Abstract
It is well known that Src tyrosine kinase, insulin-like growth factor 1 receptor (IGF-IR), and focal adhesion kinase (FAK) play important roles in prostate cancer (PrCa) development and progression. Src, which signals through FAK in response to integrin activation, has been implicated in many aspects of tumor biology, such as cell proliferation, metastasis and angiogenesis. Furthermore, Src signaling is known to crosstalk with IGF-IR, which also promotes tumor growth and angiogenesis. In this study, we demonstrate that c-Src, IGF-IR and FAK are packaged into exosomes (Exo), c-Src in particular being highly enriched in Exo from the androgen receptor (AR)-positive cell line C4-2B and AR-negative cell lines PC3 and DU145. Furthermore, we show that the active phosphorylated form of Src (SrcpY416) is co-expressed in Exo with phosphorylated FAK (FAKpY861), a known target site of Src, which enhances proliferation and migration. We further demonstrate for the first time exosomal enrichment of G-protein-coupled receptor kinase (GRK) 5 and GRK6, both of which regulate Src and IGF-IR signaling and have been implicated in cancer. Finally, SrcpY416 and c-Src are both expressed in Exo isolated from the plasma of prostate tumor-bearing TRAMP mice, and those same mice have higher levels of exosomal c-Src than their wild-type counterparts. In summary, we provide new evidence that active signaling molecules relevant to PrCa are enriched in Exo, and this suggests that the Src signaling network may provide useful biomarkers detectable by liquid biopsy, and may contribute to PrCa progression via Exo.
Keywords: Src, Exosomes, Vesicles, Prostate cancer, Signaling, G-protein-coupled receptor kinase, Focal Adhesion Kinase
Introduction
In the United States, 180,890 new prostate cancer (PrCa) cases are estimated for 2016, which is the highest for new diagnoses among different cancer types in American men [Siegel et al., 2016]. The aggressive metastatic disease has the poorest prognosis and, given the heterogeneous nature of these tumors, is difficult to study [Brocks et al., 2014; Gerlinger et al., 2014]. Altered interactions between the tumor, tumor microenvironment and extracellular matrix (ECM) promote the ability of cancer cells to migrate and metastasize [Alva and Hussain, 2013; Colombo et al., 2014]. Exosomes (Exo) are an important part of the tumor microenvironment, contribute to metastasis, and contain signaling molecules paramount to cancer progression [Azmi et al., 2013; Quail and Joyce, 2013]. The area of research of PrCa exosomal cellular communication in promoting this devastating disease is underinvestigated.
Exo are membrane vesicles derived from the intraluminal budding of multivesicular bodies and are about 30-150 nm in size [Colombo et al., 2014]. Through fusion with the plasma membrane of the cell, Exo are constitutively released into the extracellular environment. Exo can be further defined by the specific enrichment of tetraspanins such as CD9, CD63 and CD81 and other lipid-raft proteins such as flotillin-1 [Colombo et al., 2014; Thery et al., 2006]. It is known that most cells secrete Exo [Colombo et al., 2014] and that they are mediators of cell-cell communication via internalization by recipient cells and transfer of their contents, including DNA, microRNA, mRNA, lipids and proteins, to target recipient cells [Simons and Raposo, 2009]. For this reason, exosomal cell-cell communication is an important aspect of tumor progression [Azmi et al., 2013; Quail and Joyce, 2013]. Through the transfer of proteins, microRNA and mRNA, Exo mediate cell communication to favor the progression of cancer and metastasis and are repositories for potential biomarkers [Azmi et al., 2013; Harris et al., 2015; Junker et al., 2016 In press; Quail and Joyce, 2013; Skog et al., 2008; Soekmadji et al., 2013; Valadi et al., 2007]. In particular, Peinado et al. showed that melanoma-derived Exo can “educate” bone marrow progenitor cells to become pro-vascularogenic and increase metastasis of primary tumors [Peinado et al., 2012]. Additionally, our group has shown that integrin αvβ6 is transferred between PrCa cells and functionally enhances adhesion and migration of recipient cells [Fedele et al., 2015]. The study of exosomal composition is important for pinpointing the mechanisms of PrCa disease progression as well as for therapeutic or biomarker discovery.
Src-family kinases (SFKs) are normally expressed in prostatic epithelium and have been reported to transform normal cells when constitutively active [Tatarov et al., 2009; Varkaris et al., 2014]. Src is also upregulated in hormone refractory PrCa and is important to both the initiation and progression of the disease [Tatarov et al., 2009; Varkaris et al., 2014]. Src is normally maintained in an inactive, “closed” conformation when phosphorylated at tyrosine 530 by C-terminal Src kinase (CSK) [Roskoski, 2015]. When Src is activated in response to extracellular signals through integrins and other receptors, phosphatases dephosphorylate tyrosine 530, allowing for autophosphorylation at the tyrosine 416 residue in the kinase domain, which stabilizes the active conformation, allowing Src to bind to its target substrates with much higher affinity [Roskoski, 2015; Varkaris et al., 2014]. By directly interacting with Focal Adhesion Kinase (FAK), Src promotes detachment of integrins from their ligands and the formation of focal adhesions at the leading edges of cells to promote migration [Varkaris et al., 2014]. In response to extracellular signals through integrins, active Src (SrcpY416) forms a complex with active FAK (FAKpY397) [Varkaris et al., 2014]. Additionally, SrcpY416 phosphorylates FAKpY397 at multiple sites, including tyrosine 861, and this Src-FAK complex activates downstream molecules modulating a variety of functions such as migration and invasion [Varkaris et al., 2014]. Src has also been shown to downregulate the proapoptotic tumor suppressor prostate apoptosis response-4 (PAR-4), promoting cellular transformation [Hebbar et al., 2012]. Furthermore, expression of Src in Exo has been reported in colorectal cancer (v-Src) and melanoma [Ji et al., 2013; Lazar et al., 2015]. In particular, the study of colorectal cancer Exo demonstrated higher v-Src levels in Exo from a metastatic versus non-metastatic cell line [Ji et al., 2013], indicating a role for exosomal Src in cancer progression.
Growth factor receptors modulate multiple signaling pathways and their inhibition is of interest for PrCa therapy [Wu and Yu, 2014]. The insulin-like growth factor I receptor (IGF-IR) is a tetrameric transmembrane receptor tyrosine kinase and has an important role in PrCa by promoting cell survival, proliferation and inhibition of apoptosis [Baserga et al., 2003]. While inhibiting IGF-IR is investigated as a PrCa therapy, the development of resistance is an issue. There are numerous reports from our laboratory and others on the crosstalk between integrin and IGF-IR signaling [Alam et al., 2007; Sayeed et al., 2012; Sayeed et al., 2013]. Our group has reported a regulatory relationship between α5β1 and IGF-IR expression in which IGF-IR promotes PrCa growth by stabilizing α5β1 and activating androgen receptor (AR) in a α5β1-dependent manner [Sayeed et al., 2012; Sayeed et al., 2013]. Furthermore, crosstalk between Src and IGF-IR has been demonstrated in a study of non-small cell lung cancer (NSCLC) in which Src and IGF-IR are mutually activated in response to IGF-1 and together promote resistance to tyrosine kinase inhibition therapy [Min et al., 2015]. Another study by Dayyani et al. demonstrates that dual inhibition of both IGF-IR and Src shows promise as a therapeutic approach to prevent tyrosine kinase inhibition resistance through AKT activation [Dayyani et al., 2012], which has been associated with a poor clinical outcome in PrCa [Kreisberg et al., 2004].
It has been shown that G-protein-coupled receptor kinases (GRKs) crosstalk with both Src and IGF-IR and contribute to PrCa growth. In one report, it was demonstrated that GRKs phosphorylate IGF-IR, which recruits β-arrestin and subsequently modulates downstream ERK and AKT-mediated signaling as well as IGF-IR degradation. The type of signaling modulation is dependent on the specific GRK that is recruited (GRK6 or GRK2) [Zheng et al., 2012]. Additionally, GRK5 is overexpressed in PC3 cells and important for xenograft tumor growth [Kim et al., 2012]. GRK5 and GRK6 have been reported to target the β1-adrenergic receptor, causing β-arrestin and Src to be recruited to the membrane, resulting in MMP-induced EGF shedding and transactivation of EGFR [Noma et al., 2007]. Finally, in an analysis of urine Exo from PrCa patients, GPCRs were found to be upregulated in patients versus healthy donor samples; however, GRKs were not reported [Overbye et al., 2015]. Proteomic analysis indicates that GRK5 is detectable in Exo from normal thymic cells [Skogberg et al., 2013]. However, we are the first to report both GRK5 and GRK6 enrichment in cancer Exo.
We hypothesize that Exo represent an active signaling compartment, which contain proteins that promote cell migration and proliferation and PrCa progression. In this study, we demonstrate the presence and enrichment inside Exo from human PrCa cells of multiple signaling molecules paramount to cancer progression. In addition to this, we show for the first time that Exo from PrCa cells are enriched in c-Src, SrcpY416, IGF-IR, GRK5 and GRK6 and additionally express FAK and the specific Src target FAKpY861. This strongly suggests their potential for altering the tumor microenvironment in favor of tumor growth and metastasis. Finally, we demonstrate in a mouse model the potential for future studies on exosomal SrcpY416 and c-Src as easily attainable PrCa biomarkers.
Experimental Procedures
Cell Lines and Culture Conditions
PC3, DU145 and C4-2B PrCa cell lines and culture conditions have been previously described [Trerotola et al., 2013].
Exosome Isolation and Characterization
Exo were isolated from cell culture medium of DU145, PC3 and C4-2B cell lines by stepwise ultracentrufugation as previously described [Fedele et al., 2015]. TRAMP mice (n=4) were subjected to intracardiac puncture immediately after euthanasia for blood withdrawal at 32.6-37.5 weeks of age along with the same number of age-matched wild-type mice. The blood was collected in 3.8% Na-citrate and centrifuged to obtain plasma. To isolate Exo from TRAMP mice plasma, Exoquick™ solution (System Biosciences) was added in a ratio of 120μl of Exoquick™ solution for every 500μl of plasma. This ratio was adjusted to the volume of plasma that was obtained from each mouse. Plasma and Exoquick™ solution were incubated together for 2 h at 4°C, then spun down to pellet Exo for 30 min at 1,500 g at 4°C. Exo pellets were then resuspended in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl at pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, and 1% sodium deoxycholate) supplemented with protease inhibitors as previously described [Trerotola et al., 2013]. Immunoblotting (IB) was then performed (described below).
Transmission Electron Microscopy
Transmission electron microscopy (TEM) was performed as previously described [Fedele et al., 2015]
Sucrose Density Gradient
The sucrose density gradient analysis of Exo samples was conducted as previously described [Fedele et al., 2015].
Nano-Particle Tracking Analysis
Based on manufacturer's instructions, Exo samples were diluted 1:1,000 in PBS and subjected to nanoparticle tracking analysis (NTA). Dynamic light scattering was used to measure the Brownian motion and particle size was determined using the Stokes-Einstein equation. NTA 3.1 Build 3.1.46 software was used to interpret the results.
Immunoblotting
Biochemical Exo characterization was carried out by IB analysis. Exo and cells were lysed with RIPA buffer. The amount of protein present in Exo was determined using a BCA protein assay kit (Pierce). Equal amounts of protein were separated by SDS/PAGE and transferred to PVDF membranes (Millipore). After blocking with either 5% milk/TBS-T or 5% BSA/TBS-T, membranes were probed with primary antibodies. The SrcpY416, FAKpY861 and rat monoclonal antibodies (Ab) were diluted in 5% BSA while all other antibodies were diluted in 5% milk. Membranes were blocked in the same solution as the primary antibody dilution. Densitometric analysis was performed using ImageJ64 software.
Antibodies
Blocked membranes were probed with either rabbit polyclonal antibodies (Abs) to calnexin (sc713), c-Src (sc18), FAK (sc558), ERK1/2 (sc292838), IGF-IR-β (sc713) (Santa Cruz), flotillin-1 (ab41927; Abcam), and FAKpY861 (Invitrogen 700154), rabbit monoclonal (m) Ab to SrcpY416 (Cell Signaling 2101), mouse mAbs to c-Src (sc8056), CD9 (sc13118) (Santa Cruz), GRK4/5/6 (Millipore 05-466), CD81 (ab23505) and CD63 (ab8219) (Abcam), or a rat mAb antibody to CD9 (Santa Cruz sc18869).
Mice
We used TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) mice, which express the SV40 early region genes (T/t antigens; Tag) in the prostate under the control of the rat probasin promoter on a C57BL/6 background and also maintained wild-type mice of the same background under the same conditions. Based on the wealth of information available in the literature with this model, the histopathology, kinetics and penetrance of lesion formation are well known [Greenberg et al., 1995]. The plasma samples were processed for isolation of Exo using ExoQuick™ as per the manufacturer's instructions (Systems Biosciences). TRAMP mouse colony maintenance was performed as previously described [Goel et al., 2013]. Care of animals was in compliance with standards established by the office of laboratory animal welfare, Department of Health and Human Services at NIH. The Institutional Animal Care and Use Committee, Thomas Jefferson University, approved experimental protocols.
Results
c-Src and IGF-IR Are Present In Prostate Cancer Exosomes
IGF-IR and Src are critical mediators of PrCa progression [Riedemann and Macaulay, 2006; Varkaris et al., 2014; Wu and Yu, 2014]. We hypothesize that Exo may represent an active signaling compartment containing signaling molecules critical to PrCa. In order to biochemically characterize Exo protein content and investigate enrichment of signaling molecules, Exo and total cell pellets were lysed and proteins were separated on SDS-PAGE for IB analysis. The endoplasmic reticulum protein calnexin (CANX) is not present in Exo and is used as a control to test for purity of the Exo sample and loading control for the total cell lysate (TCL) (Fig. 1A). In addition, we tested for exosomal markers CD63 and CD81, and find them enriched in Exo (Fig. 1A). Next, we performed NTA on Exo derived from PC3 cells to analyze the particle size distribution of our sample. It is accepted that Exo range in size from 30-150 nm [Colombo et al., 2014] and our results demonstrate a vesicle preparation predominantly in this range from both DU145 (left) and PC3 (right) cells (Fig. 1B). TEM also confirms this size range (Fig. 1C). To further characterize our Exo preparations, the PC3 Exo sample was spun in a sucrose density gradient. It is accepted that Exo equilibrate at densities between 1.13 to 1.19 g/ml [Colombo et al., 2014]. We observe that CD63, CD81, CD9, IGF-IR and c-Src all co-sediment in the same fractions within the accepted density range of 1.15 to 1.17 g/ml (Fig. 2). Because NTA confirms a predominant vesicle population between 94 and 134 nm in size from PC3 and DU145 cells (Fig. 1B), our sucrose gradient analysis indicates that c-Src and IGF-IR are predominantly present in Exo and not a significantly larger extracellular vesicle (EV).
Fig. 1.
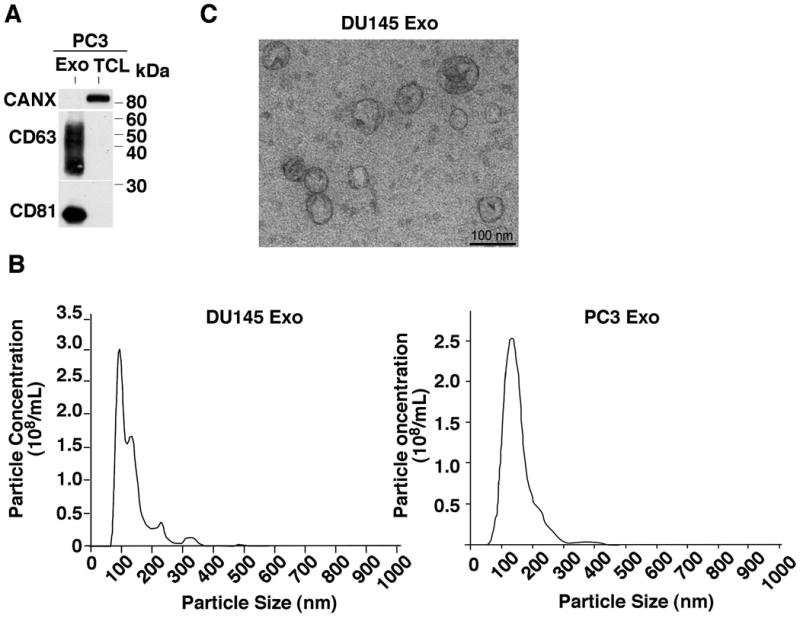
Characterization of prostate cancer exosomes. (A) Immunoblotting (IB) analysis of PC3 total cell lysates (TCL) and exosomal lysates (Exo). 10 μg of protein were loaded in each lane and 3 preparations were analyzed; a representative image is shown. CD63 and CD81 are used as exosomal markers enriched in the Exo samples. The endoplasmic reticulum protein calnexin (CANX) is not present in Exo and used as a negative control. Proteins were separated under non-reducing conditions. (B) TEM image of DU145 Exo. Scale bar = 100nm. (C) Graphical representation for NTA of DU145 (left) and PC3 (right) Exo preparations obtained via differential ultracentrifugation.
Fig. 2.
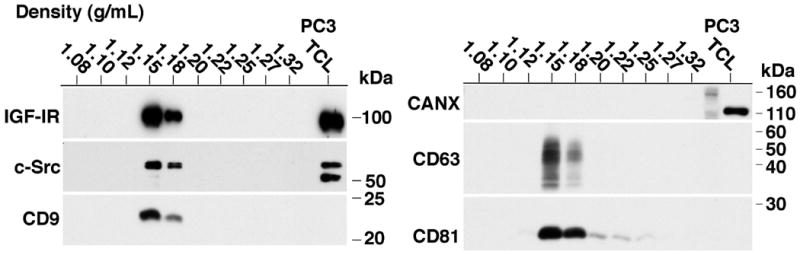
Sucrose gradient analysis and exosomal expression of c-Src and IGF-IR in PC3-derived exosomes. 80 μg PC3 Exo were used as input material. IB analysis for CD63, CD81, CANX, IGF-IR, and c-Src of each density fraction is shown. Results shown in the left and right panels are from two gels run in reducing and non-reducing conditions, respectively. The rightmost lane of each gel shows 10 μg of PC3 TCL used as a positive control. N=3; a representative image is shown.
Exosomal Enrichment of c-Src, Srcpy416, IGF-IR, GRK5 and GRK6 is Observed Across a Variety of Prostate Cancer Cell Lines
We next investigated the levels of IGF-IR and c-Src in different PrCa cell lines. In particular, we analyzed the AR-positive cell line C4-2B and AR-negative cell lines PC3 and DU145 to test a range of PrCa cell types. We probed for c-Src and SrcpY416 and observe that the C4-2B and DU145 cell lines exhibit a robust exosomal enrichment of both SrcpY416 and c-Src (Fig. 3A). The presence of exosomal SrcpY416 indicates that there are active kinases present in Exo. In addition to the specific band for c-Src at 60 kDa, we also observe a band at 50 kDa, recognizing a different SFK. ERK1/2 was used to represent proper loading of the total cell lysate (TCL), as it is not well expressed in Exo (Fig. 3A). Additionally, we observe expression of SrcpY416 in PC3 Exo (Fig. 3B), but not as robust as in Exo from DU145 and C4-2B cells (Fig. 3A). Over a total of 8 samples analyzed, Exo from PC3 cells show a range of exosomal c-Src enrichment from 1.5 to 4.5-fold higher than TCL levels (Figs. 3 and 4). Across 8 Exo samples from PC3, 5 Exo samples from DU145, and 3 Exo samples from C4-2B cells, there is a range of exosomal SrcpY416 enrichment from 0.4 to 5.7-fold, the highest being from DU145 cells. The differences in exosomal SrcpY416 enrichment between cell lines indicate that exosomal SrcpY416 levels are not entirely dependent on total c-Src levels, as those do not seem to change between cell lines. We further observe that there is a strong enrichment of IGF-IR in Exo compared to the corresponding TCL in all three cell lines, ranging from 1.3 to 2.4-fold increase (Fig. 3A and B). This result was repeated in a total of 8 Exo preparations encompassing three different cell lines. Since we hypothesize that Exo represent an active signaling compartment, we probed for FAK and FAKpY861, a downstream target of Src [Varkaris et al., 2014]. Both total FAK and FAKpY861 are detected in PC3 Exo, suggesting that active Src signaling may be occurring in PrCa Exo (Fig. 3C). This result was repeated in a total of 8 Exo preparations from PC3 cells. We then looked for further regulators of Src and IGF-IR signaling in Exo. We examined GRK5 and GRK6 expression in Exo from two PrCa cell lines. There is a significant enrichment of both GRK5 and GRK6 in PC3 Exo, while in C4-2B Exo there is a much higher enrichment of GRK6 than GRK5 (Fig. 4). This result suggests that exosomal composition may be dependent on the cell type and basal protein expression levels. GRK6 also exhibited a robust exosomal enrichment of 14.2-fold.
Fig. 3.
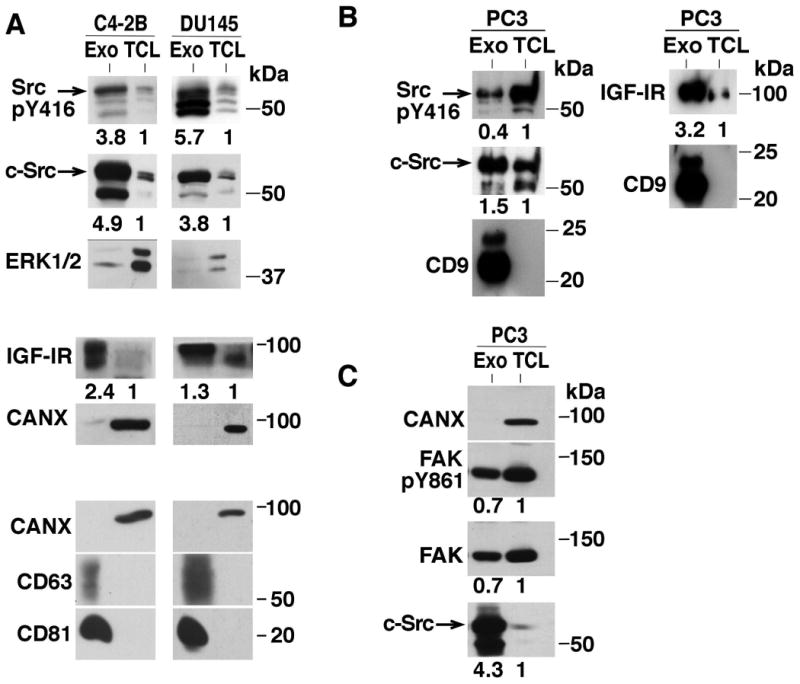
Exosomes from multiple prostate cancer cell lines contain active signaling molecules. (A)-(C), IB analysis for CD63, CD81, CANX, IGF-IR, c-Src, FAK, SrcpY416 and FAKpY861 of Exo and TCL from PC3, DU145 and C4-2B cell lines. Representative images are shown from a minimum of 3 Exo preparations and replicates from each cell line. 10 μg of protein were loaded in each lane of panels (A) and (B), and panel (C) results were obtained using 50 μg of protein in each lane. Fold changes in expression between TCL and Exo samples are shown below each panel.
Fig. 4.
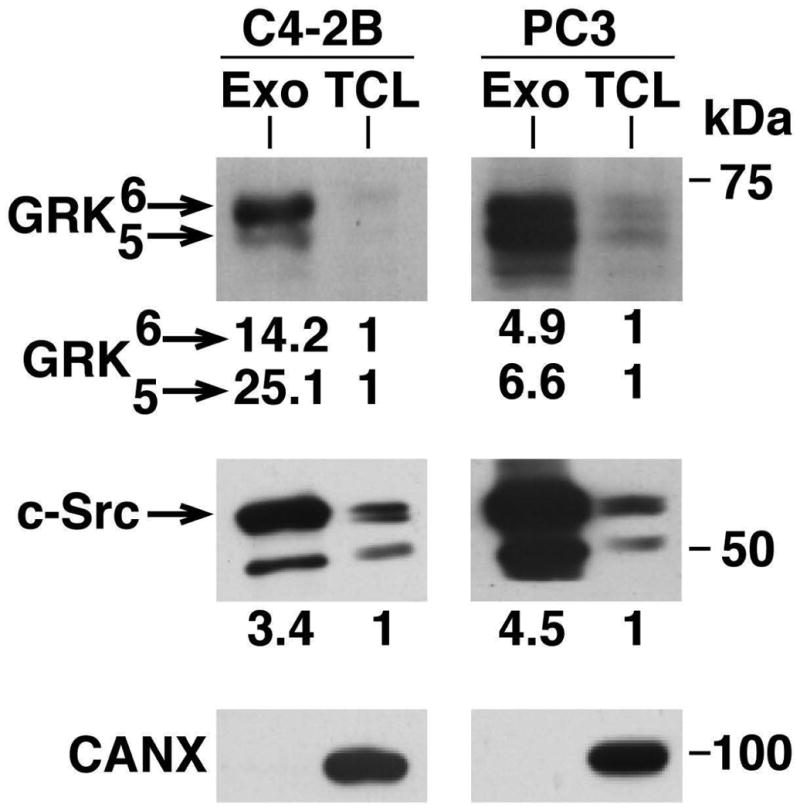
GRK5 and GRK6 are enriched in exosomes from PC3 and C4-2B cells. GRK5 and GRK6 both show enrichment in Exo versus TCL. Equal amounts of protein from Exo and TCL samples were loaded. 2 preparations were analyzed; a representative image is shown. CANX was used as a TCL loading control. Fold changes in expression between TCL and Exo samples are shown below each panel.
In a separate study of PC3 cell-derived Exo, proteomic analysis revealed a 13.9-fold enrichment of c-Src in Exo, along with a 1.3-fold increase of FAK (unpublished observation1), confirming the patterns we have demonstrated via IB. Most strikingly, but still in pattern with our findings, exosomal GRK6 was enriched in the same proteomic analysis 27.9-fold in Exo from PC3 cells (unpublished observation1).
Srcpy416 and Total c-Src are Detectable in Plasma Exosomes of Tumor-Bearing Tramp Mice
To continue our study of c-Src as a potential biomarker, Exo were isolated from the plasma of TRAMP mice using the ExoQuick™ isolation kit, and then IB analysis was performed. Based on previous study of this mouse model in our laboratory, we estimate that untreated, non-castrated TRAMP mice will develop intraepithelial neoplasia lesions by 12-16 weeks, adenocarcinoma by 18 weeks and metastasis by 24 weeks [Goel et al., 2013]. Mice with sizable tumors were used for this study and were compared to age-matched wild-type mice of the same genetic background. The plasma Exo were characterized by IB for the exosomal markers flotillin-1 and CD9 (Fig. 5A-C). Expression of both SrcpY416 and c-Src in Exo from TRAMP mouse plasma is observed. Additionally, when compared to their disease-free counterparts, TRAMP mice contained higher levels of total exosomal c-Src (Fig. 5B). In densitometric arbitrary units (A.U.), TRAMP mice exhibited a range of c-Src signal intensity between 25-60 A.U. compared to a range of 11-38 A.U. in Exo from wild-type mouse plasma. Our results indicate that the TRAMP mouse model is of value for future exosomal Src studies in PrCa and that the results obtained thus far in cell culture are transferrable to a novel pre-clinical scenario.
Fig. 5.
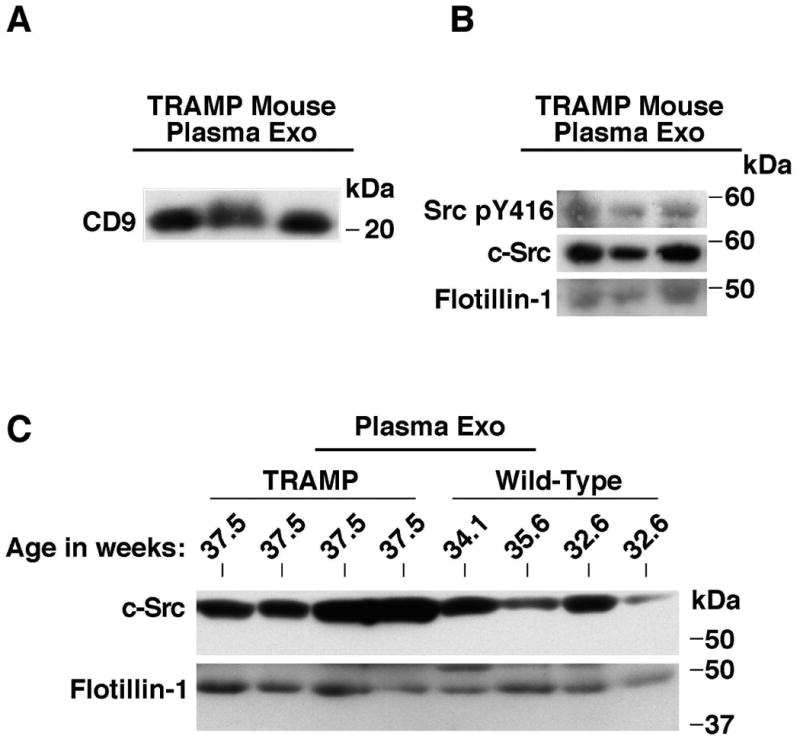
c-Src and SrcpY416 are present in plasma-derived exosomes of tumor-bearing TRAMP mice. (A) and (B) IB analysis for CD9, flotillin-1, c-Src and SrcpY416 of TRAMP mouse plasma Exo; 100 μg of protein were loaded in each lane. Mice were 37.5 weeks of age at the time of analysis. CD9 and flotillin-1 were used as exosomal markers. (B) SrcpY416 and c-Src are detected in mouse plasma Exo; 100 μg of protein were loaded in each lane. (C) IB analysis of TRAMP and non-TRAMP mouse plasma Exo for c-Src and the exosomal marker flotillin-1. Equal amounts of protein were loaded in each lane. Each experiment was repeated 3 times.
Discussion
We demonstrate for the first time in this study that c-Src, IGF-IR, SrcpY416, GRK5 and GRK6 are enriched in Exo from multiple PrCa cell lines and that both SrcpY416 and c-Src are expressed in the Exo isolated from the plasma of TRAMP mice, with total c-Src being increased in Exo compared to wild-type mice. Additionally, we are the first to show high levels of FAK, FAKpY861, SrcpY416 in the same Exo preparation as compared to the TCL. While proteomics has identified Src in Exo before [Ji et al., 2013; Lazar et al., 2015], we are the first to show this level of exosomal enrichment across multiple PrCa cell lines, elevated c-Src levels in TRAMP versus wild-type mouse plasma Exo, and strong detection of SrcpY416 in TRAMP mouse plasma Exo. There is a significant body of evidence surrounding the relevance of Exo to the progression of cancer and the development of metastasis [Azmi et al., 2013; Costa-Silva et al., 2015; Peinado et al., 2012]. In particular, it was recently shown that pancreatic cancer Exo initiate a pre-metastatic niche in the liver by causing an increase in fibronectin deposition [Costa-Silva et al., 2015]. By packaging particular proteins and RNA species into Exo and, in many cases, transferring these molecules to recipient cells, Exo contribute to cancer progression and are valuable sources of biomarkers [Melo et al., 2015; Overbye et al., 2015; Peinado et al., 2012; Soekmadji et al., 2013].
Exhibiting active signaling molecules such as SrcpY416 and FAKpY861 in the same Exo preparation implies that signaling may occur inside of Exo autonomously of their cells of origin. It was recently reported that processing of precursor microRNA into the mature form, principally via Dicer activity, occurs in Exo autonomously of cells, so it may be hypothesized that kinase signaling occurs as well [Melo et al., 2014]. It is well known that Exo promote a more aggressive or chemoresistant phenotype for cancer cells [Azmi et al., 2013; Costa-Silva et al., 2015; Peinado et al., 2012]. It is possible that signaling molecules may be transferred to recipient cells in their active state, therefore altering signaling and associated functions in the recipient cell more than if the molecules were transferred in an inactive state. Constitutive Src activation in LNCaP cells has been associated with a resistance to androgen independence, indicative of a more aggressive, anti-androgen therapy-resistant phenotype [Culig and Bartsch, 2006]. Furthermore, SFK activity is associated with bone metastasis and may represent a useful therapeutic target for bone-metastatic PrCa [Deng et al., 2014]. Future studies will determine the exosomal transfer of c-Src and other associated kinases in the IGF-IR/Src signaling axis (Fig. 6) and their functional effects on recipient cells of the tumor microenvironment.
Fig. 6.
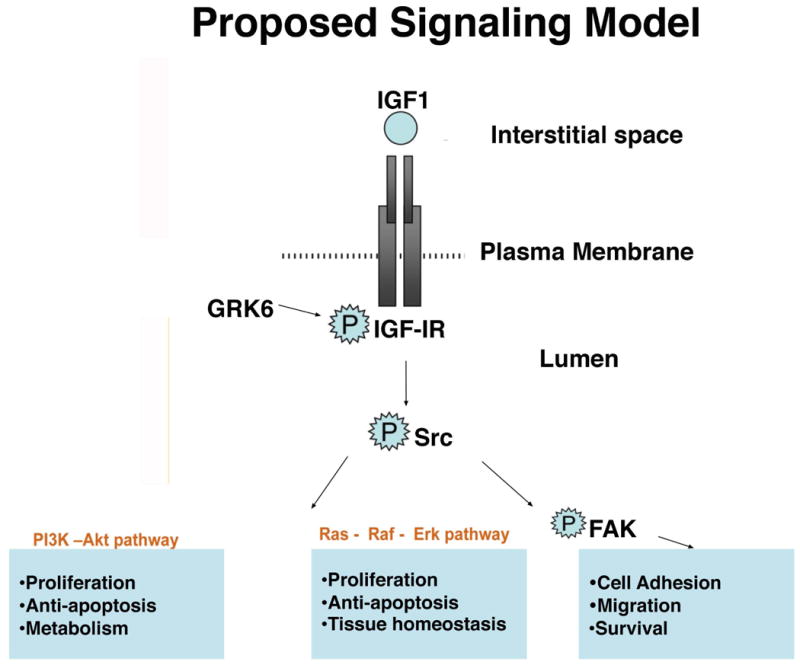
Proposed model of the Src/IGF-IR signaling axis present in both prostate cancer Exo and cells. Our model shows signal propagation and crosstalk between IGF-IR and Src, leading to FAK activation by Src and downstream activation of proliferative signals from the PI3K/AKT pathway and the Ras-MAPK pathway. There is also potential regulation by GRK6, as all of these proteins are highly enriched in Exo. This signaling axis may be active inside of Exo and may be activated by Exo in recipient cells.
Several studies have shown Exo interacting with multiple cell types to contribute to cancer progression. PrCa Exo are taken in directly by other PrCa cancer cells, increasing their migration [Fedele et al., 2015]. Additionally, pancreatic cancer Exo are preferentially taken in by Kupffer cells of the liver, leading to a metastatic niche formed early on in disease progression [Costa-Silva et al., 2015]. Additionally, Exo from melanoma cells interact with bone marrow (BM) progenitor cells, which are known to contribute to premetastatic niche formation [Peinado et al., 2012]. This BM “education” also leads to vascular leakiness at the future metastatic site, which was the lung in this study [Peinado et al., 2012]. Increased angiogenesis is an aspect of tumor biology and leads to continuous vascular remodeling and vascular leakiness [Weis and Cheresh, 2011]. Angiogenesis is a critical aspect of prostate tumor growth and disease progression [Nicholson and Theodorescu, 2004; Weis and Cheresh, 2011]. It has also been shown in multiple studies that Exo from cancer cells enhance angiogenic and metastatic potential [Azmi et al., 2013; Skog et al., 2008]. Specifically, melanoma Exo were shown to interact with and modify endothelial cells [Hood et al., 2009]. Another study by Park et al. demonstrated the pro-angiogenic effects of Exo released from hypoxic tumor cells [Azmi et al., 2013; Park et al., 2010]. It has also been demonstrated that Exo derived from chronic myeloid leukemia cells promote angiogenesis in a Src-dependent manner, however they did not specifically demonstrate a dependence on exogenous exosomal Src [Mineo et al., 2012]. Src is known to stimulate transcription of VEGF and modulate angiogenesis [Marx et al., 2001; Varkaris et al., 2014] and it is believed that any regulator of Src may also affect angiogenesis. Because SrcpY416 and c-Src are present in plasma Exo from tumor-bearing TRAMP mice, it is possible that Src-enriched Exo are promoting tumor angiogenesis in vivo. In the proteomic analysis of PC3 Exo, exosomal enrichment of the SFK members Lyn and Yes was also observed by 45- and 16-fold, respectively (unpublished observation2). The Ab to SrcpY416 may be detecting other phosphorylated SFKs such as Yes and Lyn, and we cannot ignore the potential contribution of these other exosomal SFKs in PrCa progression and tumor angiogenesis. IGF-IR, enriched in PrCa Exo, has also been shown to induce VEGF-C expression and stimulate angiogenesis in a mouse model of pancreatic cancer [Lopez and Hanahan, 2002; Wu and Yu, 2014]. Additionally, GPCRs stimulate angiogenesis by promoting endothelial cell proliferation or secretion of pro-angiogenic factors such as VEGF into the tumor microenvironment [O'Hayre et al., 2014]. It is extremely likely that PrCa Exo enriched in c-Src, FAK, IGF-IR, GRK5, and GRK6 will stimulate endothelial cell angiogenic activity in the tumor microenvironment. Future studies will elucidate the exact transfer of these signaling molecules and their functional implications in tumor progression. These discoveries will provide a platform for new therapeutic strategies, which may be more efficient when targeting Exo rather than cancer cells alone.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant numbers: R01-CA109874, R01-CA089720, PO1 CA140043-Project 2 (LRL), R01 GM044944 (JLB), R01 CA47282 (RVI)
This work was supported by the following grants: NIH R01 CA109874, CA89720, PO1 CA140043-Project 2 (L.R. Languino), R01 GM044944 (J. Benovic), R01 CA47282 (R.V. Iozzo). This project is also funded, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.); the Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The Sidney Kimmel Cancer Center Flow Cytometry Facilities (used for NTA) were supported by the NCI, National Institutes of Health, under Award P30CA056036. We would like to kindly acknowledge C. Fedele, S. Kurtoglu, S.A. Sayeed, and A. Duffy of the Languino laboratory for constructive discussion.
Footnotes
Unpublished observation by Senem Kurtoglu, Carmine Fedele, Hsin-Yao Tang, David W. Speicher, Dario C. Altieri and LRL.
Unpublished observation by Senem Kurtoglu, Carmine Fedele, Hsin-Yao Tang, David W. Speicher, Dario C. Altieri and LRL.
Conflict of Interest: The authors declare that they have no conflicts of interest with the contents of this article.
References
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- Alva A, Hussain M. The changing natural history of metastatic prostate cancer. Cancer J. 2013;19:19–24. doi: 10.1097/PPO.0b013e318281197e. [DOI] [PubMed] [Google Scholar]
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- Brocks D, Assenov Y, Minner S, Bogatyrova O, Simon R, Koop C, Oakes C, Zucknick M, Lipka DB, Weischenfeldt J, Feuerbach L, Cowper-Sal Lari R, Lupien M, Brors B, Korbel J, Schlomm T, Tanay A, Sauter G, Gerhauser C, Plass C. Intratumor DNA methylation heterogeneity reflects clonal evolution in aggressive prostate cancer. Cell Rep. 2014;8:798–806. doi: 10.1016/j.celrep.2014.06.053. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99:373–381. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- Dayyani F, Parikh NU, Varkaris AS, Song JH, Moorthy S, Chatterji T, Maity SN, Wolfe AR, Carboni JM, Gottardis MM, Logothetis CJ, Gallick GE. Combined Inhibition of IGF-1R/IR and Src family kinases enhances antitumor effects in prostate cancer by decreasing activated survival pathways. PLoS One. 2012;7:e51189. doi: 10.1371/journal.pone.0051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, He G, Liu J, Luo F, Peng X, Tang S, Gao Z, Lin Q, Keller JM, Yang T, Keller ET. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40:730–738. doi: 10.1016/j.ctrv.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The αvβ6 Integrin Is Transferred Intercellularly via Exosomes. J Biol Chem. 2015;290:4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M, Catto JW, Orntoft TF, Real FX, Zwarthoff EC, Swanton C. Intratumour Heterogeneity in Urologic Cancers: From Molecular Evidence to Clinical Implications. Eur Urol. 2014;67:729–737. doi: 10.1016/j.eururo.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Goel HL, Sayeed A, Breen M, Zarif MJ, Garlick DS, Leav I, Davis RJ, Fitzgerald TJ, Morrione A, Hsieh CC, Liu Q, Dicker AP, Altieri DC, Languino LR. β1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase 1. J Cell Physiol. 2013;228:1601–1609. doi: 10.1002/jcp.24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, Taraska JW. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One. 2015;10:e0117495. doi: 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar N, Wang C, Rangnekar VM. Mechanisms of apoptosis by the tumor suppressor Par-4. J Cell Physiol. 2012;227:3715–3721. doi: 10.1002/jcp.24098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, Xue Y, Xu T, Zhu HJ, Simpson RJ. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13:1672–1686. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- Junker K, Heinzelmann J, Beckham C, Ochiya T, Jenster G. Extracellular Vesicles and Their Role in Urologic Malignancies. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.02.046. In press. [DOI] [PubMed] [Google Scholar]
- Kim JI, Chakraborty P, Wang Z, Daaka Y. G-protein coupled receptor kinase 5 regulates prostate tumor growth. J Urol. 2012;187:322–329. doi: 10.1016/j.juro.2011.09.049. [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- Lazar I, Clement E, Ducoux-Petit M, Denat L, Soldan V, Dauvillier S, Balor S, Burlet-Schiltz O, Larue L, Muller C, Nieto L. Proteome characterization of melanoma exosomes reveals a specific signature for metastatic cell lines. Pigment Cell Melanoma Res. 2015;28:464–475. doi: 10.1111/pcmr.12380. [DOI] [PubMed] [Google Scholar]
- Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Marx M, Warren SL, Madri JA. pp60(c-src) modulates microvascular endothelial phenotype and in vitro angiogenesis. Exp Mol Pathol. 2001;70:201–213. doi: 10.1006/exmp.2001.2358. [DOI] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HY, Yun H, Lee JS, Lee HJ, Cho J, Jang HJ, Park SH, Liu D, Oh SH, Lee JJ, Wistuba II, Lee HY. Targeting the insulin-like growth factor receptor and Src signaling network for the treatment of non-small cell lung cancer. Mol Cancer. 2015;14:113. doi: 10.1186/s12943-015-0392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, Kohn EC. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson B, Theodorescu D. Angiogenesis and prostate cancer tumor growth. J Cell Biochem. 2004;91:125–150. doi: 10.1002/jcb.10772. [DOI] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. β-arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol. 2014;27:126–135. doi: 10.1016/j.ceb.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, Sandvig K, Llorente A. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357–76. doi: 10.18632/oncotarget.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(Suppl 1):S33–S43. doi: 10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. 2015;94:9–25. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Sayeed A, Alam N, Trerotola M, Languino LR. Insulin-like growth factor 1 stimulation of androgen receptor activity requires β1A integrins. J Cell Physiol. 2012;227:751–758. doi: 10.1002/jcp.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed A, Fedele C, Trerotola M, Ganguly KK, Languino LR. IGF-IR promotes prostate cancer growth by stabilizing α5β1 integrin protein levels. PLoS One. 2013;8:e76513. doi: 10.1371/journal.pone.0076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogberg G, Gudmundsdottir J, van der Post S, Sandstrom K, Bruhn S, Benson M, Mincheva-Nilsson L, Baranov V, Telemo E, Ekwall O. Characterization of human thymic exosomes. PLoS One. 2013;8:e67554. doi: 10.1371/journal.pone.0067554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekmadji C, Russell PJ, Nelson CC. Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers (Basel) 2013;5:1522–1544. doi: 10.3390/cancers5041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:3540–3549. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. John Wiley & Sons, Inc; 2006. p Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A, Languino LR. Trop-2 promotes cancer metastasis by modulating β1 integrin functions. Cancer Res. 2013;73:3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Varkaris A, Katsiampoura AD, Araujo JC, Gallick GE, Corn PG. Src signaling pathways in prostate cancer. Cancer Metastasis Rev. 2014;33:595–606. doi: 10.1007/s10555-013-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Wu J, Yu E. Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy. Cancer Metastasis Rev. 2014;33:607–617. doi: 10.1007/s10555-013-9482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Worrall C, Shen H, Issad T, Seregard S, Girnita A, Girnita L. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA. 2012;109:7055–7060. doi: 10.1073/pnas.1118359109. [DOI] [PMC free article] [PubMed] [Google Scholar]


