., Xiao H., Guo R., Li Y., Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg. Microbes. Infect. 2015;4:e28. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Z.X., Jones J.E., Anderson G.P., Gualano R.C. Oseltamivir treatment of mice before or after mild influenza infection reduced cellular and cytokine inflammation in the lung. Influenza Other Respir. Viruses. 2011;5:343–350. doi: 10.1111/j.1750-2659.2011.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Shi D., Fang D., Guo F., Guo J., Huang F., Chen Y., Lv L., Li L. A new perspective on C-reactive protein in H7N9 infections. Int. J. Infect. Dis. 2016;44:31–36. doi: 10.1016/j.ijid.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Yen H.L., Monto A.S., Webster R.G., Govorkova E.A. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis. 2005;192:665–672. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]
- Yu H., Gao Z., Feng Z., Shu Y., Xiang N., Zhou L., Huai Y., Feng L., Peng Z., Li Z., Xu C., Li J., Hu C., Li Q., Xu X., Liu X., Liu Z., Xu L., Chen Y., Luo H., Wei L., Zhang X., Xin J., Guo J., Wang Q., Yuan Z., Zhou L., Zhang K., Zhang W., Yang J., Zhong X., Xia S., Li L., Cheng J., Ma E., He P., Lee S.S., Wang Y., Uyeki T.M., Yang W. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One. 2008;3:e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Moore T.A., Newstead M.W., Deng J.C., Kunkel S.L., Luster A.D., Standiford T.J. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect. Immun. 2005;73:8226–8236. doi: 10.1128/IAI.73.12.8226-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.P., Zeng Y.M., Lin Z.S., Chen W., Liang J.S., Zhang H., Huang W.R. Clinical characteristics and therapeutic experience of case of severe highly pathogenic A/H5N1 avian influenza with bronchopleural fistula. Zhonghua Jie He He Hu Xi Za Zhi. 2009;32:356–359. [PubMed] [Google Scholar]
- Zhou J., Wang D., Gao R., Zhao B., Song J., Qi X., Zhang Y., Shi Y., Yang L., Zhu W., Bai T., Qin K., Lan Y., Zou S., Guo J., Dong J., Dong L., Zhang Y., Wei H., Li X., Lu J., Liu L., Zhao X., Li X., Huang W., Wen L., Bo H., Xin L., Chen Y., Xu C., Pei Y., Yang Y., Zhang X., Wang S., Feng Z., Han J., Yang W., Gao G.F., Wu G., Li D., Wang Y., Shu Y. Biological features of novel avian influenza a (H7N9) virus. Nature. 2013;499:500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material