Abstract
Objectives
The aim of this study was to systematically review data regarding pharmacokinetic (PK)-pharmacodynamic (PD) parameters from randomized controlled trials relating to interactions between herbal medicines and warfarin.
Methods
Three electronic databases were searched to identify relevant trials. Two reviewers independently performed the study selection and data extraction. The risk of bias and reporting quality were also assessed independently by two reviewers using the Cochrane risk of bias tool and the consolidated standards of reporting trials (CONSORT). Outcomes were measured for all reported PK-PD parameters and adverse events.
Results
Nine randomized controlled trials met our inclusion criteria. Most of the included studies were unclear regarding the risk of bias and had a low quality of methodology. Using CONSORT, the reporting percentages for the articles ranged from 36.5% to 61.5% and the mean percentage for all articles was 45.6%. St John’s wort and echinacea affected the PK parameters of warfarin. Ginseng, ginger, garlic, and cranberry had no significant effect on the PK parameters. American ginseng altered the PD parameters of warfarin. St John’s wort, ginseng, Korea red ginseng, ginkgo, ginger, garlic, aged garlic, and echincea did not significantly alter the PD parameters. Studies of ginkgo and cranberry showed conflicting results on the PK parameters and PD parameters, respectively. The incidence of adverse events in all trials was low and no major adverse events were reported.
Conclusions
It was difficult to determine whether ten herbal medicines had significant effects on the PK-PD parameters of warfarin. Low quality of evidence, different compounds within and different compositions of the herbs, and methodological limitations of the crossover study, which is a clinical study in which subjects receive a sequence of different interventions, made it difficult to form conclusions. Additional studies that remedy these vulnerabilities are necessary to verify these results.
Introduction
Warfarin is the most common oral anticoagulant used for treating or preventing thromboembolic disorders. It has a narrow range between therapeutic and toxic doses, suggesting that warfarin should be administered after calculating the optimal dose.
Patients taking warfarin should also be aware of its interaction with other drugs and foods, including herbal medicines, because the concomitant use of these agents might alter the metabolism and action of warfarin, necessitating an adjustment to the dose of warfarin for its safe and effective administration [1, 2]. Close monitoring of the anticoagulant effect of warfarin is recommended through the international normalized ratio (INR) in clinical practice [1–3].
Herbal medicines are often used by patients receiving anticoagulants. Nearly 40% of patients with cardiovascular disease have used complementary and alternative medicine, including herbal medicine, concomitantly with their prescribed medications [4]. Those who used herbal medicine for health management perceived herbal medicine to be helpful for their cardiac condition [5]. In fact, some herbs, such as ginger, ginkgo, and garlic, have antiplatelet and anticoagulant activity [6–11]. However, the mechanism of action of herbal medicines is difficult to study in vitro and in vivo because these medicines comprise complex mixtures of various compounds [12], which may simultaneously exhibit multiple physiological activities. Therefore, patients taking herbal medicine with warfarin are more likely to be exposed to potential herb-drug interactions [13].
Previous studies have revealed some of the mechanisms of interaction of warfarin with herbal medicines via clinical reports [2, 14]. Each herb has a different chemical composition, hindering generalizations about herb-warfarin interactions. Although narrative reviews on herb-warfarin interactions are available [1, 5], no study has systematically reviewed them based on changes in pharmacokinetic (PK)-pharmacodynamic (PD) parameters.
The aim of this article was to systematically review clinical data, including PK-PD parameters, from randomized controlled trials (RCTs) and to discuss interactions between herbal medicines and warfarin.
Methods
Sources of information and search strategies
Clinical trials were searched for and retrieved from core electronic databases, including PubMed, EMBASE, and CINAHL. The last search of the databases was performed in December 2015.
Search terms consisted of text terms and controlled vocabulary, such as medical subject headings (MeSH). Three types of search terms were used: warfarin-related terms, herb-related terms, and interaction-related terms. Article type or study design-related terms were not included in the search terms. The search strategy for PubMed is stated below. The search terms for the two other databases were similar.
#1 Warfarin [MeSH Terms]
#2 Warfarin [Title/Abstract]
#3 1–2 /or
#4 Dietary supplementations [MeSH Terms]
#5 Dietary supplement* [Title/Abstract]
#6 Plant, medicinal [MeSH Terms]
#7 Phytotherapy [MeSH Terms]
#8 Medicine, traditional [MeSH Terms]
#9 Pharmacognosy [MeSH Terms]
#10 Plant extracts [MeSH Terms]
#11 Ethnobotany [MeSH Terms]
#12 Ethnopharmacology [MeSH Terms]
#13 Diet, Food, and Nutrition [MeSH Terms]
#14 Plant* [Title/Abstract]
#15 Herb* [Title/Abstract]
#16 4-15/or
#17 Drug interactions [MeSH Terms]
#18 Interaction* [Title/Abstract]
#19 17-18/or
#20 3 AND 16 AND 19
Study selection
Two reviewers (SIC and UMJ) reviewed the titles and abstracts of the studies retrieved from the electronic searches to identify studies that met the inclusion criteria. Disagreements were resolved by discussion between the two reviewers or consultation with a third reviewer (DSO). No language restriction was applied. The inclusion criteria were as follows:
Type of study. All relevant RCTs that reported interactions between herbal medicines and warfarin were included.
Type of participant. Studies that evaluated subjects who received herbal medicine concomitantly with warfarin were included.
Type of intervention. Trials using warfarin alone or warfarin with placebo drug versus warfarin with herbal medicine were included. An herb was defined as a product or an extract originating from a single botanical source. The definition of herb included raw or manufactured single or complex medicinal plants, plant extracts, and dietary supplements. However, single or synthesized substances from plant material were excluded.
Type of outcome measures. Studies that measured more than one PK or PD parameter for herb-warfarin interactions were included. Because the inhibition of the metabolism of S-warfarin is clinically more important than the inhibition of the metabolism of R-warfarin, the PK of R-warfarin were not investigated in this study [15, 16]. The PK parameters included time to maximum plasma concentration (Tmax), maximum plasma concentration at steady state (Cmax), apparent volume of distribution after extra vascular administration (V/F), fraction of total drug unbound in plasma (fu), terminal half-life (T1/2), apparent plasma clearance of drug after extra vascular administration (CL/F), and area under the plasma concentration-time curve from zero to infinity (AUCinf). The PD parameters included all outcomes that reflected the biochemical and physiological effects of warfarin on the human body.
Data extraction and quality assessment
Data were extracted from the titles and abstracts of the searched studies independently by two reviewers. The study selection and data extraction used standard eligibility inclusion criteria as determined by two reviewers. The quality of methodology in all included studies was independently assessed according to the Cochrane Collaboration’s seven criteria: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, 6) selective reporting, and 7) other bias (defined as baseline data comparability). For each domain, the evaluation was denoted as low risk, high risk, or unclear risk, according to the description of the methods used in each study.
We also assessed the reporting quality of all included studies based on the Consolidated Standards of Reporting Trials (CONSORT) [17]. We used the CONSORT 2010 checklist and the extension of the CONSORT statement simultaneously for trials of herbal medicinal interventions. The Consort 2010 checklist is the latest version for assessing reporting quality. The elaborated CONSORT statements for trials of herbal interventions enhance the checklist items regarding the relevance to trials of herbal interventions [18, 19].
Data analysis
Study design and herbal medicines were analyzed among the included studies. Results of PK or PD parameters and type and proportion of adverse events in concurrent use of herbal medicine and warfarin groups were compared with those in warfarin alone groups to identify whether herbal medicine significantly affected the PK or PD parameters of warfarin. Quantitative data synthesis was planned in a meta-analysis when the study design, type of herbal medicine, and outcomes of the included studies were homogeneous; otherwise, we suggested results in a narrative synthesis without meta-analysis [20].
Results
Description of included studies
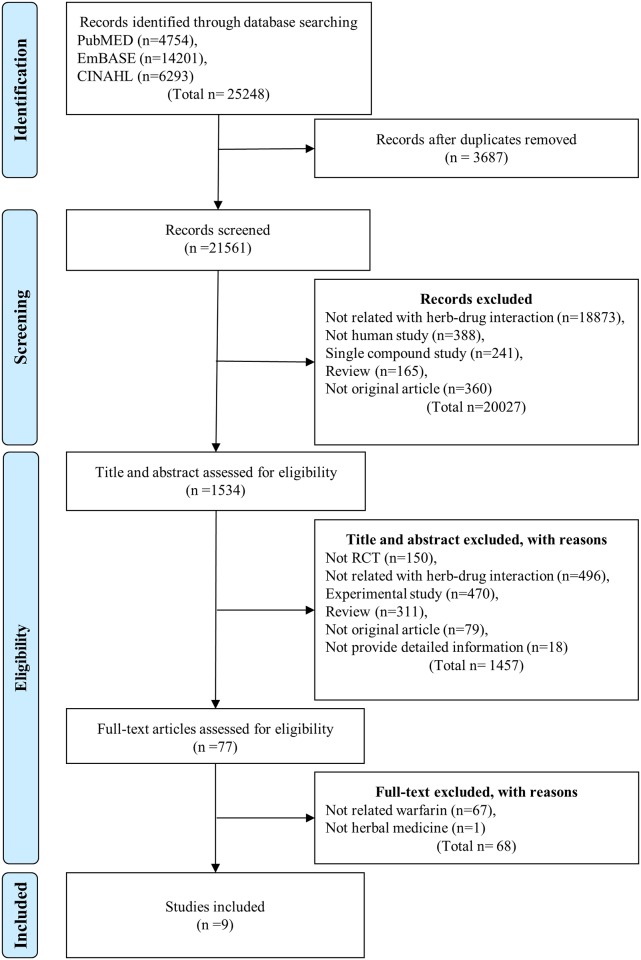
The search generated a total of 4437 potentially relevant studies; 295 duplicate and 4065 irrelevant studies were excluded by screening the titles and abstracts. Of the remaining studies, 77 full-text articles were reviewed and 9 studies [21–29] met our eligibility criteria. The PRISMA diagram of the search process and study selection is presented in Fig 1.
Fig 1. PRISMA flow diagram for selecting related articles.
Of these trials, three [23–25] were conducted in the United States, four [21, 22, 26, 28] were conducted in Australia, and one [27] was conducted in Korea. One trial [29] was conducted in China and published in Chinese. Four studies [21, 22, 26, 28] had a three-way cross-over randomized design and compared two different herbal medicine plus warfarin groups with a warfarin alone group in healthy subjects. Three studies [23, 25, 29] used a placebo-controlled parallel design and compared the concomitant administration of herbal medicine and warfarin with the concomitant administration of placebo and warfarin. Two studies [24, 27] used a double-blind crossover design. The study sample sizes ranged from 7 to 48 and a total of 160 subjects were involved in the nine trials. Thirty participants were reported to have dropped out of the nine studies. The key data from the included RCTs are summarized in Table 1.
Table 1. Characteristics of the included studies (n = 9).
| 1stauthor, Country | Jiang [21], Australia | Jiang [22], Australia | Yuan [23], United States | Li [24], United States | Macan [25], United States | Abdul [26], Australia | Lee [27], Republic of Korea | Abdul [28], Australia | Zhou [29], China |
|---|---|---|---|---|---|---|---|---|---|
| Study design (RCT) | Open-label, three-way crossover | Open-label, three-way crossover | Double-blind, placebo-controlled | Double-blind, crossover | Double-blind, placebo-controlled | Open-label, three- way treatment crossover | Double-blind, crossover | Open-label, three-way treatment crossover | Two-way treatment, placebo-controlled crossover |
| The number of subjects(The number of male subjects) | 12(12) | 12(12) | 20(9) | 7(7) | 48(30) | 12(12) | 25(4) | 12(12) | 12(6) |
| Type of subjects | Healthy volunteers | Healthy volunteers | Healthy volunteers | Atrial fibrillation patients | Deep vein thrombosis, cerebro-vascular accident, thrombosis, valvular heart disease, atrial fibrillation, or prosthetic heart valves patients | Healthy volunteers | Cardiac valve replacement patients | Healthy volunteers | Healthy volunteers |
| Age | 20–40 | 20–36 |
|
68.8 (Mean) | 56 (Mean) | 18–34 | - | 18–34 | 19–24 |
| Intervention in treatment group (+warfarin) |
|
|
America Ginseng | Cranberry | Aged Garlic |
|
Korea red ginseng | Echinacea | Ginkgo |
| Intervention in control group (+warfarin) | None | None | Placebo | Placebo | Placebo | None | Placebo | Placebo | Placebo |
| Treatment Period (1st/2nd period, if cross-over) | 2weeks/1week | 1week/1week | 4weeks | 1week/1week | 12weeks | 2weeks/1week | 6weeks | 2week/1week | 5weeks |
| Washout Period | 2weeks | 2weeks | Not applicable | 1week | Not applicable | 2weeks | 3weeks | 2weeks | - |
| The number of observed adverse events | 3 | 1 | 0 | Not reported | 0 | 4 | Not reported | 0 | 0 |
| Results reported |
|
No affect the pharmacokinetics or pharmacodynamics of either S-warfarin and coagulation status | Reduces the anticoagulant effect of warfarin | No any significant interaction | No increase in the incidence of hemorrhages |
|
Not enhance the anticoagulation effect | No affected warfarin pharmacodynamics, platelet aggregation or baseline clotting status | No effects on the pharmacodynamics of single dose warfarin |
Abbreviations: INR, International Normalized Ratios.
Herbal medicine
Nine herbal medicines were identified in the included studies: Panax ginseng, Panax quinquefolius, Allium sativum, Gingko biloba, Vaccinium macrocarpon, Hypericum perforatum, Echinacea angustifolia, Echinacea purpurea, and Zingiber officinale. Ginseng [21, 23, 27] was administered in three studies, but three ginsengs that have different scientific names were used: Korean ginseng root (Panax ginseng) [21], American ginseng root (Panax quinquefolius) [23], and Korea red ginseng (steamed Panax ginseng) [27]. Garlic (Allium sativum)was administered in two studies, but each study used garlic manufactured with a different process [25, 26]. One study [25] used an aged garlic product that was made by soaking raw garlic in ethanol, whereas the other study [26] used an enteric-coated garlic tablet. Gingko (Gingko biloba) [22, 29] and cranberry (Vaccinium macrocarpon) [24, 26] were used in two studies and St John’s wort (Hypericum perforatum) [21], echinacea (Mixture of Echinacea angustifolia and Echinacea purpurea) [28], and ginger (Zingiber officinale) [22] were administered in one study. In addition, policosanol [28] was mentioned in one study. However, policosanol is a complex mixture of fatty alcohols derived from sugar cane wax and was not included in the inclusion criteria. Extraction and formulation method, composition, and bioanalytical data regarding the herbal preparations from the included RCTs are summarized Table 2.
Table 2. Herbal preparations of the included studies (n = 9).
| 1stauthor | Jiang [21] | Jiang [22] | Yuan [23] | Li [24] | Macan [25] | Abdul [26] | Lee [27] | Abdul [28] | Zhou [29] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material (Scientific name) | St John's wort (Hypericum perforatum) | Panax Ginseng (Panax ginseng) | Ginkgo (Gingko bilba) | Ginger (Zingiber officinale) | America Ginseng (Panax quinquefolius) | Cranberry (Vaccinium macrocarpon) | Garlic (Allium sativum) | Cranberry (Vaccinium macrocarpon) | Garlic (Allium sativum) | Red ginseng (Panax ginseng) | Echinacea (Echinacea angustifolia and Echinacea purpurea) | Ginkgo (Gingko biloba) |
| Preprocessing of material | ND | ND | ND | ND | Grinding | ND | Slicing | ND | ND | Steaming | ND | ND |
| Type of extract | Dry extract | Dry extract | Dry extract | ND | None | Juice | Long-term maceration | ND | ND | Decoction | ND | ND |
| Solvent | ND | ND | ND | ND | None | ND | Aqueous ethanol | ND | ND | Water | ND | ND |
| Formulation type | Tablet | Capsule | Tablet | Capsule | Capsule | Packaged liquid | Solid | Capsule | Tablet | Powder | Tablet | Tablet |
| Commerical product | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | ND | Yes | ND |
| Constituents & Qualty Control |
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: ND, not described
Risk of bias assessment
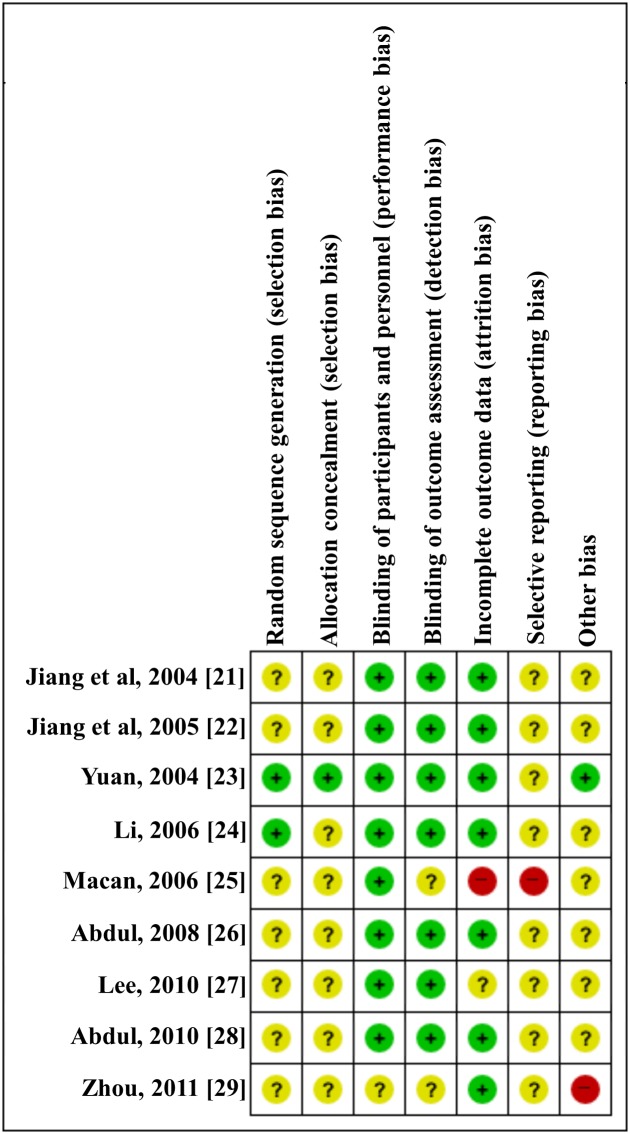
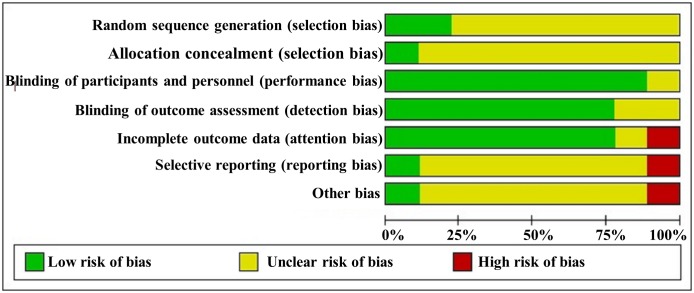
No study had a low risk of bias in all seven domains. For random sequence generation, two [23] of the nine studies (22%) used a random table, whereas the other studies (78%) did not report a specific method of random sequence generation. For allocation concealment, one study [23, 24] (11%) used an opaque envelope method, whereas the other studies (89%) did not report any information about concealment. For blinding, four studies [23–25, 27] (44%) used a double-blinding method by blinding participants and researchers and four studies [21, 22, 26, 28] had an open-label design. One study [29] did not provide information about blinding. For incomplete outcome data, seven trials [21–24, 26, 28, 29] reported detailed information regarding attrition by describing the number and reasons for withdrawal. For selective outcome reporting, only one study [26] presented the clinical trial identifier number, whereas the other trials did not report registration information. Therefore, we could not compare the protocols and trial reports. Information for other risks of bias was not reported in the studies, except for one study [29] that was at high risk. The risk of bias assessment information is presented in Figs 2 and 3.
Fig 2. Risk of bias summary.
Review of authors' judgments about each risk of bias item for all nine included studies. Plus (+) marked circle, Low risk of bias; Question (?) marked circle, Unclear risk of bias; Minus (-) marked circle, High risk of bias.
Fig 3. Risk of bias graph.
Review of authors' judgments about each risk of bias item presented as percentages across all included studies.
Reporting quality
Based on the two CONSORT statements, the reporting percentage for each of the articles ranged from 36.5% to 61.5% and the mean percentage for all articles was 45.6%. All RCTs described the eligibility criteria, participant flow, and interpretation of the results. No trials provided information about the qualitative testing of interventions, periods of recruitment, or follow up. Detailed results are presented in the S1 Table.
Because the two CONSORT statements were developed with the aim of evaluating and reporting quality for parallel design and two treatment groups, they may be insufficient to evaluate and reflect the characteristics of the crossover design. However, most of the items in the CONSORT checklist apply to all trial designs because they reflect the characteristics of RCTs rather than those of parallel design. Therefore, the CONSORT statements were used to assess the reporting quality of all the included trials.
Outcomes
There are many different methods of measuring outcome parameters. Because the aim of this review was to assess herb-warfarin interactions, various types of PK-PD parameters were evaluated (S2 Table).
Five trials [21, 22, 26, 28, 29] reported PK data based on the absorption, distribution, metabolism, or elimination of warfarin when herbal medicine was co-administered with warfarin. There were three herbs that affected the PK of warfarin in healthy subjects. St John’s wort [21] increased S-warfarin clearance and reduced R-warfarin clearance. Echinacea [28] increased the apparent clearance of warfarin. One study [29] reported that ginkgo significantly increased Cmax, AUCinf, and T1/2 and decreased the CL/F of warfarin, whereas another study [22] reported that ginkgo did not markedly change the PK parameters of warfarin. There were no significant changes in the PK parameters of warfarin when ginseng [21], ginger [22], garlic [26], and cranberry [26] were co-administered. Garlic and cranberry did not affect the S-warfarin clearance in subjects with different genotypes of cytochrome P450 2C9 (CYP2C9) [26]. Whereas garlic increased the half maximal effective concentration (EC50) of S-warfarin in the subjects with the CC genotype of vitamin K epoxide reductase complex (VKORC1), cranberry decreased the EC50 of S-warfarin in the subjects with the CT or TT genotype of VKORC1 [26].
The PD parameters of warfarin were reported when ten herbal medicines, including ginkgo, ginger, ginseng, St John’s wort, echinacea, cranberry, Korean red ginseng, American ginseng, garlic, and aged garlic were co-administered with warfarin. The AUC of INR for time to treatment was used in six studies [21–23, 26, 28, 29], INR max [26, 28] was evaluated in two studies, and INR change was reviewed in two studies [24, 27]. Five studies [21, 22, 26, 28, 29] used area under concentration-time curves until the last concentration observation (AUCobs) as an outcome measure. Platelet aggregation [21, 22], peak INR change [23], vitamin K intake [23], prothrombin time [29], and incidence of hemorrhage [25] were also included as PD parameters. The results of two studies [23, 26] indicated that co-administration of herbal medicine altered the PD parameters of warfarin. The results of one study indicated that cranberry significantly increased the area under the INR-time curve when administered with warfarin in healthy subjects [26]. However, the results of another study indicated that cranberry did not markedly change INR values in patients with atrial fibrillation [24]. American ginseng also reduced the anticoagulant effect of warfarin [23]. There were no significant changes in the PD parameters of warfarin when St John’s wort [21], ginseng [21], Korea red ginseng [27], ginkgo [22, 29], ginger [22], garlic [26], aged garlic [25], and echinacea [28] were co-administered with warfarin.
Adverse events
All trials evaluated adverse events (AEs). As shown in Table 1, AEs were reported for three studies and none of the events was major. Among these events, one study [22] reported that one subject experienced gastrointestinal side effects, including constipation, during the first two days of ginkgo pre-treatment and mild diarrhea during the first two days of ginger pre-treatment. One study [21] reported that three subjects experienced changes in sleeping habits during St John’s wort treatment and one study [26] reported rashes in two subjects using cranberry-warfarin. In studies including garlic, one subject had evidence of nasal bleeding and one subject reported lip dryness.
Discussion
In this review, the interaction between ten herbs and warfarin, as indicated by changes in the PK and PD parameters of warfarin, was analyzed based on published evidence. We assessed the methodological quality of RCTs using the Cochrane risk of bias tool and CONSORT and the results of quality assessments were reflected in the interpretation of this study. Two herbs (St John’s wort and echinacea) affected the PK parameters of warfarin, whereas four herbs (ginseng, ginger, garlic, and cranberry) did not. There were conflicting results as to whether ginkgo affected the PK parameters of warfarin. American ginseng changed the PD parameters of warfarin, but eight herbal medicines (St John’s wort, ginseng, Korea red ginseng, ginkgo, ginger, garlic, aged garlic, and echinacea) did not. There were mixed results as to whether cranberry changed the PD parameters of warfarin. There was a low risk of AEs after co-administration of herbs and warfarin. However, most of the included studies had low reporting quality and a crossover design that was unsuitable for meta-analysis. There were also inconsistent results from several studies that used the same herbal medicine.
The use of herbal medicine is rapidly expanding and many reports have raised concerns about possible herb-drug interactions. Herb-drug interactions may be categorized as either PK or PD interactions. PK interactions include changes in absorption, distribution, metabolism, and elimination [30]. PD interactions result from synergistic, additive, or antagonistic effects of herbs when co-administered with drugs. Well-aligned PK-PD data provides information regarding clinical efficacy and safety outcomes and guides the selection of doses and dosing schedules for clinical trials [31].
All included studies investigated the PD interactions of warfarin with herbal medicines, but different parameters were used in each study. Only four studies reported PK interaction parameters between an herbal medicine and warfarin, whereas the five remaining studies did not measure PK parameters. There were inconsistent results among those that used the same herbal medicine. Two studies that evaluated the interaction between cranberry and warfarin reported contradictory PD effects, possibly because one study [26] investigated healthy subjects and the other [24] included patients with atrial fibrillation. The results of two studies that assessed the PK-PD interactions between warfarin and ginkgo also differed. One study [22] reported no significant differences in PK-PD parameters, whereas another [29] observed that ginkgo had limited effects on PK parameters. These studies used different types of clinical designs and subject conditions. Three studies [21, 23, 27] investigated the interaction between ginseng and warfarin and the results differed according to the type of ginseng used (Korean ginseng root (Panax ginseng), American ginseng root (Panax quinquefolius), or Korea red ginseng (steamed Panax ginseng)). Korean ginseng and American ginseng have different ginsenoside profiles [32] and Korea red ginseng contains converted ginsenosides transformed from the ginsenosides in fresh ginseng [33]. The heterogeneous composition of compounds among the three types of ginseng might have led to different results.
Several case reports have pointed to the risk associated with concomitant herb and warfarin use. There were two case reports of an increased INR after co-administration of warfarin with cranberry juice [34, 35]. Interactions between ginseng and warfarin were also mentioned in one case report [36]. Two case reports suggested that a warfarin-St John’s wort interaction was associated with a change in INR [37, 38]. These relevant case reports indicated a potential herb-warfarin interaction, but it was difficult to identify a causal relationship. Suspected herb-warfarin interactions are primarily limited to anecdotal case reports. In addition, these case reports did not provide sufficient information about the patients’ medical records and compounding factors may have existed, such as administration of other medications, dietary supplements, foods, or alcohol intake. Some studies pointed out such limitations, that is, that case reports often result in misleading conclusions for multiple reasons [39, 40]. There have been previous experiments and clinical studies of platelet aggregation caused by herbs. Several studies have suggested that herbal constituents may affect PK-PD and alter the anticoagulant and platelet aggregation effects of warfarin [41, 42].
Other studies have shown conflicting results as to whether herb-warfarin interactions were associated with increased risks. Garlic and ginger are known potent inhibitors of platelet aggregation [43]. One review article reported that spontaneous bleeding occurred during the concurrent use of warfarin and these herbs. Conversely, some cases did not indicate a significant inhibition of platelet function [44]. One in vitro study suggested that gingko contributed to the altered platelet aggregation [45]. In contrast, a clinical study confirmed that gingko did not change platelet aggregation. Furthermore, it was difficult to determine whether the combined use of warfarin and herbs led to increased platelet aggregation [46].
Warfarin is predominantly metabolized via CYP2C9 and changes in CYP2C9 may significantly alter the PK-PD parameters of warfarin [35, 39]. However, an in vivo study indicated that echinacea did not significantly affect the metabolism of drugs metabolized by CYP2C9 [47]. Another in vivo study reported that gingko induced CYP enzyme activity in a dose-dependent manner, but did not cause hepatic damage [48]. Two clinical trials also evaluated the effects of ginkgo in healthy volunteers and the results indicated that warfarin concentrations did not significantly change with concomitant administration of ginkgo [22, 29]. These results implied that concerns regarding increased hemorrhagic complications resulting from an herb-warfarin interaction were unfounded. This study also indicated that herbal medicine might not lead to a clinically significant change in the PK-PD parameters of warfarin. Furthermore, herbal medicine did not significantly alter the anticoagulant effects of warfarin and no severe AEs were reported.
There were four limitations of the current review. First, the rationale for determining the washout period in each study was lacking. Several studies adopted a crossover design, which has been used by many researchers to investigate potential drug-drug interactions. Because a crossover trial carries the risk of a carry-over effect, trials with a crossover design should use a sufficient washout period. Otherwise, the effect of the first period treatment may persist into the subsequent period [49]. The average washout period in seven studies was two weeks and one study did not mention the washout period. To minimize the carry-over effect, the washout period in a crossover study should be at least five times the half-life of the drug [50]. The mean range of T1/2 was 29.2 to 38.7 hours for the control group and 27.2 to 76.6 hours for the experimental group. The washout period was calculated to be 6–8 days for the control group and 6–16 days for the experimental group based on these results. These results included studies for which it was difficult to determine if the washout period was sufficient.
Second, clinical data are lacking in order to provide synthesized evidence for herb-drug interactions from crossover trials. We attempted to conduct a meta-analysis using five trials that assessed the effects of herbs combined with warfarin versus warfarin alone or warfarin combined with placebo. A meta-analysis for crossover trials can be conducted if one of the following three measurements is available: 1) individual subject data, 2) the mean and standard deviation (or standard error) of the subject-specific differences between the experimental group and control group, 3) the mean difference and variables from a paired t-test (28). However, no studies were reported these data. Therefore, it was impossible to synthesize the data into a meta-analysis.
Third, the reporting quality of the included studies was poor based on CONSORT 2010 and the extension of the CONSORT statement for trials of herbal medicine interventions. This CONSORT extension enhances the checklist items regarding the relevance of herbal interventions to trials [19]. Several studies did not describe some of the items in this elaborated statement. For example, some studies autonomously prepared the intervention drug or herbal medicine without a quality control, making it difficult to report the characteristics of the herbal product, including the concentration of the extraction solvent, the method of the authentication of raw material, fingerprinting, and standardization. The majority of studies also did not discuss randomization, including sequence generation, allocation, and concealment. The four crossover design studies did not report how the treatment group and control group were crossed. Therefore, the response rate for each article was less than 50%.
Finally, all included RCTs focused on changes in the PK-PD parameters of warfarin and not those of the herbal medicine. Each single herb has a variety of biochemical compounds and the composition of components in the herb could vary upon cultivation, delivery, and product manufacturing conditions. These uncertainties and complexities make it difficult to determine standards for the PK-PD parameters of herbal medicines. Therefore, this study could not include PK-PD evaluation of herbal medicines themselves and did not reflect the purpose of the administration and intention for use of the herbal medicine.
Further studies evaluating interactions between warfarin and herbal medicines should avoid the limitations mentioned in this study. The details of the information for a trial should be clearly described and fully reported. The crossover design is a common study design, but is inappropriate for obtaining valid evidence through meta-analysis. We recommend using high-quality RCTs to confirm herb-warfarin interactions. In addition, to ensure the quality of the clinical trials, randomization and allocation concealment procedures should be performed to minimize bias.
Although there are some limitations, to our knowledge, this study was the first systematic review of the clinical outcomes and PK-PD effects of herb-warfarin interactions. We deliberately selected related studies after searching a wide range of databases and this analysis was based on available clinical trials that evaluated herb-warfarin interactions.
Conclusions
It was difficult to decide whether ten herbal medicines significantly affected the PK-PD parameters of warfarin. Low quality of evidence, herbal uncertainties and complexities of different compounds and their compositions, and methodological limitations of the crossover study made it difficult to form conclusions. Further studies with an appropriate study design and reporting quality are necessary to verify herb-warfarin interactions.
Supporting information
(DOCX)
(DOCX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Research and development of concomitant administration on combinatory effect of herbal and Western medicine (No. K17252, grant recipient DSO) and development of Korean Medicine contents for clinical practice (No.K17124, grant recipient UMJ) of the Korea Institute of Oriental Medicine. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
References
- 1.Ge B, Zhang Z, Zuo Z. Updates on the clinical evidenced herb-warfarin interactions. Evid Based Complement Alternat Med. 2014;2014:957362 Epub 2014/05/03. doi: 10.1155/2014/957362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nutescu EA, Shapiro NL, Ibrahim S, West P. Warfarin and its interactions with foods, herbs and other dietary supplements. Expert Opin Drug Saf. 2006;5(3):433–51. Epub 2006/04/14. doi: 10.1517/14740338.5.3.433 . [DOI] [PubMed] [Google Scholar]
- 3.Nutescu EA, Shapiro NL, Chevalier A, Amin AN. A pharmacologic overview of current and emerging anticoagulants. Cleve Clin J Med. 2005;72 Suppl 1:S2–6. . [DOI] [PubMed] [Google Scholar]
- 4.Yeh GY, Davis RB, Phillips RS. Use of complementary therapies in patients with cardiovascular disease. Am J Cardiol. 2006;98(5):673–80. doi: 10.1016/j.amjcard.2006.03.051 . [DOI] [PubMed] [Google Scholar]
- 5.Sanjai KS, Aiswarya P. Warfarin interactions with complementary medicines, herbs, and dietary supplements. Journal of Chemical and Pharmaceutical Research. 2015;7(6):71–5. [Google Scholar]
- 6.Rui TQ, Zhang L, Qiao HZ, Huang P, Qian S, Li JS, et al. Preparation and Physicochemical and Pharmacokinetic Characterization of Ginkgo Lactone Nanosuspensions for Antiplatelet Aggregation. J Pharm Sci. 2016;105(1):242–9. doi: 10.1016/j.xphs.2015.10.002 . [DOI] [PubMed] [Google Scholar]
- 7.Ryu KH, Han HY, Lee SY, Jeon SD, Im GJ, Lee BY, et al. Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb Res. 2009;124(3):328–34. Epub 2009/04/08. doi: 10.1016/j.thromres.2009.02.010 . [DOI] [PubMed] [Google Scholar]
- 8.Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardiol. 2009;131(3):408–9. Epub 2007/11/27. doi: 10.1016/j.ijcard.2007.07.107 . [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez M, Ringstad L, Schafer P, Just S, Hofer HW, Malmsten M, et al. Reduction of atherosclerotic nanoplaque formation and size by Ginkgo biloba (EGb 761) in cardiovascular high-risk patients. Atherosclerosis. 2007;192(2):438–44. doi: 10.1016/j.atherosclerosis.2007.02.021 . [DOI] [PubMed] [Google Scholar]
- 10.Lau AJ, Toh DF, Chua TK, Pang YK, Woo SO, Koh HL. Antiplatelet and anticoagulant effects of Panax notoginseng: comparison of raw and steamed Panax notoginseng with Panax ginseng and Panax quinquefolium. J Ethnopharmacol. 2009;125(3):380–6. Epub 2009/08/12. doi: 10.1016/j.jep.2009.07.038 . [DOI] [PubMed] [Google Scholar]
- 11.Yun YP, Do JH, Ko SR, Ryu SY, Kim JH, Song HC, et al. Effects of Korean red ginseng and its mixed prescription on the high molecular weight dextran-induced blood stasis in rats and human platelet aggregation. J Ethnopharmacol. 2001;77(2–3):259–64. Epub 2001/09/06. . [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia. 2014;92:133–47. doi: 10.1016/j.fitote.2013.10.010 . [DOI] [PubMed] [Google Scholar]
- 13.Tsai HH, Lin HW, Lu YH, Chen YL, Mahady GB. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One. 2013;8(5):e64255 Epub 2013/05/15. doi: 10.1371/journal.pone.0064255 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69(13):1777–98. doi: 10.2165/11317010-000000000-00000 . Language: English. Entry Date: 20091106. Revision Date: 20110520. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh J. Current anticoagulant therapy—Unmet clinical needs. Thrombosis Research. 2003;109(SUPPL.):S1–S8. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Blair EY, McLachlan AJ. Investigation of the effects of herbal medicines on warfarin response in healthy subjects: a population pharmacokinetic-pharmacodynamic modeling approach. J Clin Pharmacol. 2006;46(11):1370–8. Epub 2006/10/20. doi: 10.1177/0091270006292124 . [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869 doi: 10.1136/bmj.c869 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol. 2006;59(11):1134–49. doi: 10.1016/j.jclinepi.2005.12.020 . [DOI] [PubMed] [Google Scholar]
- 19.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144(5):364–7. . [DOI] [PubMed] [Google Scholar]
- 20.Ferrer P, Ballarin E, Sabate M, Vidal X, Rottenkolber M, Amelio J, et al. Antiepileptic drugs and suicide: a systematic review of adverse effects. Neuroepidemiology. 2014;42(2):107–20. doi: 10.1159/000356807 . [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57(5):592–9. Epub 2004/04/20. doi: 10.1111/j.1365-2125.2003.02051.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59(4):425–32. Epub 2005/04/02. doi: 10.1111/j.1365-2125.2005.02322.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan CS, Wei G, Dey L, Karrison T, Nahlik L, Maleckar S, et al. Brief communication: American ginseng reduces warfarin's effect in healthy patients: a randomized, controlled Trial. Ann Intern Med. 2004;141(1):23–7. Epub 2004/07/09. . [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Seeram NP, Carpenter CL, Thames G, Minutti C, Bowerman S. Cranberry does not affect prothrombin time in male subjects on warfarin. Journal of the American Dietetic Association. 2006;106(12):2057–61. doi: 10.1016/j.jada.2006.09.012 . Language: English. Entry Date: 20070202. Revision Date: 20120302. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 25.Macan H, Uykimpang R, Alconcel M, Takasu J, Razon R, Amagase H, et al. Aged garlic extract may be safe for patients on warfarin therapy. J Nutr. 2006;136(3 Suppl):793S–5S. Epub 2006/02/18. . [DOI] [PubMed] [Google Scholar]
- 26.Mohammed Abdul MI, Jiang X, Williams KM, Day RO, Roufogalis BD, Liauw WS, et al. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br J Pharmacol. 2008;154(8):1691–700. Epub 2008/06/03. doi: 10.1038/bjp.2008.210 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YH, Lee BK, Choi YJ, Yoon IK, Chang BC, Gwak HS. Interaction between warfarin and Korean red ginseng in patients with cardiac valve replacement. Int J Cardiol. 2010;145(2):275–6. Epub 2009/11/17. doi: 10.1016/j.ijcard.2009.09.553 . [DOI] [PubMed] [Google Scholar]
- 28.Abdul MI, Jiang X, Williams KM, Day RO, Roufogalis BD, Liauw WS, et al. Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br J Clin Pharmacol. 2010;69(5):508–15. Epub 2010/06/25. doi: 10.1111/j.1365-2125.2010.03620.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Zeng R. Effects of Ginkgo biloba extract on anticoagulation and blood drug level of warfarin in healthy wolunteers. Zhongguo Zhong Yao Za Zhi. 2011;36(16):2290–3. Epub 2011/11/22. . [PubMed] [Google Scholar]
- 30.Hussain MS. Patient counseling about herbal-drug interactions. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):152–63. doi: 10.4314/ajtcam.v8i5S.8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roskos LK, Schneider A, Vainshtein I, Schwickart M, Lee R, Lu H, et al. PK-PD modeling of protein drugs: implications in assay development. Bioanalysis. 2011;3(6):659–75. doi: 10.4155/bio.11.28 . [DOI] [PubMed] [Google Scholar]
- 32.Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34(3):160–7. doi: 10.5142/jgr.2010.34.3.160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SM, Kim SC, Oh J, Kim JH, Na M. 20(R)-Ginsenoside Rf: A new ginsenoside from red ginseng extract. Phytochemistry Letters. 2013;6(4):620–4. doi: 10.1016/j.phytol.2013.08.002 [Google Scholar]
- 34.Paeng CH, Sprague M, Jackevicius CA. Interaction between warfarin and cranberry juice. Clinical Therapeutics. 2007;29(8):1730–5. doi: 10.1016/j.clinthera.2007.08.018 . Language: English. Entry Date: 20080314. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 35.Sylvan L, Justice NP. Possible interaction between warfarin and cranberry juice. Am Fam Physician. 2005;72(6):1000; author reply Epub 2005/09/30. . [PubMed] [Google Scholar]
- 36.Janetzky K, Morreale AP. Probable interaction between warfarin and ginseng. Am J Health Syst Pharm. 1997;54(6):692–3. Epub 1997/03/15. . [DOI] [PubMed] [Google Scholar]
- 37.Yue QY, Bergquist C, Gerden B. Safety of St John's wort (Hypericum perforatum). Lancet. 2000;355(9203):576–7. doi: 10.1016/S0140-6736(05)73227-X [DOI] [PubMed] [Google Scholar]
- 38.Barnes J, Anderson LA, Phillipson JD. St John's wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53(5):583–600. Epub 2001/05/24. . [DOI] [PubMed] [Google Scholar]
- 39.Pham DQ, Pham AQ. Interaction potential between cranberry juice and warfarin. American Journal of Health-System Pharmacy. 2007;64(5):490–4. doi: 10.2146/ajhp060370 . Language: English. Entry Date: 20070525. Revision Date: 20120302. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 40.Zikria J, Goldman R, Ansell J. Cranberry juice and warfarin: when bad publicity trumps science. Am J Med. 2010;123(5):384–92. doi: 10.1016/j.amjmed.2009.08.019 . [DOI] [PubMed] [Google Scholar]
- 41.Fasinu PS, Bouic PJ, Rosenkranz B. An overview of the evidence and mechanisms of herb-drug interactions. Frontiers in Pharmacology. 2012;3 APR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izzo AA, Di Carlo G, Borrelli F, Ernst E. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol. 2005;98(1):1–14. Epub 2005/01/29. doi: 10.1016/j.ijcard.2003.06.039 . [DOI] [PubMed] [Google Scholar]
- 43.Ramsay NA, Kenny MW, Davies G, Patel JP. Complimentary and alternative medicine use among patients starting warfarin. Br J Haematol. 2005;130(5):777–80. Epub 2005/08/24. doi: 10.1111/j.1365-2141.2005.05689.x . [DOI] [PubMed] [Google Scholar]
- 44.Vaes LP, Chyka PA. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478–82. Epub 2001/01/06. doi: 10.1345/aph.10031 . [DOI] [PubMed] [Google Scholar]
- 45.Koch E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine. 2005;12(1–2):10–6. Epub 2005/02/08. doi: 10.1016/j.phymed.2004.02.002 . [DOI] [PubMed] [Google Scholar]
- 46.Bal Dit Sollier C, Caplain H, Drouet L. No alteration in platelet function or coagulation induced by EGb761 in a controlled study. Clinical and laboratory haematology. 2003;25(4):251–3. Epub 2003/08/02. . [DOI] [PubMed] [Google Scholar]
- 47.Gorski JC, Huang SM, Pinto A, Hamman MA, Hilligoss JK, Zaheer NA, et al. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75(1):89–100. doi: 10.1016/j.clpt.2003.09.013 . [DOI] [PubMed] [Google Scholar]
- 48.Umegaki K, Saito K, Kubota Y, Sanada H, Yamada K, Shinozuka K. Ginkgo biloba extract markedly induces pentoxyresorufin O-dealkylase activity in rats. Jpn J Pharmacol. 2002;90(4):345–51. Epub 2002/12/26. . [DOI] [PubMed] [Google Scholar]
- 49.Li T, Yu T, Hawkins BS, Dickersin K. Design, Analysis, and Reporting of Crossover Trials for Inclusion in a Meta-Analysis. PLoS One. 2015;10(8):e0133023 doi: 10.1371/journal.pone.0133023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhariwal K, Jackson A. Effect of length of sampling schedule and washout interval on magnitude of drug carryover from period 1 to period 2 in two-period, two-treatment bioequivalence studies and its attendant effects on determination of bioequivalence. Biopharm Drug Dispos. 2003;24(5):219–28. doi: 10.1002/bdd.359 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.