Abstract
Background
Studies in Sub Saharan Africa have shown that the Circulating Cathodic Antigen point-of-care-test (POC-CCA) is more accurate in the detections of S. mansoni than the microscopic Kato-Katz technique but less is known about the accuracy of this rapid test in detecting S. haematobium infections. This study was intended to evaluate the field accuracy of POC-CCA as a rapid test kit for schistosomiasis mapping in The Gambia.
Methods
This prospective study was conducted in 4 regions in the country. Ten schools were randomly selected from each region, and a total of 2018 participants whose ages range from 7 to 14 years were enrolled in the study. Stool and urine samples were collected from each participant from May to June 2015, and tested for S. haematobium and S. mansoni infections in field and laboratory settings. The tests conducted included POC-CCA, double Kato-Katz slides, urine filtration and dipstick for hematuria.
Results
Of the 1954 participants that had complete data, the mean age of participants was 9.9 years. The prevalence of children infected with S. haematobium, using urine filtration technique was 10.1% (95% CI: 8.87–11.55). Central River Region had the highest level of urinary schistosomiasis with a prevalence of 28.0% (24.13–32.12).The lowest urinary schistosomiasis prevalence of 0.6% (0.12–1.86) was found in Lower River Region and North Bank Region had no cases of schistosomiasis detected. Only 5 participants were infected with S. mansoni. Using urine filtration as reference standard for the detection of S. haematobium, the sensitivity and specificity of POC-CCA was 47.7% and 75.8%. Whilst sensitivity and specificity of POC-CCA for detecting S. mansoni were 60.0% and 71.2% using double Kato-Katz as reference standard.
Conclusion
This study showed lower sensitivity of POC-CCA in detecting S. haematobium. Therefore POC-CCA is less useful for rapid diagnosis of urinary schistosomiasis.
Background
Schistosomiasis is a chronic infection endemic in over 74 tropical and sub-tropical countries; more than 200 million people are infected and 650 million people thought to be at risk[1]. Sub-Saharan Africa carries the highest burden (90%) of schistosomiasis and both S. mansoni and S. haematobium infections are prevalent [1]. Schistosomiasis is caused by trematode parasites belonging to the genus Schistosoma with S. haematobium, S. mansoni, and S. japonicum representing the species that typically infect humans. The move to control schistosomiasis and other NTDs has gained momentum after the London declaration of 30th January 2012, which advocated for the elimination of schistosomiasis in some countries [2, 3]. World Health Assembly Resolution WHA 65.21 has resulted in scaled up of efforts worldwide to control morbidity of the disease so that the target for elimination can be met. Preventive chemotherapy, or mass drug administration (MDA), using praziquantel is the recommended method of control for schistosomiasis by the World Health Organization (WHO). Before the implementation of MDA campaigns, infection prevalence should be assessed to guide program decision making. This is usually achieved through surveys based on the use of traditional parasitological methods (urine filtration for S. haematobium and Kato-Katz thick smears for S. mansoni infections) [4]. These methods are useful for detecting active infection, but are insensitive and often miss light-intensity infections [5, 6]. This resulted to increased efforts to develop more sensitive methods, including molecular techniques and more rapid techniques such as the point- of- care (POC) test for circulating cathodic antigen (CCA) [7, 8, 9] Traditional parasitological methods of diagnosis (urine filtration and Kato-Katz) are useful in areas of high endemicity. However, they are less sensitive for diagnosis in populations where schistosomiasis prevalence is low [6, 10]. Since preventive chemotherapy-based schistosomiasis control programmes are due to be scaled up [11], low infection intensity is expected in areas where MDA campaigns are successful. More sensitive methods of diagnosis will be needed to monitor prevalence and intensity of infection following MDA. Furthermore, the WHO has recently recommended that preschool-aged children be included in treatment programmes [12]. A significant proportion of these children harbor light infections [13] and are therefore more likely to be missed by traditional parasitological methods.
POC-CCA is a rapid, sensitive, user-friendly, equipment-free and deliverable tool and it has been found that a single POC-CCA was nearly three times more sensitive than four Kato-Katz preparations for the diagnosis of S. mansoni [9]. However, studies have found that POC-CCA was less sensitive for the diagnosis of S. haematobium [9,14,15]. However, more evaluation studies on the field sensitivity of POC-CCA as a rapid mapping tool for schistosomiasis are recommended [15]. In The Gambia, there is limited information on the current endemicity of schistosomiasis [16,17, 18,19]. Neither historical nor clinical data provide reliable and adequate information on the endemicity of schistosomiasis in The Gambia, thus, new surveys are needed[19,20].This study evaluated the field accuracy of POC-CCA as tool for mapping of schistosomiasis endemic regions in the country.
Materials and methods
Ethical issues
The study was approved by the Gambia Government and Medical Research Council Joint Ethical Committee with a number of SCC1415. Written consent forms were signed on behalf of all the students whose parents or guardians were contacted by the head master/mistress. In addition, informed consent was obtained from each study participant as they were enrolled by the data collection teams.
Study design
The survey was a prospective and cross-sectional study that assessed the sensitivity and specificity of the POC-CCA compared to traditional tools in determining the prevalence of schistosomiasis.
Study site, population and participant selection
The study was conducted in 4 regions across the country, namely: North Bank Region (NBR), Lower River Region (LRR), Central River Region (CRR) and Upper River Region (URR) (Fig 1). Ten schools were randomly selected from each region, including CRR and URR, which are the historically high endemic regions of schistosomiasis, LRR which has moderate endemicity and NBR which was considered to be non-endemic. Fifty students (25 boys and 25 girls) between the age of 7 and 14 years were randomly selected from each school. In total, 2018 children from 40 schools were enrolled for the study. Stool, urine and blood samples were collected from each participant; 1954 participants have complete data record and included for the analysis. The sample size determination was based on the WHO recommended protocols with modifications for NTD mapping in the WHO AFRO Region Enrolled participants were students who lived in the selected district and attended the selected school, were between 7and 14 years of age, provide informed consent and submitted all requested specimens. Students absent from school during the study period were excluded from the study.
Fig 1. A map showing the four regions (coloured) in which the study had been conducted.
Sample collection
To obtain consent, head teachers in schools were informed and obtained consent from parents, guardians or wards of the students prior to the survey exercise. Written consent forms were signed on behalf of all the students whose parents or guardians were contacted by the head master/mistress. In addition, informed consent was obtained from each study participant as they were enrolled by the data collection teams. Participants who gave their consent were given two containers to voluntarily donate their urine and stool samples, which were collected in the morning between the hours 8:00am and 12:00 mid-day.
Parasitological investigations
Each participant’s urine sample was analyzed on site using POC-CCA according to manufacturer’s instructions[21]. Two Kato-Katz slides were prepared from each stool sample and a examine with a microscopic to S. mansoni eggs. Also, each urine samples was adequately shaken before 10 ml being passed through the urine filtration kit and the filter transferred to a slide for microscopic examination for S. heamatobium eggs. Haematuria level was investigated using a rapid urine dipstick (Hemastix) for the samples.
Data analysis
Prevalence estimates with 95% CIs and 2x2 tables comparing POC-CCA results to Kato-Katz, urine filtration, and dipstick methods were computed using SAS University Edition. Due to the proportions being sometimes close to 0, the Agresti-Coull method was used to calculate 95% CIs.
Results
Population characteristics and prevalence of schistosomiasis
A total of 2018 participants were enrolled in the study. 1954 participants had complete data that was included in the final analysis (Table 1). The final sample included 975 boys and 979 girls between the ages of 7 to 14 years from across the four regions of the study area. The mean age of participants was 9.9 ± 0.05 years.
Table 1. Population characteristics and prevalence of schistosomiasis.
| Filtration | KK | POC-CCA | Dipstick | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | no. children investigated | no. infected | prevalence of infection % (95% CI) | no. infected | prevalence of infection % (95% CI) | no. infected | prevalence of infection % (95% CI) | no. infected | prevalence of infection % (95% CI) |
| CRR | 483 | 135 | 27.95 (24.13–32.12) | 4 | 0.83 (0.24–2.19) | 147 | 30.43 (26.50–34.68) | 185 | 38.30 (34.07–42.71) |
| LRR | 492 | 3 | 0.61 (0.12–1.86) | 0 | 0 (0.00–0.03) | 85 | 17.28 (14.18–20.88) | 29 | 5.89 (4.11–8.36) |
| NBR | 494 | 0 | 0 (0.00–0.93) | 0 | 0 (0.00–0.93) | 91 | 18.42 (15.24–22.09) | 49 | 9.92 (7.57–12.89) |
| URR | 485 | 60 | 12.37 (9.72–15.62) | 1 | 0.21 (0.00–1.28) | 133 | 27.42 (23.64–31.56) | 71 | 14.64 (11.76–18.08) |
| Total | 1954 | 198 | 10.13 (8.87–11.55) | 5 | 0.26 (0.09–0.62) | 456 | 23.34 (21.51–25.26) | 334 | 17.09 (15.49–18.83) |
Using the urine filtration technique, 198 school children of the 1954 children (10.1% prevalence; 95% CI: 8.87–11.55) were positive for S. haematobium, infections (Table 1). Central River Region (CRR) has the highest prevalence of 27.95% (95% CI: 24.13–32.12) followed by Upper River Region (URR) (12.4%(95% CI: 9.72–15.62)). Lower River Region (LRR) had only 3 positive cases (0.6% prevalence (Table 1). Only 5 cases of S. mansoni were detected out of the 1954 participants tested using the double Kato-Katz technique. Four of the 5 positive cases were seen in CRR and the fifth was seen in URR (Table 1). This presented a prevalence of 0.3% (95% CI: 0.1–0.6) of intestinal schistosomiasis in the study area. One out of the 5 positive cases had 25 egg/ gram of faeces while the remaining 4 counted only 1 egg/slide.
Prevalence of intensity of schistosomiasis
No case of S. haematobium was detected among the 494 participants tested in North Bank Region (NBR). Of the 198 infected children, 2.7% (95% CI 2.07–3.54) had heavy infection and majority of whom were found in CRR (8.3%; 95% CI 6.12–11.10)(Table 2). The mean egg count for S. haematobium was 17.8 eggs/10 ml urine in CRR, 6.1 eggs/10 ml urine in URR and 0.4 eggs/10 ml urine in LRR (Table 2).
Table 2. Infection intensity and mean egg count by urine filtration and Kato-Katz by region.
| Region | no. children investigated | no. heavy infected | prevalence of heavy infection % (95% CI) | Mean egg count (95%CI) | |||
|---|---|---|---|---|---|---|---|
| KK | Filter | KK | Filter | KK | Filter | ||
| CRR | 483 | 0 | 40 | 0 (0.00–0.95) | 8.28 (6.12–11.10) | 0.01 (0.00–0.02) | 17.83 (11.70–23.95) |
| LRR | 492 | 0 | 1 | 0 (0.00–0.03) | 0.20 (0.00–1.26) | 0 | 0.39 (0.00–1.15) |
| NBR | 494 | 0 | 0 | 0 (0.00–0.93) | 0 (0.00–0.93) | 0 | 0 |
| URR | 485 | 0 | 12 | 0 (0.00–0.95) | 2.47 (1.37–4.32) | 0.05 (0.00–0.15) | 6.07 (2.61–9.53) |
| Total | 1954 | 0 | 53 | 0 (0.00–0.24) | 2.71 (2.07–3.54) | 0.01 (0.00–0.04) | 6.01 (4.24–7.79) |
Calculated for all children investigated, irrespective of their infection status
Students were considered to have heavy infection for S. haematobium if they have at least 50 eggs/10 ml of urine and for S. mansoni more than 399 eggs/gram of faeces.
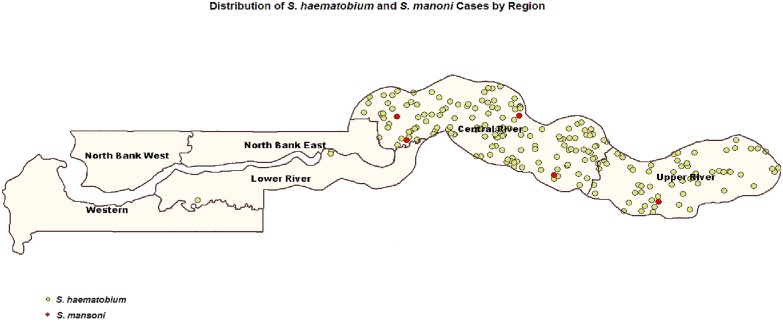
Co-endemicity of S. haematobium and S. mansoni
Three of the 5 participants that tested positive for S. mansoni were co-infected with S. haematobium. The existence of these few cases of S. mansoni in CRR and URR where S. haematobium is prevalent demonstrated that the two diseases are co-endemic in these regions (Fig 2). The prevalence of antigen to schistosomiasis infection (S. haematobium and S. mansoni) tested by POC-CCA was 23.3% (95% CI: 21.5–25.3). The prevalence of micro-hematuria was highest in CRR 38.30% and lowest in LRR with 5.89% (Table 1).
Fig 2. Co-endemicity of S. haematobium and S. mansoni.
Sensitivity and specificity of POC-CCA
Using urine filtration as a standard, POC-CCA had a sensitivity of 47.7% in the high endemic regions whilst 66.7% in the low endemic regions(CRR and URR), and 47.98% in all 4 regions. In the high endemic area, 24.2% of children who were egg negative by filtration were CCA positive. In the low endemic area, 17% of egg-negative children were CCA positive (Table 3). The sensitivity of CCA for the schistosomiasis using kato- Katz as standard was 60% (3/5 children). The specificity of the CCA was 75.8%, 82.3% and 79.4% in the high endemic regions, low endemic regions and all 4 regions using filtration as standard, respectively (Table 3). The sensitivity and specificity of POC-CCA using the Dipstick method as the gold standard were 47% and 81.5% in all the participants tested, respectively.
Table 3. Sensitivity and specificity of POC-CCA against urine filtration, Kato-Katz and dipstick test techniques by endemicity.
| Region | Number tested per region | POC-CCA neg/pos | Filtration—(#/%) | Filtration + (#/%) | Sensitivity/specificity | KK-(#/%) | KK+ (#/%) | Sensitivity/specificity | Dipstick- (#/%) | Dipstick+ (#/%) | Sensitivity/specificity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High endemic region | 968 | POC-CCA neg | 586 (75.8%) | 102(52.3%) | 47.69/75.81 | 686(71.2%) | 2(40%) | 60.00/71.24 | 572(80.3%) | 116(45.3%)54.68/80.34 | 54.69/80.34 |
| POC-CCA pos | 187(24.2%) | 93(47.7%) | 277 (28.8%) | 3 (60%) | 140 (19.7%) | 140 (54.7%) | |||||
| Low endemic region | 968 | POC-CCA neg | 809 (82.3%) | 1 (33.3%) | 66.67/82.30 | 810 (82.2%) | 0 | 749 (82.5%) | 61 (78.2%) | 21.79/82.49 | |
| POC-CCA pos | 174 (17.7%) | 2 (66.7%) | 176 (17.8%) | 0 | 159 (17.5%) | 17 (21.8%) | |||||
| All 4 regions | 1954 | POC-CCA neg | 1395 (79.4%) | 103 (52.0%) | 47.98/79.44 | 1496 (76.8%) | 2 (40.0%) | 60.00/76.76 | 1321 (81.5%) | 177 (53.0%) | 47.01/81.54 |
| POC-CCA pos | 361 (20.6%) | 95 (48.0%) | 453 (23.2%) | 3 (60.0%) | 299 (18.5%) | 157 (47.0%) |
Discussion
There have been increased efforts to develop more rapid and accurate techniques to achieve the WHO 2020 goals and WHA Resolution 65.21. New rapid mapping tools that can be used at the point of care are required to accelerate mapping process in endemic regions towards the achievement of schistosomiasis elimination targets. These tools need to be accurate and sensitive, since prevalence and intensity of infection will decrease as we move towards elimination of the disease. In this study we evaluated the field performance of POC-CCA for prevalence mapping of schistosomiasis at point-of–care for the first time in a country which is endemic for S. haematobium. A total of 40 schools were mapped across four regions in The Gambia. From the study findings, POC-CCA prevalence was 23.3% (95% CI, 21.5–25.3) which was two times higher than the prevalence based on egg-detection for S. haematobium 10.1% (95% CI 8.9–11.6) and hundred times S. mansoni (; 0.26%, (95% CI, 0.09–0.62) respectively.
Sensitivity of POC-CCA against urine filtration technique in detecting S. haematobium in the four regions was 47.98% which was comparable to the sensitivity in the high endemic regions (47.69%). The specificity of POC-CCA technique was 79.44% for the four regions, 82.30% for the low endemic regions and 75.81% for the high endemic regions. The sensitivity of POC-CCA is generally low in this study, which is in line with findings of previous studies that found POC-CCA not very efficient in detecting S. haematobium [22]
Using double Kato-Katz as a reference standard for S. mansoni detection, the sensitivity of the POC-CCA proved to be relatively higher (60.0%) compare to the POC-CCA against urine filtration technique, although few infections were found in the southern region of the country. Coulibaly and others reported a sensitivity of 75.0% for double CCA when compared to four Kato-Katz as reference standard in detecting S. mansoni [9]. Also the 2 Kato-Katz positive cases that were reported negative by the POC-CCA both had 1 egg/slide. This low egg count might be the reason they were missed by the rapid test. This is in concordance with previous findings by Coulibaly and others [9], who found the prevalence of S. mansoni estimated by a single POC-CCA to be 51.7% compared to 20.3% prevalence given by the egg-detection methods. Another study in Ethiopia reported a prevalence of 65.9% by POC-CCA and prevalence of 43.1% by double Kato-Katz in an area of moderate prevalence for S. mansoni which was similar finding s to another study in Kenya [23,24]. Colley et al also observed higher prevalence based in single POC-CCA than in a single Kato-katz in a study conducted in five African countries in 2013 [25]. Studies employing serological methods have yielded higher prevalence estimates than traditional parasitological methods for diagnosis of schistosomiasis [4, 9, 14, 26]. Furthermore, a meta-analysis found that many studies conclude antigen testing for S. haematobium yielded poor sensitivity and specificity, however antigen testing was found as an effective tool rapid diagnosis of S. mansoni [15,23].
According urine filtration and dipstick techniques are more sensitive and specific than the POC-CCA in detecting S. haematobium infection in high endemic regions. Previous findings had indicated POC-CCA to be less sensitive in detecting S. haematobium compared to other techniques [26,22].POC-CCA is a rapid, simple and easy-to-use technique and efficient in diagnosing S. mansoni infection [27]. However, it proved not to be a very useful tool in detecting S. haematobium in this study as concluded in the meta-analysis of CCA for diagnosis of schistosomiasis [14, 15]. This makes it a less favorable tool for mapping urinary schistosomiasis in endemic countries.
Conclusion
This study evaluated the potential of the rapid test kit in detecting schistosomal antigens in Lower Basic School going children. A total of 40 schools were surveyed for the prevalence and intensity of urinary and intestinal schistosomiasis. According to the results obtained by the antigen detecting POC-CCA, prevalence of schistosomiasis was two times higher than the prevalence reported by egg-detection methods. Of the four regions in the study, CRR has the highest prevalence of urinary schistosomiasis. From the 1954 individuals sampled, 198 were infected with S. haematobium. Although The Gambia is known to be endemic for only S. haematobium, yet 5 subjects were found to harbor S. mansoni. These 5 individuals were from CRR and URR, which are the high endemic schistosomiasis regions. Three individuals were co-infected with S. haematobium and S. mansoni. Sensitivity of POC-CCA kit in detecting S. haematobium was lower whilst the sensitivity in detecting S. mansoni was relatively higher. Specificity of the kit was generally higher. The sensitivity of the tool was also observed to be higher in low urinary schistosomiasis endemic regions than in high endemic regions. POC-CCA could be a useful tool for the rapid mapping of intestinal schistosomiasis. However, from the outcome of the current study it may not be an ideal technique for mapping in urinary schistosomiasis endemic regions.
Also, in future evaluations of POC-CCA, other rapid diagnostic test kits can be used alongside in similar large sample size studies. More accurate techniques such as ELISA and PCR should be employed to further confirm the field performance of the POC-CCA.
KK: Kato-Katz
Acknowledgments
We are thankful to Dr Sharmila Lariff Jah of World Health Organization country Office the Gambia for technical support during the study. We are also grateful to Dr Patrick Lammie of Taskforce for Global Health for editorial work on the manuscript. We thanks the field study teams, education directors, school masters and teachers, parents and guardians and participating school children and Ministry of Health and Social welfare for payment of vehicle milages and other logistic assistance during the surveys.
Data Availability
Data gathered from the survey will be managed with utmost confidentiality and be made accessible to only the Ministry of Health personnel and partners such as WHO and The Task Force for Global Health before official publication. Since we have pledged to protect the data generated from this study, anonymized data will be available from Mr Bakary Sanneh, National Public Health Laboratories, The Gambia (email: sheikbakary@yahoo.com), and Kisito Ogoussan, Deputy NTD mapping Director, Taskforce for Global Health, USA (email kogoussan@taskforce.org).
Funding Statement
This work was supported by the Task Force for Global Health, INC (A992A78-CC70-4846-AF50BE65573CA71B) to BS.
References
- 1.WHO: Working to overcome the global impact of neglected tropical diseases, First WHO report on neglected tropical diseases. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 2.WHA: Elimination of schistosomiasis (WHA 65.21). Geneva: WHO; 2012.
- 3.WHO: Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation. Geneva: WHO; 2012.
- 4.Nausch N, Dawson EM, Midzi N, Mduluza T, Mutapi F, Doenhoff MJ. Field evaluation of a new antibody-based diagnostic for Schistosoma haematobium and S. mansoni at the point-of-care in northeast Zimbabwe. BMC Infect Dis. 2014;14(1):165 doi: 10.1186/1471-2334-14-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmeier H, Poggensee G. Diagnostic techniques in schistosomiasis control–a review. ActanTrop 1993, 52:205–220. [DOI] [PubMed] [Google Scholar]
- 6.Alarcon de Noya BA, Ruiz R, Losada S, Colmenares C, Contreras R, Cesari IM, et al. : Detection of schistosomiasis cases in low-transmission areas based oncoprologic and serologic criteria–the Venezuelan experience. Acta Trop 2007, 103:41–49. doi: 10.1016/j.actatropica.2007.04.018 [DOI] [PubMed] [Google Scholar]
- 7.Enk MJ, Oliveira e Silva G, Rodrigues NB. Diagnostic accuracy and applicability of a PCR system for the detection of Schistosoma mansoni DNA in human urine samples from an endemic area. PLoS One 2012, 7:e38947 doi: 10.1371/journal.pone.0038947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibironke OA, Phillips AE, Garba A, Lamine SM, Shiff C. Diagnosis of Schistosoma haematobium by detection of specific DNA fragments fromfiltered urine samples. Am J Trop Med Hyg 2011, 84:998–1001. doi: 10.4269/ajtmh.2011.10-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulibaly JT, Goran EKN, Utzinger J, Doenhoff MJ, Dawson EM. A new rapid diagnostic test for detection of anti-Schistosoma mansoni and anti-Schistosoma haematobium antibodies. 2013:1–8. doi: 10.1186/1756-3305-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies?. Trends in parasitology. 2004. January 31;20(1):35–9. [DOI] [PubMed] [Google Scholar]
- 11.WHO: Schistosomiasis: population requiring preventive chemotherapy and number of people treated in 2010. WklyEpidemiol Rec 2012, 87:33–44. [PubMed] [Google Scholar]
- 12.WHO: Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children. Geneva: WHO; 2011.
- 13.Sousa-Figueiredo JC, Pleasant J, Day M, Betson M, Rollinson D, Montresor A, et al. Treatment of intestinalschistosomiasis in Ugandan preschool children: best diagnosis, treatment efficacy and side-effects, and an extended praziquanteldosing pole. Int Health 2010, 2:103–113. doi: 10.1016/j.inhe.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochodo EA, Gopalakrishna G, Spek B, Reitsma JB, van Lieshout L, Polman K, et al. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database of Systematic Reviews,2015. Issue 3 Art. No.: CD009579 doi: 10.1002/14651858.CD009579.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danso-Appiah A, Minton J, Boamah D, Otchere J, Asmah RH, Rodgers M, et al. Accuracy of point-of-care testing for circulatory cathodic antigen in the detection of schistosome infection: systematic review and meta-analysis.Bull World Health Organ 2016;94:522–533A| http://dx.doi.org/10.2471/BLT.15.158741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack, A. D., Chemotherapy in the Control of Schistosoma haematobium infections in The Gambia: A Primary Health Care Approach. Ph.D doctoral dissertation, London School of Hygiene and Tropical Medicine– 1989.
- 17.Wilkins H. A., Goll P. H., Marshall T. D. C., & Moore P. J. Dynamics of Schistosoma haematobium infection in a Gambian community. I. The pattern of human infection in the study area. Transactions of the Royal Society of Tropical Medicine and Hygiene, (1984). 78(2), 216–221. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins H. A., Goll P. H., Marshall T. D. C., & Moore P. J. Dynamics of Schistosoma haematobium infection in a Gambian community. I. The pattern of human infection in the study area. Transactions of the Royal Society of Tropical Medicine and Hygiene, (1984). 78(2), 216–221. [DOI] [PubMed] [Google Scholar]
- 19.Gambia Ministry of Health and Social Welfare. Neglected Tropical Diseases Situational Analysis Report EDC,2014
- 20.Gambia Ministry of Health and Social Welfare, National Master Plan Neglected Tropical Diseases 2015–2020, 2014
- 21.Urine cca test for schistosomiasis (bilharzia) manufacture instruction guide (http://www.rapid-diagnostics.com/downloads/RMD%20Pamphlet%20Just%2030.09.08.pdf
- 22.Coulibaly JT, Knopp S, N'Guessan NA, Silué KD, Fürst T, Lohourignon LK, et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS neglected tropical diseases. 2011. November 22;5(11):e1384 doi: 10.1371/journal.pntd.0001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PN, et al. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS neglected tropical diseases. 2011. January 25;5(1):e951 doi: 10.1371/journal.pntd.0000951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erko B, Medhin G, Teklehaymanot T, Degarege A, Legesse M. Evaluation of urine-circulating cathodic antigen (Urine-CCA) cassette test for the detection of Schistosoma mansoni infection in areas of moderate prevalence in Ethiopia. Trop Med Int Health. 2013;18(8):1029–1035. doi: 10.1111/tmi.12117 [DOI] [PubMed] [Google Scholar]
- 25.Colley D. G., Binder S., Campbell C., King C. H., Tchuem Tchuenté L.-A., N’Goran E. K., et al. (A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. The American Journal of Tropical Medicine and Hygiene 2013, 88(3), 426–32. doi: 10.4269/ajtmh.12-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stothard JR, Sousa-Figueiredo JC, Standley C, Van Dam GJ, Knopp S, Utzinger J, et al. An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta tropica. 2009. July 31;111(1):64–70. doi: 10.1016/j.actatropica.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Tchuenté LA, Fouodo CJ, Ngassam RI, Sumo L, Noumedem CD, Kenfack CM, et al. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS neglected tropical diseases. 2012. July 31;6(7):e1758 doi: 10.1371/journal.pntd.0001758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data gathered from the survey will be managed with utmost confidentiality and be made accessible to only the Ministry of Health personnel and partners such as WHO and The Task Force for Global Health before official publication. Since we have pledged to protect the data generated from this study, anonymized data will be available from Mr Bakary Sanneh, National Public Health Laboratories, The Gambia (email: sheikbakary@yahoo.com), and Kisito Ogoussan, Deputy NTD mapping Director, Taskforce for Global Health, USA (email kogoussan@taskforce.org).