Abstract
Background
The utility of intramuscular (IM) oxytocin for the prevention of postpartum hemorrhage in resource-poor settings is limited by the requirement for temperature-controlled storage and skilled staff to administer the injection. We evaluated the safety, tolerability and pharmacokinetics (PK) of a heat-stable, inhaled (IH) oxytocin formulation.
Methods
This phase 1, randomized, single-center, single-blind, dose-escalation, fixed-sequence study (NCT02542813) was conducted in healthy, premenopausal, non-pregnant, non-lactating women aged 18–45 years. Subjects initially received IM oxytocin 10 international units (IU) on day 1, IH placebo on day 2, and IH oxytocin 50 μg on day 3. Subjects were then randomized 4:1 using validated GSK internal software to IH placebo or ascending doses of IH oxytocin (200, 400, 600 μg). PK was assessed by comparing systemic exposure (maximum observed plasma concentration, area under the concentration-time curve, and plasma concentrations at 10 and 30 min post dose) for IH versus IM oxytocin. Adverse events (AEs), spirometry, laboratory tests, vital signs, electrocardiograms, physical examinations, and cardiac telemetry were assessed.
Findings
Subjects were recruited between September 14, 2015 and October 12, 2015. Of the 16 subjects randomized following initial dosing, 15 (IH placebo n = 3; IH oxytocin n = 12) completed the study. IH (all doses) and IM oxytocin PK profiles were comparable in shape. However, systemic exposure with IH oxytocin 400 μg most closely matched IM oxytocin 10 IU. Systemic exposure was approximately dose proportional for IH oxytocin. No serious AEs were reported. No clinically significant findings were observed for any safety parameters.
Interpretation
These data suggest that similar oxytocin systemic exposure can be achieved with IM and IH administration routes, and no safety concerns were identified with either route. The inhalation route may offer the opportunity to increase access to oxytocin for women giving birth in resource-poor settings.
Keywords: Inhaled oxytocin, Placebo, Postpartum hemorrhage, Phase 1
Highlights
-
•
First-in-human study of a heat-stable, dry powder oxytocin formulation for oral inhalation using a simple inhaler device
-
•
Data suggest that similar oxytocin systemic exposure can be achieved with intramuscular and inhaled administration routes.
-
•
This could provide an effective delivery system for prevention of postpartum hemorrhage in women in resource-poor settings.
Intramuscular or intravenous oxytocin is the gold standard preventative therapy for postpartum hemorrhage (PPH). However, the utility of intramuscular oxytocin in resource-poor settings is limited by the requirement for temperature-controlled storage and skilled staff to administer the injection. The Innovation Countdown 2030 Initiative has estimated that the introduction of alternative, heat-stable formulations of oxytocin could prevent 146,000 maternal deaths over a period of 8 years. This study provides encouraging preliminary evidence that inhaled oxytocin can be delivered using a heat-stable dried powder for inhalation with no safety concerns. Establishing the clinical utility of this potential medicine will require further studies.
1. Introduction
Oxytocin is a neuropeptide involved in the onset and progression of labor (Arrowsmith and Wray, 2014, Blanks and Thornton, 2003). Oxytocin acts as a potent endogenous uterotonic (Arrowsmith and Wray, 2014), causing an increase in uterine contraction intensity and frequency, which facilitates labor and delivery (Blanks and Thornton, 2003). Following delivery, the mother is, however, at risk of postpartum hemorrhage (PPH), defined as blood loss of ≥ 500 mL within 24 h after birth (World Health Organization, 2012), most commonly due to uterine atony (Weeks, 2015). In the 25 years between 1990 and 2015, an estimated 10.7 million women worldwide died in pregnancy and childbirth (World Health Organization, 2015a). Of the estimated 303,000 women who died during and following pregnancy and childbirth in 2015, 99% were from low-income countries, with approximately two-thirds of deaths occurring in sub-Saharan Africa and one-third in Asia (World Health Organization, 2015a). PPH is estimated as the single largest contributor to maternal mortality, responsible for 19.7% of maternal deaths worldwide (Say et al., 2014). Many of these deaths could be prevented by effective access to treatments to reduce PPH (World Health Organization, 2012). Active management of the third stage of labor, including prophylactic administration of injectable oxytocin immediately after delivery, has been shown to significantly reduce primary blood loss ≥ 500 mL compared with expectant management (Begley et al., 2015).
Intramuscular (IM) oxytocin (10 international units [IU]) was identified as one of 13 life-saving commodities for women and children in a 2012 United Nations (UN) Commission Report (United Nations, 2012), and was included in the World Health Organization (WHO) model list of essential medicines (World Health Organization, 2015b). Oxytocin is supplied as an aqueous solution in an ampoule and requires cold-chain storage, sterile needles with sharps disposal, and trained healthcare professionals for administration. This significantly limits access in resource-poor settings, where many women cannot attend medical facilities, their birth attendants are not allowed to administer injections, or the oxytocin cannot be refrigerated, leading to use of material of degraded quality. A review of studies assessing the quality of oxytocin ampoules for injection in low- and middle-income countries found that 58% and 22% of ampoules collected in Africa and Asia, respectively, contained less than the specified content of oxytocin, according to pharmacopoeial limits (Torloni et al., 2016).
Inhaled (IH) delivery of heat-stable oxytocin could offer a practical means to deliver this medicine without the need for cold-chain storage and provide access in those resource-poor settings of greatest unmet need. The Innovation Countdown 2030 Initiative has estimated that the introduction of non-injectable, heat-stable formulations of oxytocin could prevent 146,000 maternal deaths over a period of 8 years (Innovation Countdown 2030, 2015).
We have developed a heat-stable dry powder formulation of oxytocin for inhalation and sought to evaluate the safety, tolerability and pharmacokinetics (PK) in this study. Subjects also received IM oxytocin 10 IU for PK comparison.
2. Materials and Methods
2.1. Study Design
This phase 1, randomized, single-center, single-blind, ascending dose-escalation, fixed-sequence study with IH oxytocin (NCT02542813) was conducted at the GSK Clinical Unit Cambridge, Addenbrooke's Centre for Clinical Investigation (Addenbrooke's Hospital, UK). Ethical approval for the study was obtained from the Office for Research Ethics Committees Northern Ireland. The study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice, all applicable subject privacy requirements, and the ethical principles outlined in the Declaration of Helsinki 2013. The full protocol can be accessed at https://gsk-clinicalstudyregister.com/search/ps/1/?study_ids=201558.
2.2. Subjects
Subjects eligible for inclusion in the study were healthy, premenopausal, non-pregnant, non-lactating women aged 18–45 years inclusive, with a body mass index of 18–30 kg/m2, who were taking an estrogen-containing oral contraceptive pill both prior to and for the duration of the study. Key exclusion criteria included a history of chronic lung conditions, respiratory tract infection within 4 weeks of screening, and history of smoking within 1 year of screening. A full list of exclusion criteria are included in Supplementary Appendix A. Potential subjects were identified from the volunteer panel of the GSK Unit and by advertising. Written informed consent was obtained prior to the performance of any study-specific procedures.
2.3. Randomization and Masking
Suitable subjects were enrolled for the study by the principal investigator or designee based on the inclusion and exclusion criteria. Subjects were randomized 1:4 to receive either IH placebo or ascending doses of IH oxytocin (50, 200, 400, 600 μg). Subjects were assigned to study treatment in accordance with the randomization schedule generated by GSK prior to the start of the study using validated internal software. Dosing with IH placebo and IH oxytocin was single blind (blinded to subjects only) for all subjects throughout the study. The excipients in the placebo formulation had not, to our knowledge, previously been included in an inhaled product in this combination. Although the respiratory risk was deemed to be low, the investigator remained unblinded to allow differentiation of adverse events related to inhaled placebo from inhaled oxytocin in order to monitor the tolerability of the study medication and the safety of the subjects. Blinding for subjects was maintained by the use of a matched inhaler device and capsule presentation for both IH placebo and IH oxytocin. The timings of all procedures after IH placebo and IH oxytocin were identical.
2.4. Procedures
2.4.1. Interventions
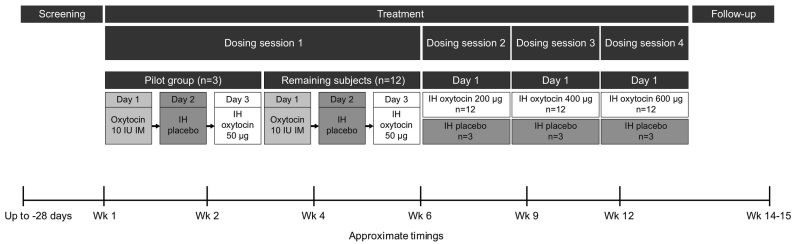
In dosing session one, all subjects received oxytocin 10 IU (17 μg) (EVER Neuro Pharma GmbH, Unterach, Austria) by IM injection in the thigh on day 1. The excipients in the dry powder IH formulation capsules have been used previously in other IH products, but not in the combination used in this study. Therefore, the safety and tolerability of the excipients alone (IH placebo) were tested in all subjects on day 2. IH oxytocin was then administered to all subjects at a dose of 50 μg on day 3. All IH treatment capsules were administered by oral inhalation using a Modified Air Inlet ROTAHALER™ Dry Powder Inhaler. Study investigators were trained to use the inhaler, and trained subjects prior to use. The first dosing session employed a sentinel cohort approach, in which a pilot group of three subjects was evaluated first, before the remaining 12 subjects were dosed in a staggered fashion, with no more than four subjects dosed per day.
The doses of IH oxytocin selected for investigation were expected to encompass the likely IH therapeutic dose (ie, with a similar systemic exposure to IM oxytocin 10 IU). Dose selection considerations encompassed the predicted amount of drug delivered to the lungs (% nominal) for the current dry powder formulation delivered via the ROTAHALER™ inhaler device, and assumptions around the likely range of relative bioavailability values of IH oxytocin compared with recently reported exposure values following IM administration of oxytocin 10 IU to healthy subjects. Lung bioavailability was assumed to be between 10% and 150% of IM availability, with the upper bound of the range reflecting lung bioavailability reported for other low molecular weight proteins, such as leuprolide (Adjei and Garren, 1990) and insulin (Patton et al., 2004).
In dosing sessions two, three, and four, subjects randomized to placebo received IH placebo (excipients only), while subjects randomized to IH oxytocin received escalating doses of IH oxytocin: 200 μg, 400 μg, and 600 μg (administered as 200 μg and 400 μg capsules using one ROTAHALER™ inhaler device per capsule). The decision to proceed to the next increased dose level was made by the dose-escalation committee based on the available safety and tolerability data from not less than nine subjects receiving oxytocin at the prior dose level, as well as any available PK data from the previous dose levels. A follow-up visit was conducted 7–21 days after the last IH oxytocin or IH placebo administration. An overview of the study design is shown in Fig. 1.
Fig. 1.
Study design.
IH = inhaled; IM = intramuscular; IU = international units.
2.4.2. Assessments and Analyses
2.4.2.1. Safety Assessments
Adverse events (AEs) and serious AEs (SAEs) were monitored throughout the study. Spirometry, clinical laboratory tests, vital signs, electrocardiograms (ECGs) (PR, QRS, QT, corrected QT [QTc] intervals), physical examinations, and cardiac telemetry were assessed at specified times after dosing.
2.4.2.2. PK Assessments
Blood samples were drawn pre-dose and 3, 5, 10, 20, 30, and 45 min, 1, 1.5, 2, 2.5, 3, 4, and 8 h after dosing. An additional 24-h post-dose blood sample was drawn in dosing session one for the pilot group and for the first three subjects at each new dose level in dosing sessions two, three, and four. Plasma samples were taken via an indwelling cannula (or by direct venepuncture), collected into tri-potassium ethylenediaminetetraacetic acid (K3 EDTA) tubes and placed immediately on ice prior to centrifugation. Supernatant plasma was then prepared and analyzed for oxytocin by inVentiv Health Clinique Inc. (Québec, Canada) using a validated analytical method based on automated solid phase extraction, followed by liquid chromatography/mass spectrometry/mass spectrometry analysis. The range of quantification for oxytocin was 2–500 pg/mL using a 350 μL aliquot of human plasma.
The PK parameters determined for both IM oxytocin and IH oxytocin were maximum observed plasma concentration (Cmax), time to Cmax (tmax), apparent terminal phase heat stable, inhaled (IH) oxytocin formulation area under the concentration-time curve from time zero (pre-dose) to last time of quantifiable concentration (AUC[0–t]), area under the concentration-time curve from zero (pre-dose) to 3 h post dose (AUC[0 − 3h]), observed plasma concentration at 10 min (nominal time) post-dose (Cp10), and 30 min (nominal time) post-dose (Cp30).
2.5. Outcomes
The objectives and endpoints of the study were to evaluate the safety and tolerability of IH oxytocin by monitoring AEs and changes over time in hematology, clinical chemistry, urinalysis, vital signs, spirometry, cardiac telemetry, and 12-lead ECG parameters from pre-dose values; evaluate the safety and tolerability of the five excipients in the placebo by monitoring for respiratory AEs by spirometry, including forced expiratory volume in 1 s (FEV1), and pulse oximetry; and to establish the PK characteristics of oxytocin by assessing the plasma concentration profile (Cmax, tmax, AUC, and t1/2) for IH and IM oxytocin at the specified doses.
IM oxytocin is reported to have a rapid onset (3–5 min) and limited duration (2 − 3h) of uterine activity (Pitocin prescribing information). To assess whether therapeutic concentrations of oxytocin were achieved rapidly and maintained for a sufficient duration post-inhalation compared with the IM route of administration, Cp10 and Cp30 [representing the median and upper bound of the range of tmax following IM administration in a previous study with IM oxytocin (Wong et al., 2016)] were estimated, representing the onset of uterine activity, along with systemic exposure over the 0–3-h post-dose period (AUC[0 − 3h]), representing the duration of uterine activity.
2.6. Statistical Analysis
The sample size was based on feasibility. The study populations assessed included the all subjects/safety population, which included all subjects who received at least one dose of study medication; and the PK population, which included all subjects who received at least one dose of study medication and had at least one PK sample analyzed.
For PK comparisons (IH vs IM), point estimates and two-sided 90% confidence intervals (CI) were computed using a mixed-effect model. Loge-transformed AUC[0–t], AUC[0–3h], Cp10, Cp30, and Cmax were analyzed with a fixed-effect term for treatment and a random-effect term for subject. Point estimates and CIs were exponentially back-transformed to obtain adjusted (least squares) geometric means, point estimates and associated 90% CI for the ratios. Summary statistics were carried out using SAS v9.3 (Cary, CA, USA). Plasma oxytocin concentration-time data were analyzed by non-compartmental methods with WinNonlin v6.3 (Pharsight Corporation, St. Louis, MO, USA). Safety data were summarized descriptively.
3. Results
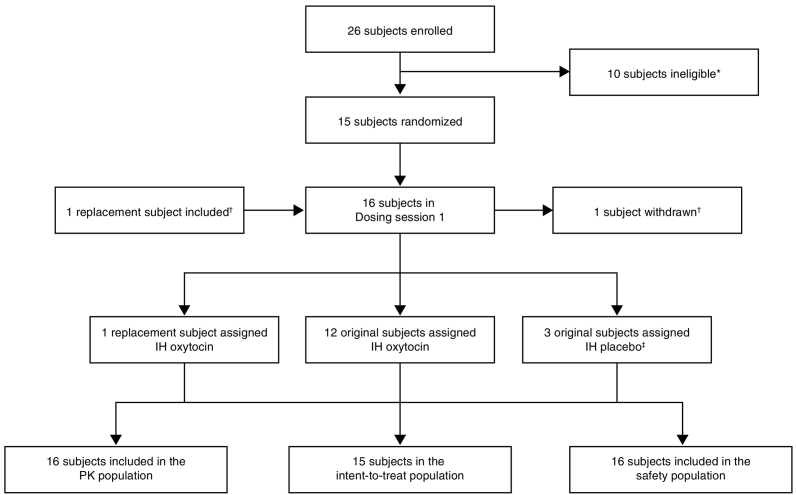
The study was conducted between September 14, 2015 (first subject first visit) and December 16, 2015 (last subject last visit). Subjects were recruited between September 14, 2015 and October 12, 2015. A total of 26 subjects were enrolled, of whom 16 received study treatment and 15 completed the study (trial profile Fig. 2). In the pilot group, two subjects were randomized to receive IH oxytocin and one subject to IH placebo. Among the other subjects, ten subjects were randomized to receive IH oxytocin and two subjects to IH placebo. One subject was withdrawn prior to dosing, at the investigator's discretion, due to inability to cannulate. This subject was replaced and assigned the same randomization sequence. All 16 subjects received IM oxytocin 10 IU and were included in the safety and PK populations.
Fig. 2.
Trial profile.
*Ineligible subjects: did not meet inclusion/met exclusion criteria (n = 5); subject withdrew consent (n = 2); reserve, not used (n = 2); investigator discretion (n = 1). †One subject was withdrawn at the investigator's discretion due to inability to cannulate. She received IM oxytocin only. This subject was replaced and assigned the same randomization sequence, thus, a total of 16 subjects were randomized; ‡subjects had received IM oxytocin and IH oxytocin (50 μg) in the initial dosing session. IH = inhaled; PK = pharmacokinetics.
Baseline demographics and characteristics are shown in Table 1. The mean (range) age of the study population was 28.5 (23–35) years, and all subjects were of White/Caucasian/European heritage.
Table 1.
Baseline demographics and characteristics.
| Characteristic | Total population (N = 16) |
|---|---|
| Age (years), mean (range) | 28.5 (25–35) |
| Sex, female, n (%) | 16 (100) |
| BMI (kg/m2), mean (range) | 22.8 (18.3–28) |
| Height (cm), mean (SD) | 166.5 (5.7) |
| Weight (kg), mean (SD) | 63.3 (8.7) |
| Race, White/Caucasian/European Heritage, n (%) | 16 (100) |
BMI = body mass index; SD = standard deviation.
A total of 13 (81%) subjects experienced at least one AE during the study. AEs occurred in four (25%) subjects who received oxytocin IM 10 IU; five (33%) subjects who received IH oxytocin 50 μg; three (25%) subjects who received IH oxytocin 200 μg; five (42%) subjects who received IH oxytocin 400 μg; six (50%) subjects who received IH oxytocin 600 μg; and five (33%) subjects who received IH placebo. Overall, 45 AEs were reported, of which seven were moderate in intensity, and none were severe. The most common AE was headache, reported in six (38%) subjects. Nine AEs reported in five subjects were determined by the investigator to be drug-related. Of these drug-related AEs, headache and cough were the most common drug-related AEs (three subjects each, 19%). Drug-related AEs occurred in one (6%) subject who received oxytocin IM 10 IU (headache); three (25%) subjects who received IH oxytocin 400 μg (cough and headache in two subjects [17%] each); three (25%) subjects who received IH oxytocin 600 μg (cough, headache and flushing in one [6%] subject each); and one (6%) subject who received IH placebo (cough). No trends or clinically important differences in AEs were noted between groups or with escalating doses. There were no discontinuations or withdrawals from the study due to AEs, and no deaths or SAEs were reported (Table 2). No trends or clinically significant abnormal spirometry, laboratory, vital signs, ECG, or telemetry findings were reported or noted during review of the data.
Table 2.
Adverse event summary (safety population).
| IM oxytocin 10 IU (17 μg) |
IH oxytocin 50 μg |
IH oxytocin 200 μg |
IH oxytocin 400 μg |
IH oxytocin 600 μg |
IH placebo |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of subjects N = 16 |
Number of AEs | Number of subjects N = 15 |
Number of AEs | Number of subjects N = 12 |
Number of AEs | Number of subjects N = 12 |
Number of AEs |
Number of subjects N = 12 |
Number of AEs | Number of subjects N = 15 |
Number of AEs | Number of subjects N = 16 |
Number of AEs | |
| All AEs | ||||||||||||||
| Any | 4 (25%) | 5 (33%) | 3 (25%) | 5 (42%) | 6 (50%) | 5 (33%) | 13 (81%) | 45 | ||||||
| Drug-related | 1 (6%) | 0 | 0 | 3 (25%) | 3 (25%) | 1 (7%) | 5 (31%) | 9 | ||||||
| Cough | 0 | 0 | 0 | 2 (17%) | 2 | 1 (8%) | 1 | 1 (7%) | 1 | 3 (19%) | 3 | |||
| Headache | 1 (6%) | 1 | 0 | 0 | 2 (17%) | 2 | 1 (8%) | 1 | 0 | 3 (19%) | 3 | |||
| Flushing | 0 | 0 | 0 | 0 | 1 (8%) | 1 | 0 | 1 (6%) | 1 | |||||
| Led to discontinuation of study drug or withdrawal from the study | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAEs | ||||||||||||||
| Non-fatal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AE = adverse event; IH = inhaled; IM = intramuscular; IU = international units; SAE = serious adverse event.
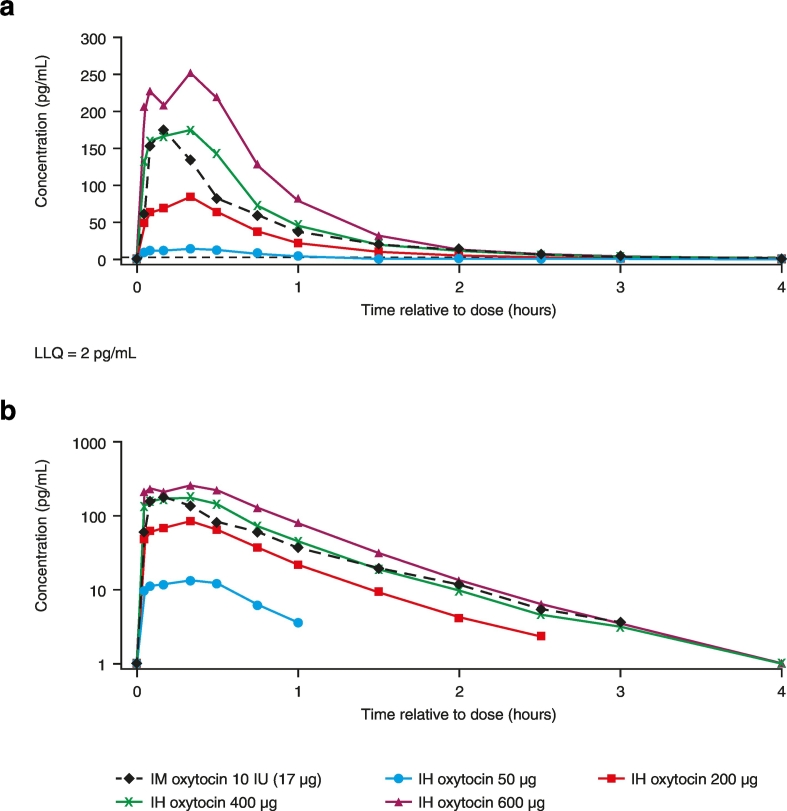
The shape of the observed PK concentration-time profile following IH administration was consistent with the IM PK profile (Fig. 3). Plasma oxytocin was detectable in all subjects at the first sampling time (3 min post-dose) and remained quantifiable in 5/16 subjects up to 4 h following IM administration and in at least half of subjects for 1 h with the 50 μg IH dose and 2.5 to 4 h with the higher IH doses (200 μg, 400 μg, and 600 μg). Following each route of administration (IH or IM) there was rapid absorption of oxytocin into plasma (tmax ~ 10 min following IM administration and tmax ~ 8–20 min following IH oxytocin; Table 3). Thereafter, plasma oxytocin concentrations declined rapidly, irrespective of administration route or dose, with the apparent terminal elimination half-life comparable for IH versus IM oxytocin (Table 3). Following IH oxytocin, Cmax and AUC(0–t) increased in an approximately linear manner between 200 μg and 600 μg (Table 4).
Fig. 3.
Median oxytocin plasma concentration-time profile on A) non-logarithmic and B) logarithmic scales (PK population).
IH = inhaled; IM = intramuscular; IU = international units; LLQ = lower limit of quantification; PK = pharmacokinetic.
Table 3.
Summary of plasma oxytocin tmax and t1/2 (PK population).
| Parameter | Number of subjects (n/N) | Oxytocin dose and formulation | |
|---|---|---|---|
| Tmax (h), median (min, max) | 16/16 | 10 IU IM | 0.17 (0.09, 0.34) |
| 15/15 | 50 μg IH | 0.32 (0.06, 0.34) | |
| 12/12 | 200 μg IH | 0.33 (0.05, 0.50) | |
| 12/12 | 400 μg IH | 0.33 (0.05, 0.34) | |
| 12/12 | 600 μg IH | 0.14 (0.05, 0.34) | |
| T1/2 (h), geometric mean (95% CI) | 13/16 | 10 IU IM | 0.64 (0.55, 0.74) |
| 8/15 | 50 μg IH | 0.36 (0.30, 0.44) | |
| 12/12 | 200 μg IH | 0.45 (0.38, 0.54) | |
| 9/12 | 400 μg IH | 0.55 (0.43, 0.70) | |
| 12/12 | 600 μg IH | 0.60 (0.49, 0.74) |
CI = confidence interval; IH = inhaled; IM = intramuscular; IU = international units; PK = pharmacokinetics; t1/2 = terminal phase half-life; tmax = time to maximum observed plasma concentration.
Table 4.
Statistical analysis of oxytocin PK parameters (PK population).
| Parameter | Comparison (IH oxytocin vs IM oxytocin 10 IU) | Adjusted geometric mean |
Adjusted geometric mean ratio for IH vs IM oxytocin (90% CI) | |
|---|---|---|---|---|
| IH oxytocin | IM oxytocin | |||
| Cmax (pg/mL)a | 50 μg IH | 15.3 | 189.96 | 0.08 (0.06, 0.10) |
| 200 μg IH | 103.03 | 0.54 (0.41, 0.71) | ||
| 400 μg IH | 255.95 | 1.35 (1.03, 1.77) | ||
| 600 μg IH | 365.42 | 1.92 (1.47, 2.52) | ||
| AUC(0–3 h) (pg·h/mL)a | 50 μg IH | 14.75 | 127.61 | 0.12 (0.10, 0.14) |
| 200 μg IH | 69.78 | 0.55 (0.46, 0.65) | ||
| 400 μg IH | 161.67 | 1.27 (1.07, 1.50) | ||
| 600 μg IH | 234.91 | 1.84 (1.56, 2.18) | ||
| AUC(0–t) (pg·h/mL)a |
50 μg IH | 9.16 | 119.83 | 0.08 (0.06, 0.10) |
| 200 μg IH | 65.02 | 0.54 (0.42, 0.70) | ||
| 400 μg IH | 153.83 | 1.28 (1.00, 1.66) | ||
| 600 μg IH | 224.34 | 1.87 (1.45, 2.41) | ||
| Cp10 (pg/mL)a | 50 μg IH | 12.01 | 171.24 | 0.07 (0.05, 0.09) |
| 200 μg IH | 72.71 | 0.42 (0.33, 0.55) | ||
| 400 μg IH | 188.13 | 1.10 (0.85, 1.43) | ||
| 600 μg IH | 258.31 | 1.51 (1.16, 1.96) | ||
| Cp30 (pg/mL)a | 50 μg IH | 9.58 | 82.16 | 0.12 (0.09, 0.15) |
| 200 μg IH | 58.63 | 0.71 (0.56, 0.91) | ||
| 400 μg IH | 135.57 | 1.65 (1.30, 2.09) | ||
| 600 μg IH | 190.96 | 2.32 (1.83, 2.95) | ||
%CVb = inter-subject variability; AUC(0–3 h) = area under the concentration-time curve from zero (pre-dose) to 3 h; AUC(0–t) = area under the concentration-time curve from time zero (pre-dose) to last time of quantifiable concentration; CI = confidence interval; Cmax = maximum observed plasma concentration; Cp10 = quantified concentration at 10 min (nominal time) post-dose; Cp30 = quantified concentration at 30 min (nominal time) post-dose; IH = inhaled; IM = intramuscular; IU = international units; PK = pharmacokinetics.
%CVb for IM oxytocin and IH oxytocin, respectively: Cmax: 47% and 61–98%; AUC(0–3 h): 27% and 41–61%; AUC(0–t): 41% and 43–72%; Cp10: 45% and 64–92%; Cp30: 45% and 42–58%.
PK exposure (as defined by Cp10 and Cp30, AUC(0–3 h) and Cmax) was higher with IH oxytocin 400 μg and 600 μg and much lower with 50 μg and 200 μg IH compared with IM oxytocin. Oxytocin exposure with IH oxytocin 400 μg most closely matched that with IM oxytocin 10 IU, based on the geometric mean ratio for IH versus IM for the PK parameters Cp10, Cp30, and AUC(0–3 h) (Table 4).
4. Discussion
PPH is a major cause of maternal death with 99% of these deaths occurring in resource-poor settings (World Health Organization, 2015a). Oxytocin is considered the gold standard preventative treatment for PPH, but the requirement for refrigerated storage and needle-based administration for the currently available IM formulations substantially limits its utility in these settings. Other treatments, such as ergometrine or misoprostol, are used but are not considered to be as effective as oxytocin and have contraindications to their use (World Health Organization, 2012). The WHO states that recommendations concerning alternative uterotonics should not detract from the objective of increasing access to oxytocin (World Health Organization, 2012) and in response to this, several initiatives have been launched. The Uniject single-use prefilled syringe, which does not require additional sterile equipment, eliminates the need for dosage estimation and can be used by unskilled personnel (Diop et al., 2016, Tsu et al., 2003, Tsu et al., 2009, Althabe et al., 2011, Strand et al., 2005, Stanton et al., 2013). However, the Uniject devices still require maintenance of cold-chain supply, and a study in rural Ghana highlighted that the single-use prefilled syringe was highly sensitive to small changes in ambient temperature and could expire in as few as 6 days (Mullany et al., 2014). The preferred approach would be the development of a heat-stable, non-parenteral oxytocin formulation as identified by the UN Commission on Life-saving Commodities for Women and Children (United Nations, 2012). A heat-stable oxytocin analog, carbetocin is in development, but it will still require parenteral administration (Boucher et al., 2004). Non-parenteral routes of administration include sublingual, IH, and intranasal administration. A PK study carried out in male volunteers showed that sublingual oxytocin had poor bioavailability, a long lag time and long absorption half-life and was therefore not a reliable formulation for accurate dosing for immediate prevention of PPH (De Groot et al., 1995). We selected the IH route, as this has proven a reliable route for other potent drugs and offers the potential for rapid absorption (Cazzola et al., 2013). Additionally, the formulation of peptides and proteins in a spray-dried matrix for IH delivery is known to confer enhanced thermostability compared with aqueous solutions (Vehring, 2008). This approach has been applied to the formulations used in this study: robust heat stability was demonstrated at 30 °C and at 40 °C during product development (Supplementary Fig. 1) and recent data support a minimum 30 month shelf-life at 25 °C (approved by the UK Medicines & Healthcare products Regulatory Agency for clinical supply). An in vitro preclinical study further demonstrated that dry powder formulations of oxytocin suitable for oral inhalation can be prepared and are stable after extended storage under tropical and subtropical conditions (Fabio et al., 2015). The potential for an IH powder form of oxytocin was also demonstrated in an in vivo preclinical study, which showed that administration of an ultra-fine oxytocin powder to the airways of postpartum sheep led to rapid absorption and comparable uterine electromyographic activity to that observed with IM administration (Prankerd et al., 2013).
This study reports successful administration of oxytocin by the IH route in humans. The five non-pharmacologically active components contained in the IH oxytocin formulation are approved individually for use in other IH products. However, they had never been used previously in this particular combination. Administration of the excipients alone was not associated with features of irritancy (eg, coughing and bronchospasm) in this study. Indeed, the IH route of delivery was well tolerated in this small-scale study.
Since IM oxytocin reaches its site of action, the uterus, via the plasma it is proposed that demonstrating a comparable plasma drug profile following IH and IM routes of administration allows for extrapolation from the extensive clinical safety and efficacy data for the approved IM oxytocin product for the prevention of PPH to the IH product. Overall, the shape of the plasma PK profile following IH administration was similar to that following IM administration. Both administration routes demonstrated rapid absorption of oxytocin into plasma (median tmax 8–20 min), followed by rapid elimination, with an apparent t1/2 consistent across administration routes. Oxytocin exposure with IH oxytocin was approximately linear over the 200–600 μg dose range, while dose normalized exposure at 50 μg was lower. Lower exposure (AUC) following 50 μg may in part reflect censoring of the PK profile; since concentrations of oxytocin fell below the lower limit of quantification in at least half the subjects by 1 h post-dose. The dose range of IH oxytocin assessed in this study was predicted to cover the therapeutic range and the 400 μg dose of IH oxytocin was found to result in systemic exposure most similar to that of IM oxytocin 10 IU; with the rate and extent of absorption of oxytocin into plasma most consistent with that observed following IM oxytocin 10 IU (as reflected by the geometric mean ratio for IH versus IM for the PK parameters Cp10, Cp30, and AUC[0–3h]). However, between subject variability tended to be higher following IH compared with IM dosing.
This study provides important preliminary evidence in humans that a heat-stable, dry powder IH formulation has the potential to deliver a systemic profile of oxytocin comparable with parenteral (IM) administration, although important questions remain. This study was conducted in healthy non-pregnant women. The physiological changes associated with pregnancy, including higher levels of circulating oxytocin, increased renal/hepatic blood flow, increased blood/plasma volume and cardiac output, increased total body water, and decreased plasma proteins have the potential to alter the PK of oxytocin (Sheffield et al., 2014, Prevost et al., 2014). However, unless affecting lung absorption, these factors would be expected to affect IH and IM administration equally. To determine whether there are differences in PK between pregnant and non-pregnant women, investigations are ongoing to study women in third stage of labor. We conducted an enabling study in women in the third stage of labor and observed that their inspiratory profile using the ROTAHALER™ inhaler device was not materially different from non-pregnant volunteers (Wong et al., 2016). However, any effect of altered inspiratory profile during labor on the PK of IH oxytocin will need to be determined in future investigations in women in the third stage of labor. In this study IH oxytocin was not associated with evidence of respiratory tract irritancy, but the information is preliminary; an evaluation of tolerability in smokers and people with asthma would form part of future investigations.
The device for delivery was selected on the basis of its simplicity. The capsule is pushed into the device, the device twisted once to break open the capsule and the powder IH. There has been over 20 years of experience with this type of device, which has optimized and simplified the design to support practical use. Effective delivery in the field will depend on appropriate educational materials and training; in use studies are planned as part of the development program.
This study provides proof-of-concept that an IH oxytocin formulation resistant to thermo-degradation can achieve systemic exposure comparable with IM delivery. If confirmed in women in the third stage of labor with an optimized formulation and delivery device, this approach has the potential to address one of the most important unmet health needs in developing countries.
Role of the Funding Source
GSK, the funder of this study (NCT02542813), was involved in the study design, data collection, data analysis, data interpretation, and writing of the study report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit. The McCall MacBain Foundation and Grand Challenges Canada (funded by the Government of Canada) provided funding support to the project team at Monash University and had no role in the design, conduct, interpretation, or reporting of this study.
Acknowledgments
Conflicts of Interest
Disala Fernando, Sarah Siederer, Sunita Singh, Ian Schneider, Ashutosh Gupta, Marcy Powell and Duncan Richards are employees of GSK. Sarah Siederer, Sunita Singh, Ian Schneider, Ashutosh Gupta, Marcy Powell are shareholders of GSK. Susan Fowles was an employee and shareholder of GSK during the conduct of the study and during the initial stages of manuscript development. Peter Lambert has no conflicts of interest to declare. Michelle McIntosh has a patent application for a method and formulation for inhalation (WO2013/016754 A1 [PCT/AU2011001430]) pending.
Author Contributions
Disala Fernando, Sarah Siederer, Sunita Singh, and Marcy Powell contributed to the study concept and design, data acquisition, and analysis and interpretation. Ian Schneider contributed to study concept and design, and data acquisition. Ashutosh Gupta and Duncan Richards contributed to analysis and interpretation. Michelle McIntosh and Peter Lambert contributed to the study concept and design and interpretation. Susan Fowles contributed to study concept and design, and analysis and interpretation. All authors, apart from Susan Fowles, were involved in the preparation and review of the manuscript and approved the final version to be submitted. Posthumous authorship was granted for Susan Fowles as she was involved in the initial stages of manuscript development prior to her death in September 2016.
Acknowledgments
The authors would like to dedicate this manuscript to Susan Fowles, who died during the initial stages of manuscript development. This study 201558 (NCT02542813) was funded by GSK. The contributions of Michelle McIntosh and Peter Lambert were undertaken with the financial support of the McCall MacBain Foundation, Grand Challenges Canada and the Government of Canada (TTS 0609-05). The authors thank Simon Parry from the GSK drug analysis/DMPK group and Kimberley Hacquoil from the GSK clinical statistics group, and Sujith Madhaven, Swethajit Biswas, David Neil, and Subramanya Kumar for their contributions throughout the study. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors comments, grammatical editing and referencing) was provided by Elizabeth Hutchinson, PhD, CMPP, at Fishawack Indicia Ltd., UK, and was funded by GSK.
Footnotes
Funding: GSK (Study 201558/NCT02542813).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.07.020.
Contributor Information
Disala Fernando, Email: disala.x.fernando@gsk.com.
Sarah Siederer, Email: Sarah.K.Siederer@gsk.com.
Sunita Singh, Email: sunita.p.singh@gsk.com.
Ian Schneider, Email: ian.j.schneider@gsk.com.
Ashutosh Gupta, Email: drashutoshgupta@outlook.com.
Marcy Powell, Email: marcy.x.powell@gsk.com.
Duncan Richards, Email: Duncan.B.Richards@gsk.com.
Michelle P. McIntosh, Email: michelle.mcintosh@monash.edu.
Peter Lambert, Email: pete.lambert@monash.edu.
Appendix A. Supplementary data
Supplementary figure 1. Heat stability of oxytocin formulation at (a) 30 °C and (b) 40 °C during product development.
References
- Adjei A., Garren J. Pulmonary delivery of peptide drugs: effect of particle size on bioavailability of leuprolide acetate in healthy male volunteers. Pharm. Res. 1990;7:565–569. doi: 10.1023/a:1015853824722. [DOI] [PubMed] [Google Scholar]
- Althabe F., Mazzoni A., Cafferata M.L., Gibbons L., Karolinski A., Armbruster D., Buekens P., Belizan J.M., Oxytocin in Uniject Study, G Using Uniject to increase the use of prophylactic oxytocin for management of the third stage of labor in Latin America. Int. J. Gynaecol. Obstet. 2011;114:184–189. doi: 10.1016/j.ijgo.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Arrowsmith S., Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014;26:356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- Begley C.M., Gyte G.M., Devane D., Mcguire W., Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst. Rev. 2015;3 doi: 10.1002/14651858.CD007412.pub4. CD007412. [DOI] [PubMed] [Google Scholar]
- Blanks A.M., Thornton S. The role of oxytocin in parturition. BJOG. 2003;110(Suppl. 20):46–51. doi: 10.1016/s1470-0328(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Boucher M., Nimrod C.A., Tawagi G.F., Meeker T.A., Rennicks White R.E., Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery:a double-blind randomized trial. J. Obstet. Gynaecol. Can. 2004;26:481–488. doi: 10.1016/s1701-2163(16)30659-4. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Segreti A., Matera M.G. New developments in the combination treatment of COPD: focus on umeclidinium/vilanterol. Drug Des. Devel. Ther. 2013;7:1201–1208. doi: 10.2147/DDDT.S39449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot A.N., Vree T.B., Hekster Y.A., Pesman G.J., Sweep F.C., Van Dongen P.J., Van Roosmalen J. Bioavailability and pharmacokinetics of sublingual oxytocin in male volunteers. J. Pharm. Pharmacol. 1995;47:571–575. doi: 10.1111/j.2042-7158.1995.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Diop A., Daff B., Sow M., Blum J., Diagne M., Sloan N.L., Winikoff B. Oxytocin via Uniject (a prefilled single-use injection) versus oral misoprostol for prevention of postpartum haemorrhage at the community level: a cluster-randomised controlled trial. Lancet Glob. Health. 2016;4:e37–44. doi: 10.1016/S2214-109X(15)00219-3. [DOI] [PubMed] [Google Scholar]
- Fabio K., Curley K., Guarneri J., Adamo B., Laurenzi B., Grant M., Offord R., Kraft K., Leone-Bay A. Heat-stable dry powder oxytocin formulations for delivery by oral inhalation. AAPS PharmSciTech. 2015;16:1299–1306. doi: 10.1208/s12249-015-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innovation Countdown 2030 . 2015. The IC2030 Report. Reimagining Global Health. 30 High-impact Innovations to Save Lives. (Seattle, WA, USA) [Google Scholar]
- Mullany L.C., Newton S., Afari-Asiedu S., Adiibokah E., Agyemang C.T., Cofie P., Brooke S., Owusu-Agyei S., Stanton C.K. Cumulative effects of heat exposure and storage conditions of Oxytocin-in-Uniject in rural Ghana: implications for scale up. Glob. Health Sci. Pract. 2014;2:285–294. doi: 10.9745/GHSP-D-14-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J.S., Fishburn C.S., Weers J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- Pitocin Prescribing Information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/018261s031lbl.pdf. (accessed 27.07.17).
- Prankerd R.J., Nguyen T.H., Ibrahim J.P., Bischof R.J., Nassta G.C., Olerile L.D., Russell A.S., Meiser F., Parkington H.C., Coleman H.A., Morton D.A., Mcintosh M.P. Pulmonary delivery of an ultra-fine oxytocin dry powder formulation: potential for treatment of postpartum haemorrhage in developing countries. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost M., Zelkowitz P., Tulandi T., Hayton B., Feeley N., Carter C.S., Joseph L., Pournajafi-Nazarloo H., Yong Ping E., Abenhaim H., Gold I. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front. Public Health. 2014;2:1. doi: 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Say L., Chou D., Gemmill A., Tuncalp O., Moller A.B., Daniels J., Gulmezoglu A.M., Temmerman M., Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- Sheffield J.S., Siegel D., Mirochnick M., Heine R.P., Nguyen C., Bergman K.L., Savic R.M., Long J., Dooley K.E., Nesin M. Designing drug trials: considerations for pregnant women. Clin. Infect. Dis. 2014;59(Suppl. 7):S437–44. doi: 10.1093/cid/ciu709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C.K., Newton S., Mullany L.C., Cofie P., Tawiah Agyemang C., Adiibokah E., Amenga-Etego S., Darcy N., Khan S., Armbruster D., Gyapong J., Owusu-Agyei S. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand R.T., Da Silva F., Jangsten E., Bergstrom S. Postpartum hemorrhage: a prospective, comparative study in Angola using a new disposable device for oxytocin administration. Acta Obstet. Gynecol. Scand. 2005;84:260–265. doi: 10.1111/j.0001-6349.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Torloni M.R., Gomes Freitas C., Kartoglu U.H., Metin Gulmezoglu A., Widmer M. Quality of oxytocin available in low- and middle-income countries: a systematic review of the literature. BJOG. 2016;123:2076–2085. doi: 10.1111/1471-0528.13998. [DOI] [PubMed] [Google Scholar]
- Tsu V.D., Luu H.T., Mai T.T. Does a novel prefilled injection device make postpartum oxytocin easier to administer? Results from midwives in Vietnam. Midwifery. 2009;25:461–465. doi: 10.1016/j.midw.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Tsu V.D., Sutanto A., Vaidya K., Coffey P., Widjaya A. Oxytocin in prefilled Uniject injection devices for managing third-stage labor in Indonesia. Int. J. Gynaecol. Obstet. 2003;83:103–111. doi: 10.1016/s0020-7292(03)00186-3. [DOI] [PubMed] [Google Scholar]
- United Nations . 2012. UN Commission on Life-saving Commodities for Women and Children. Commissioners' Report. (September 2012. New York, NY, USA) [Google Scholar]
- Vehring R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008;25:999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122:202–210. doi: 10.1111/1471-0528.13098. [DOI] [PubMed] [Google Scholar]
- Wong D., Otiker T., Mahar K., Siederer S., Richards D. Evaluation of a translational oxytocin challenge paradigm to assess contractility in the non-pregnant uterus. Proc. Br. Pharmacol. Soc. 2016:2016. [Google Scholar]
- World Health Organization . 2012. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. (Geneva, Switzerland) [PubMed] [Google Scholar]
- World Health Organization . World Bank Group and the United Nations Population Division; Geneva, Switzerland: 2015. Trends in Maternal Mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA. [Google Scholar]
- World Health Organization . 19th edition. 2015. WHO Model List of Essential Medicines. (Geneva, Switzerland) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Heat stability of oxytocin formulation at (a) 30 °C and (b) 40 °C during product development.