Abstract
Objective
The phase III clinical trial PERFECT was designed to assess clinical safety and efficacy of intramyocardial CD133+ bone marrow stem cell treatment combined with CABG for induction of cardiac repair.
Design
Multicentre, double-blinded, randomised placebo controlled trial.
Setting
The study was conducted across six centres in Germany October 2009 through March 2016 and stopped due slow recruitment after positive interim analysis in March 2015.
Participants
Post-infarction patients with chronic ischemia and reduced LVEF (25–50%). Interventions: Eighty-two patients were randomised to two groups receiving intramyocardial application of 5 ml placebo or a suspension of 0.5–5 × 106 CD133+.
Outcome
Primary endpoint was delta (∆) LVEF at 180 days (d) compared to baseline measured in MRI.
Findings (prespecified)
Safety (n = 77): 180 d survival was 100%, MACE n = 2, SAE n = 49, without difference between placebo and CD133+. Efficacy (n = 58): The LVEF improved from baseline LVEF 33.5% by + 9.6% at 180 d, p = 0.001 (n = 58). Treatment groups were not different in ∆ LVEF (ANCOVA: Placebo + 8.8% vs. CD133+ + 10.4%, ∆ CD133+ vs placebo + 2.6%, p = 0.4).
Findings (post hoc)
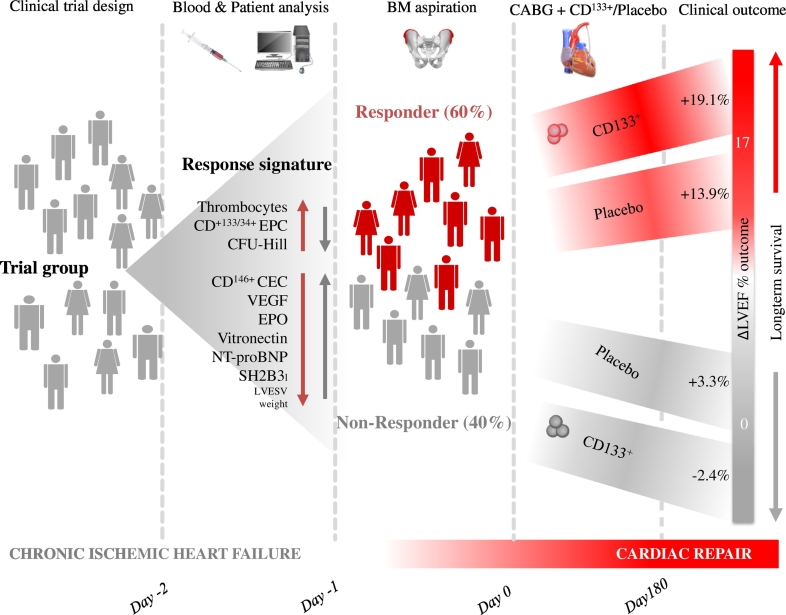
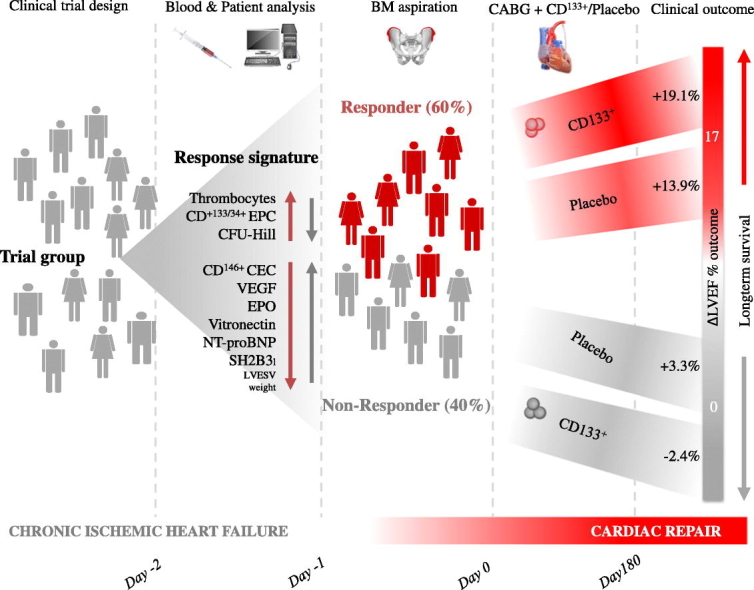
Responders (R) classified by ∆ LVEF ≥ 5% after 180 d were 60% of the patients (35/58) in both treatment groups. ∆ LVEF in ANCOVA was + 17.1% in (R) vs. non-responders (NR) (∆ LVEF 0%, n = 23). NR were characterized by a preoperative response signature in peripheral blood with reduced CD133+ EPC (RvsNR: p = 0.005) and thrombocytes (p = 0.004) in contrast to increased Erythropoeitin (p = 0.02), and SH2B3 mRNA expression (p = 0.073). Actuarial computed mean survival time was 76.9 ± 3.32 months (R) vs. + 72.3 ± 5.0 months (NR), HR 0.3 [Cl 0.07–1.2]; p = 0.067.Using a machine learning 20 biomarker response parameters were identified allowing preoperative discrimination with an accuracy of 80% (R) and 84% (NR) after 10-fold cross-validation.
Interpretation
The PERFECT trial analysis demonstrates that the regulation of induced cardiac repair is linked to the circulating pool of CD133 + EPC and thrombocytes, associated with SH2B3 gene expression. Based on these findings, responders to cardiac functional improvement may be identified by a peripheral blood biomarker signature.
TRIAL REGISTRATION: ClinicalTrials.govNCT00950274.
Keywords: Randomised double-blinded phase III multicentre trial, CD133+, CD34+, Endothelial progenitor cell (EPC), SH2B3, Lnk adaptor, Cardiac repair, Cardiac stem cell therapy, Angiogenesis
Graphical Abstract
Highlights
-
•
Heart function improvement is dependent on circulating endothelial progenitor cells.
-
•
Suppression of bone marrow response is associated to SH2B3 gene expression.
-
•
Peripheral blood angiogenesis response can be predicted by a biomarker signature.
Improvement of left ventricular heart function was studied in the randomised double-blinded placebo controlled PERFECT-trial designed to assess clinical safety and efficacy of intramyocardial CD133 + bone marrow derived cell application and CABG surgery. Cardiac function improvement was determined by circulating CD133,34 + endothelial progenitor cells and a diagnostic biomarker signature in peripheral blood. Non-response was associated to SH2B3 gene expression and bone marrow suppression. The described mechanism of bone marrow CD133+ angiogenesis response may have a pivotal role in heart function recovery. Selection of patients by specific peripheral blood biomarkers appears to be feasible and may lead to tailored therapy.
Research in Context
Evidence Before This Study
Intramyocardial CD133+ purified autologous bone marrow stem cell (BMSC) transplantation has been investigated as an adjunctive strategy to coronary artery bypass graft (CABG) revascularization in order to improve left ventricular heart function following deterioration of left ventricular ejection fraction (LVEF) after acute myocardial ST-segment elevation infarction (STEMI), and coronary artery 3-vessel disease sequentially treated by acute PCI and secondary CABG revascularization. Previous safety and efficacy (phase I, IIa, IIb) trials have demonstrated clinical safety and some evidence of therapeutic efficacy of adjunctive CD133+ BMSC treatment adjunctive to CABG coronary revascularization. The randomised double-blinded placebo controlled PERFECT-trial was designed to assess clinical safety and efficacy in a, ICH-GCP complaint study setting. Post hoc biomarker and subgroup analyses were performed to identify CD133+ bone marrow stem cell related cardiac repair mechanisms related to interventional CD133+ BMSC transplantation.
Added Value of This Study
The study demonstrates the central regulatory importance of CD133,34 + EPC response for angiogenesis, suppression of response by SH2B3, impact for cardiac tissue repair, selection of responding patients, and monitoring of angiogenesis response by combined diagnostic factors using machine learning.
Implications of All the Available Evidence
The described mechanism of suppression bone marrow CD133+ angiogenesis response may have a pivotal role in cardiovascular tissue repair. Selection of patients by specific diagnostic peripheral blood biomarkers appears to be feasible and may lead to tailored therapy in cardiovascular disease. The lack of vascular repair by reduced blood angiogenesis may be a decisive determinant for cardiovascular disease and impaired tissue repair.
1. Introduction
Reparative therapies using stem cells for the repair of heart tissue have been at the forefront of preclinical and clinical development during the past 16 years (Fisher et al., 2016). Among the different approaches, the direct implantation of bone marrow- derived cells into heart tissue still attracts the most dedicated clinical developmental attention since the first-in-man application in 2001 and several promising clinical pilot trials (Stamm et al., 2003, Tse et al., 2003, Stamm et al., 2007). Yet, in these trials, clinically relevant improvements of LVEF as well as non-responsive patients were observed both in treatment and placebo groups (Henry et al., 2016, Nasseri et al., 2014, Bartunek et al., 2016). This has raised the question of induction of reparative mechanisms independent of stem cell application and potential suppressive factors of vascular repair associated with CD34+ Endothelial Progenitor Cells (EPC) (Werner et al., 2005, Taylor et al., 2016, Bhatnagar et al., 2016, Contreras et al., 2017).
In light of this uncertainty, we have attempted to investigate the mechanism of cardiac repair and the role of bone marrow CD133 + EPC regulated angiogenesis using the results of the clinical PERFECT trial and its data recorded (Donndorf et al., 2012). Extensive additional laboratory analyses was carried out to delineate the underlying mechanisms and to develop diagnostic approaches for identifying patient (non)responsiveness to stem cell therapies by analyzing the following clinical features: 1. Baseline characteristics of treatment responders vs. non-responders; 2. Mechanism of action for cardiac regeneration and diagnostic access; 3. Relevance of LVEF endpoint for long term survival.
2. Methods
2.1. Trial Design
The PERFECT trial was a randomised, multicenter, placebo-controlled, double-blinded phase III study investigating the effects of intramyocardial CD133+ BMSC treatment in combination with coronary artery bypass graft revascularization (CABG) for post infarction myocardial ischemia (Donndorf et al., 2012). The trial performed according to ICH-GCP was listed under the EudraCT number 2006-006404-11, DRKS number DRKS00000213, and approved by the committee of the University Medicine Rostock (FK 2007-07) and all trial sites in Germany (Supplement Appendix 1). Regulatory approval was given by the Paul-Ehrlich-Institute, Langen, Germany. The trial was registered at ClinicalTrials.gov identifier: NCT00950274. Characteristics of trial design, changes to trial design, outcomes, interim analysis, and recruitment period are depicted in Appendix 2 (Supplement) and the Clinical Trial Report (Appendix 1).
Inclusion criteria of the PERFECT trial (Supplement Appendices 1 and 2) were (a) coronary artery disease after myocardial infarction with the indication for CABG surgery, (b) reduced LVEF (25–50%) and (c) presence of a localized kinetic/hypokinetic/hypoperfused area of LV myocardium defining the SC target area (Supplement Fig. 1). According to the trial flow chart (Supplement Fig. 2) assessments were performed preoperative and at days 1, 3, 10, 90, and 180 post operation. In addition, safety (MACE) follow up was performed at 24 months post-treatment.
2.2. Participants and Study Settings
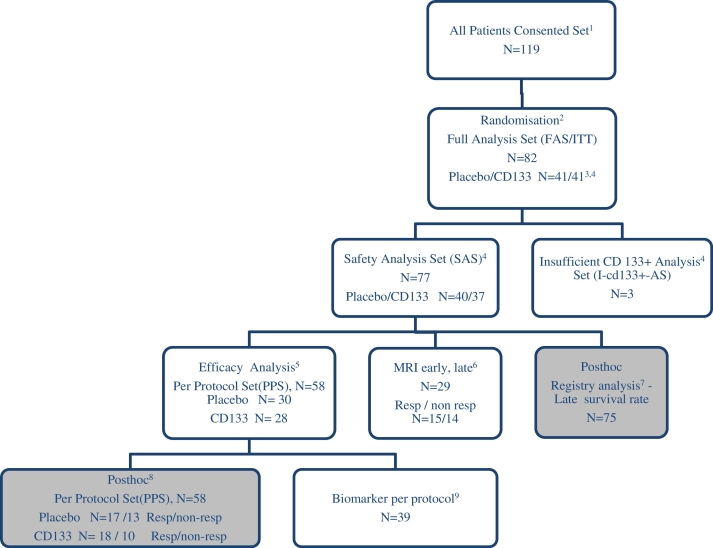
A total of 119 patients were screened in 6 centres in Germany (Fig. 1). All patients signed the informed consent form and were included in the study. Eighty-two (82) patients were randomised to active treatment or placebo. The allocation of patients to the different analysis sets is shown in Fig. 1. Initially, we evaluated the basic patient characteristics of the randomised patient groups for safety set (SAS) analysis (n = 77) and per-protocol set (PPS) efficacy analysis (n = 58) respectively for subanalysis of MRI early/late, primary endpoint responder/non-responder, biomarkers, preoperative cardiac disease state, age, sex, concomitant diseases, taking medications, operative procedures and postoperative course (Table 1).
Fig. 1.
PERFECT Trial flowchart and prespecified or post hoc analysis sets.
The randomised multicentre trial was performed double-blinded placebo controlled through six heart centres in Germany according to ICH-GCP and is depicted according to CONSORT and STARD guidelines:1 A total of 119 patients were screened in 6 centres in Germany from Sept. 2009 through June 2015. All patients signed the informed consent form and were included in the study. Thirty-seven participants were excluded before randomisation due to newly identified exclusion criteria such as severe arrhythmia. 2 Eighty-two (82) patients were randomised to active treatment or placebo. Two (Stamm et al., 2003) patients were randomised but not treated because the CD 133 + preparation did not comply with the release criteria for GMP. 3 Forty (48.8%) patients received an injection of CD133 + cells and 40 (48 ⋅ 8%) received an injection of placebo. 4 Three patients were excluded because of insufficient CD133 + cell count below minimum dosis resulting in the safety-analysis-population (n = 77). 5 After a careful review of the blinded data in a blind data review meeting conducted on the 20 May 2016 a total of 19 patients were excluded from the full analysis population due to protocol violations with incomplete MRI follow-up data leading to the Per Protocol Set (PPS) efficacy-analysis-population (n = 58). Patient distribution for PPS efficacy population by study centres: German Heart Center Berlin 8%, Medical School Hannover 28%, University Medicine Rostock 38%, Heart and Diabetes Center Bad Oeynhausen 5%, Heart Center Leipzig 13%, University Medicine Hamburg 10%. 6 Additional MRI at day 10 postoperative for subanalysis of early and late postoperative changes for subgroup analysis early and late postoperative changes. 7 Post hoc analysis for actuarial survival was performed in registry analysis 7 years after FPI on Nov. 1, 2016. 8 Post hoc analysis was additionally performed in the efficacy group (n = 58) to unravel contributing non CD133+ injection related factors of late improvement. Patients were grouped in the efficacy analysis set according to effective response in primary endpoint as responder or non-responder (Δ LVEF 180 d vs.0 responder ≥ 5% vs. non-responder < 5%). According to this post hoc analysis 35 patients from 58 (60.3%) were responders to treatment. This Responder/non-responder (R/NR) ratio was similar respectively in the placebo group 56 ⋅ 5% (R/NR 17/30 pt.) and in the CD133 + group 64% (R/NR 18/28 pt.) (Placebo vs. CD133 +; p = 0 ⋅ 373). Responder (35/58) and non-responder (23/58) analysis was performed in efficacy group (n = 58). 9 Biomarkers were studied in 39 patients of the efficacy group (n = 58) independent on placebo/CD133 + or responder/non-responder group. All laboratory tests were realized in patients located in the Rostock centre (n = 31), where immediate laboratory analysis of FACS and CFU was guaranteed. Additional patients from other centres (8/58) were evaluated also in the Biomarker cohort according to realized parameters. Biobank at time point (pre- and postoperative day) − 2, − 1, + 1, + 3, + 10, + 180: Peripheral blood MNC/FACS (CD 133, 34, 117, 184, 309, 45, 31, 14), CFU-Hill, serum analysis angiogenesis factors and cytokines; Bone-marrow MNC, Isolated CD133 + FACS (CD133, 34, 117, 184, 309, 45, 31, 14), CFU-EC, RNA-seq.
Table 1.
Patient characteristics and randomisation analysis sets.
| Patient characteristics and randomisation analysis sets I | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Safety (SAS) all | Safety (SAS) placebo | CD133 + | P | Efficacy placebo/CD133 + | CD133 + | P | Efficacy (PPS) Resp | NonResp | p | MRI early/late Plac/CD133 | Biomarker | |
| N | 77 (mean, SD, min-max, median) |
40 (mean, SD, min-max, median) |
37 (mean, SD, min-max, median) |
30 | 28 | 35 | 23 | 29 | 39 | |||
| Basic data | ||||||||||||
| Age (y) | 63.2 ± 8.37 34–79 Median: 63.0 |
62.9 ± 8.50 35–79 Median: 61.5 |
63.5 ± 8.34 34–78 Median: 65.0 |
0.751a | 63.6 ± 7.75 53–79 Median: 62 |
64.0 ± 7.20 50–78 Median: 65 |
0.853a | 62.9 ± 7.21 50–78 Median: 61 |
65.3 ± 7.68 53–79 Median: 66.0 |
0.231a | 62.9 ± 6.86 51–79 Median: 62 |
64.8 ± 7.46 53–79 Median: 66.0 |
| Sex/male% | 67 (88.2) | 34 (85) | 33 (89.2) | 0.739c | 26 (86.7) | 26 (92.9) | 0.671c | 31 (88.6) | 21 (91.3) | 1.000c | 27 (93.1) | 33 (84.6) |
| Body mass index (kg/m2) | 76, 28.8 ± 4.12 19.4–38.6 Median: 28.3 |
39, 29.1 ± 4.28 19.4–38.6 Median: 28.6 |
28.5 ± 3.98 19.6–38.0 Median: 28.1 |
0.575a | 29.0 ± 3.81 19.4–37.2 Median: 29.4 |
28.8 ± 4.11 19.6–38.0 Median: 28.4 |
0.804a | 28.7 ± 4.14 19.4–38.0 Median: 29.1 |
29.1 ± 3.64 22.9–35.2 Median: 28.4 |
0.723a | 28.7 ± 3.87 22.5–38.0 Median: 27.8 |
29.5 ± 3.90 19.6–38 Median: 30.2: |
| Last myocardial infarction | 47, ≤ 6 months: 21 (44.7) 7–12 months: 6 (12.8) >12 months: 20 (42.6) |
21, ≤ 6 months: 10 (47.6) 7–12 months: 3 (14.3) >12 months: 20 (38.1) |
26, ≤ 6 months: 11 (42.3) 7–12 months: 3 (11.5) >12 months: 12 (46.2) |
0.631b | 15, ≤ 6 months: 4 (26.7) 7–12 months: 3 (20.0) <12 months: 8 (53.3) |
19, ≤ 6 months: 8 (42.1) 7–12 months: 2 (10.5) >12 months: 9 (47.4) |
0.584b | 19, ≤ 6 months: 8 (42.31 7–12 months: 2 (10.5) >12 months: 9 (47.4) |
15, ≤ 6 months: 4 (26.7) 7–12 months: 3 (20.0) >12 months: 8 (53.3) |
0.341b | 19, ≤ 6 months: 5 (26.3) 7–12 months: 5 (26.3) >12 months: 9 (47.4) |
22, ≤ 6 months: 8 (36.4) 7–12 months: 4 (18.2) >12 months: 10 (45.5) |
| PCI prior to CABG, n% | 20 (26.0) | 12 (30.0) | 8 (21.6) | 0.445c | 9 (30.0) | 6 (21.4) | 0.554c | 5 (14.7) | 10 (41.7) | 0.074c | 8 (27.6) | 9 (23.1) |
| Diabetes(% | 42.9 | 50.0 | 35.1 | 0.198c | 46.7 | 42.9 | 0.893 | 38.2 | 56.5 | 0.190 | 37.9 | 30.8 |
| Hypert. (%) | 83.1 | 85.0 | 81.1 | 0.464c | 90.0 | 89.3 | 0.828 | 91.2 | 91.3 | 1.000 | 96.6 | 100 |
| Hyperlipidemia (%) | 61.0 | 65.0 | 56.8 | 0.491c | 60.0 | 64.3 | 0.791 | 65.7 | 56.5 | 0.583 | 86.2 | 84.6 |
| Laboratory parameters | ||||||||||||
| LDL cholesterol, mg/dl | 65, 2.88 ± 0.86 0.80–5.0 Median: 2.70 |
34, 2.92 ± 0.748 1.6–5.0 Median: 2.75 |
31, 2.84 ± 0.98 0.80–4.80 Median: 2.70 |
0.695a | 26, 2.91 ± 0.75 1.6–5.0 Median: 2.75 |
24, 2.84 ± 0.915 1.60–4.80 Median: 2.70 |
0.767a | 30, 2.90 ± 0.911 1.60–5.0 Median: 2.65 |
20, 2.84 ± 0.698 1.6–4.1 Median: 2.70 |
0.788a | 26, 2.83 ± 0.82 1.6–5.0 Median: 2.65 |
35, 2.98 ± 0.92 1.60–5.0 Median: 2.80 |
| HDL cholesterol, mg/dl | 65, 1.12 ± 0.293 0.60–1.98 Median: 1.10 |
34, 1.12 ± 0.237 0.70–1.60 Median: 1.10 |
31, 1.12 ± 0.35 0.60–1.98 Median: 1.00 |
0.476b | 26, 1.13 ± 0.218 0.80–1.5 Median: 1.10 |
24, 1.04 ± 0.286 0.60–1.70 Median: 0.900 |
0.114b | 30, 1.05 ± 0.252 0.60–1.70 Median: 0.95 |
20, 1.15 ± 0.252 0.80–1.60 Median: 1.10 |
0.177b | 26, 1.06 ± 0.239 0.60–1.50 Median: 1.05 |
35, 1.11 ± 0.253 0.80–1.70 Median: 1.10 |
| Triglycerides, mol/dl | 68, 1.81 ± 0.98 0.60–6.40 Median: 1.60 |
38, 1.99 ± 1.14 0.80–6.40 Median: 1.60 |
30, 1.59 ± 0.70 0.60–3.40 Median: 1.50 |
0.166b | 28, 2.06 ± 1.26 0.90–6.4 Median: 1.60 |
24, 1.70 ± 0.72 0.70–3.40 Median: 1.65 |
0.451b | 31, 1.86 ± 1.13 0.80–6.4 Median: 1.60 |
21, 1.94 ± 0.951 0.70–4.50 Median: 1.80 |
0.495b | 26, 1.83 ± 1.14 0.70–6.40 Median: 1.60 |
36, 2.03 ± 1.17 0.70–6.4 Median: 1.75 |
| CRP (mg/l) | 76, 0.565 ± 0.846 0.10–7.0 Median: 0.400 |
0.635 ± 1.11 0.10–7.00 Median: 0.400 |
36, 0.486 ± 0.383 0.10–1.70 Median: 0.400 |
0.983b | 0.403 ± 0.275 0.10–1.20 Median: 0.400 |
0.511 ± 0.42 0.10–1.70 Median: 0.35 |
0.504b | 0.469 ± 0.3471 0.10–1.40 Median: 0.400 |
0.435 ± 0.370 0.10–1.70 Median: 0.30 |
0.641b | 0.483 ± 0.433 0.10–1.70 Median:0.300 |
0.505 ± 0.389 0.10–1.70 Median: 0.400 |
| Creatinine (μmol/l) | 90.0 ± 22.8 48–160 Median: 87 |
90.4 ± 21.4 53–152 Median: 86 |
91.4 ± 24.4 48–160 Median: 87 |
0.992b | 91.3 ± 23.2 53–152 Median: 85.5 |
92.6 ± 25.4 48–160 Median: 89 |
0.913b | 92.0 ± 26.2 48–160 Median: 88.0 |
91.8 ± 21.1 53–132 Median: 87.0 |
0.611b | 89.4 ± 23.8 57–160 Median: 82.0 |
95.6 ± 25.2 48–160 Median: |
| Leucocytes (109/l) | 76, 8.05 ± 1.78 5.0–11.9 Med.: 7.90 |
8.06 ± 1.75 5.0–11.9 Median: 8.00 |
36, 8.04 ± 1.83 5.1–11.7 Med.: 7.90 |
0.975a | 8.03 ± 1.78 5–11.8 Median: 8.00 |
7.91 ± 1.94 5.1–11.7 Median: 7.70 |
0.807a | 7.99 ± 1.86 5.0–11.7 Median: 7.70 |
7.94 ± 1.86 5.1–11.8 Median: 7.80 |
0.921a | 8.07 ± 1.65 5.1–11.7 Median: 7.70 |
8.25 ± 1.91 5.1–11.8 Median: 8.00 |
| Thrombocytes (109/l) | 242 ± 78.2 73.620 Median:231 |
252 ± 91.4 144–620 Median: 231 |
232 ± 60.2 73–351 Median: 232 |
0.714b | 246 ± 82.3 144–620 Median: 231 |
229 ± 65.7 73–351 Median: 229 |
0.709b | 257 ± 81.5 123–620 Median: 238 |
208 ± 51.2 73–311 Median: 220 |
0.004b | 228 ± 63.8 73–351 Median: 223 |
239 ± 85.8 73–620 Median: 234 |
| NT Pro-BNP (pg/ml) | 1468 ± 1947 108–12,735 Median: 803 |
1474 ± 2378 108–12,735 Median: 646 |
1560 ± 1370 225–7230 Median: 1028 |
0.065b | 1551 ± 2647 108–12,735 Median: 681 |
1560 ± 1527 225–7230 Median: 1048 |
0.079b | 1266 ± 1469 137–8444 Median: 688 |
1925 ± 2903 108–12,735 Median: 1025 |
0.861b | 28, 1753 ± 2796 108–12,735 Med.: 687 |
1757 ± 2382 108–12,735 Median: 1063 |
| Medication | ||||||||||||
| Aspirin (%) | 97.4 | 97.5 | 97.3 | 1.000c | 96.7 | 100.0 | 1.000 | 100.0 | 95.7 | 0.397 | 29 (100) | 39 (100) |
| Statin (%) | 97.4 | 95.0 | 100.0 | 0.494c | 96.7 | 100.0 | 1.000 | 97.1 | 100.0 | 1.000 | 26 (89.7) | 33 (84.6) |
| β-blocker (%) | 98.7 | 97.5 | 100.0 | 1.000c | 100.0 | 100.0 | n/a | 100.0 | 100.0 | 1.000 | 24 (82.8) | 35 (89,7) |
| ACE inh. (%) | 81.8 | 82.5 | 81.1 | 1.000c | 83.3 | 82.1 | 1.000 | 85.7 | 78.3 | 0.496 | 21 (72.4) | 26 (66.7) |
| AT1 rec. Antag. (%) | 32.5 | 32.5 | 32.4 | 1.000c | 36.7 | 28.6 | 0.583 | 28.6 | 39.1 | 0.568 | 4 (13.8) | 6 (15.4) |
| Aldosteron Antag.(%) | 55.8 | 57.5 | 54.1 | 0.821c | 63.3 | 64.3 | 1.000 | 60.0 | 69.6 | 0.579 | 10 (34.5) | 8 (20.5) |
| Diuretic (%) | 93.5 | 92.5 | 94.6 | 1.000c | 93.3 | 96.4 | 1.000 | 97.1 | 91.3 | 0.557 | 20 (69.0) | 28 (71.8) |
| Ca-antag. (%) | 37.7 | 32.5 | 43.2 | 0.356c | 36.7 | 42.9 | 0.789 | 40.0 | 39.1 | 1.000 | 2 (6.9) | 5 (12.8) |
| Anti-arrh. (%) |
7.8 | 7.5 | 8.1 | 1.000c | 3.3 | 7.1 | 0.605 | 5.7 | 4.3 | 1.000 | 1 (3.4) | 1 (2.6) |
| Risk factors and status | ||||||||||||
| Smoking (previous) N (%) |
35 (45.5) | 20 (50) | 15 (40.5) | 0.494c | 13 (43.3) | 12 (42.9) | 1.000c | 18 (51.4) | 7 (30.4) | 0.175c | 14 (48.3) | 18 (46.2) |
| Smoking (actual) N (%) |
20 (26.0) | 8 (20) | 12 (32.4) | 0.299c | 7 (23.3) | 8 (28.6) | 0.767c | 10 (28.6) | 5 (21.7) | 0.760c | 8 (27.6) | 11 (28.2) |
| EuroScore | 4.33 ± 3.44 0.13–17.1 Median: 2.98 |
3.98 ± 3.64 0.13–17.1 Median: 2.60 |
4.69 ± 3.21 0.88–11.9 Median: 3.57 |
0.170b | 3.94 ± 3.24 1.33–16.3 Median: 2.59 |
4.80 ± 3.35 1.33–11.9 Median: 3.66 |
0.246b | 3.97 ± 2.64 1.33–11.94 Median: 2.74 |
4.95 ± 4.09 1.33–16.3 Median: 3.22 |
0.583b | 4.31 ± 3.26 1.33–16.3 Median: 3.22 |
4.77 ± 3.73 1.33–16.3 Median: 3.22 |
| NYHA (class) N(%) |
1: 10 (13.0) 2: 29 (37.7) 3: 36 (46.8) 4: 2 (2.6) |
1: 4 (10.0) 2: 16 (40.0) 3: 19 (47.5) 4: 1 (2.5) |
1: 6 (16.2) 2: 13 (35.1) 3: 17 (45.9) 4: 1 (2.7) |
0.872d | 1: 4 (13.3) 2: 9 (30.0) 3: 16 (53.3) 4: 1 (3.3) |
1: 5 (17v9) 2: 10 (35.7) 3: 12 (42.9) 4: 1 (3.6) |
0.881d | 1: 4 (11.8) 2: 8 (23.5) 3: 21 (61.8) 4: 1 (2.9) |
1: 5 (20.8) 2: 11 (45.8) 3: 7 (29.2) 4: 1 (4.2) |
0.180d | 1: 5 (17.2) 2: 10 (34.5) 3: 13 (44.8) 4: 1 (3.4) |
1: 8 (20.5) 2: 12 (30.8) 3: 17 (43.6) 4: 2 (5.1) |
| CCS (class) | 76, 1.46 ± 1.18 0–3 Median: 2 |
39, 1.62 ± 1.16 0–3 Median: 2 |
1.30 ± 1.20 0–3 Median: 1 |
0.259b | 29, 1.59 ± 1.18 0–3 Median: 2 |
1.14 ± 1.24 0–3 Median:1 |
0.199b | 34, 1.38 ± 1.21 0–3 Median: 1.5 |
1.35 ± 1.27 0–3 Median: 2 |
0.878b | 28, 1.57 ± 1.23 0–3 Median: 2 |
38, 1.61 ± 1.22 0–3 Median: 2 |
| 6MWT-baseline (meter) | 64, 372 ± 109 108–644 Median: 360 |
36, 376 ± 112 108–644 Median: 367.5 |
28, 367 ± 107 192–628 Median: 360 |
0.759a | 27, 374 ± 114 108–644 Median: 350 |
20, 376 ± 92.0 206–570 Median: 361 |
0.967a | 30, 368 ± 95.4 108–570 Median: 361 |
17 388 ± 119 206–644 Median: 360 |
0.530a | 26, 388 ± 128 108–644 Median: 365 |
32, 383 ± 114 108–644 Median: 385 |
| Patient characteristics and randomisation analysis set II | ||||||||||||
| Safety (SAS) All |
Safety (SAS) Placebo |
CD133 + | P | Efficacy (PPS) Placebo/CD133 + |
CD133 + | P | Efficacy (PPS) Resp |
NonResp | p | MRI early/late Placebo/CD133 + |
Biomarker | |
| N | 77 (mean,SD,min-max, median) |
40 (mean,SD,min-max, median) |
37 (mean,SD,min-max, median) |
30 | 28 | 35 | 23 | 29 | 39 | |||
| Myocardial function, perfusion and infarction | ||||||||||||
| Area of infarction Septal (segments 1,5,10,11) | 58, 11 (19.0) | 29, 8 (27.6) | 29, 3 (10.3) | 0.179c | 22, 2 (9.1) | 21, 3 (14.3) | 0.664c | 24, 5 (20.8) | 19, 0 (0) | 0.056c | 19, 4 (21.1) | 29, 3 (10.3) |
| Posterior (segments 2,6, 8,9,11) | 58, 26 (44.8) | 29, 13 (44.8) | 29, 14 (48.3) | 1.000c | 22, 17 (77.3) | 21, 12 (57.1) | 0.203c | 24, 9 (37.5) | 19, 18 (94.7) | < 0.001c | 19, 11 (57.9) | 29, 24 (82.8) |
| Anterior (segments 3,5,6,7,9,10,11) | 58, 24 (41.4) | 29, 13 (44.8) | 29, 8 (27.6) | 0.274c | 22, 8 (36.4) | 21, 11 (52.4) | 0.364c | 24, 11 (45.8) | 19, 7 (36.8) | 0.756c | 19, 7 (36.8) | 29, 12 (41.4) |
| LateraI (segments 4,7,8,9,10,11) | 58, 17 (29.3) | 29, 8 (27.6) | 29, 9 (31.0) | 1.000c | 22, 8 (36.4) | 21, 7 (33,3) | 1.000c | 24, 9 (37.5) | 19, 6 (31.6) | 0.755c | 19, 7 (36.8) | 29, 5 (17.2) |
| Combined (%) (score 5–11) | 58, 24 (41.4) | 29, 12 (41.4) | 29 12 (41.4) | 1.000c | 22, 11 (50.0) | 21, 9 (42.9) | 0.763c | 24, 11 (45.8) | 19, 9 (47.4) | 1.000c | 19, 8 (42.1) | 29, 16 (55.2) |
| Coronary artery stenosis > 50% LMCA N (%) |
21 (27.3) | 13 (35.1) | 8 (20.0) | 0.200c | 7 (23.3) | 10 (35.7) | 0.390c | 11 (32.4) | 6 (25.0) | 0.772c | 12 (41.4) | 11 (28.2) |
| Coronary artery stenosis > 50% RIVA N (%) |
76, 66 (86.8) | 33 (89.2) | 39, 33 (84.6) | 0.737c | 29, 24 (82.8) | 25 (89.3) | 0.706c | 30 (88.2) | 19 (82.6) | 0.697c | 22 (75.9) | 38, 35 (92.1) |
| Coronary artery stenosis > 50% RCX N (%) |
76, 58 (76.3) | 29 (78,4) | 39, 29 (74.4) | 0.790c | 29, 20 (69.0) | 25 (89.3) | 0.103c | 28 (82.4) | 23, 17 (73.9) | 1.000c | 24 (82.8) | 38, 31 (81.6) |
| Coronary artery stenosis > 50% RCA N (%) |
76, 69 (90.8) |
35 (94.6) | 39, 34 (87.2) |
0.432c | 29, 25 (86.2) |
27 (96.4) | 0.352c | 31 (91.2) | 23, 21 (91.3) |
1.000c | 27 (93.1) | 38, 35 (92.1) |
| Scar size (MRI)-baseline (g) | 70, 31.3 ± 15.7 2–89 Median: 29.5 |
37, 32.2 ± 12.6 2–56 Median: 32 |
33, 30.2 ± 18.8 4–89 Median: 29 |
0.573a | 28, 30.4 ± 12.3 2–49 |
25, 31.9 ± 20.8 4–89 |
0.755a | 33, 27.5 ± 14.6 2–59 Median: 25 |
20, 37.1 ± 18.5 14–89 Median: 36 |
0.042a | 27, 31.1 ± 17.3 6–89 Median: |
36, 30.9 ± 15.9 6–89 Median: 27.5 |
| Non-viable tissue (MRI) – baseline (g) | 69, 24.8 ± 16.2 0–70 Median: 22 |
36, 25.0 ± 14.2 0–56 Median: 25.5 |
33, 24.5 ± 18.5 0–70 Median: 20 |
0.897a | 27, 23.0 ± 13.9 0–50 Median: 22.0 |
25, 25.9 ± 20.4 0–70 Median: 19.0 |
0.551a | 33, 21.5 ± 16.2 0–62 Median: 18.0 |
19, 29.6 ± 18.1 7–70 Median: 25.0 |
0.102a | 26, 23.0 ± 17.6 4–70 Median: 19.5 |
36, 23.0 ± 15.7 3–70 Median: 18.5 |
| LV mass (MRI) (g) | 75, 182 ± 43.0 101–287 Median: 180 |
39, 179 ± 42.5 104–287 Median: 178 |
36, 185 ± 43.9 101–274 Median: 187 |
0.608a | 184 ± 44.4 104–287 Median: 183 |
183 ± 36.7 122–270 Median: 186 |
0.933a | 182 ± 38.5 104–270 Median: 186 |
186 ± 44.1 111–287 Median: 185 |
0.711a | 178 ± 47.6 104–287 Median: 178 |
188 ± 44.4 104–287 Median: 187 |
| LVEF (MRI) – baseline (%) | 76, 34.3 ± 6.42 25–49 Median: 34 |
39, 35.6 ± 6.67 25–49 Median: 36 |
32.8 ± 5.89 25–48 Median: 32 |
0.056b |
34.4 ± 6.46 25–49 Median: 35.0 |
32.5 ± 5.89 25–48 Median: 32.0 |
0.249b |
32.8 ± 5.42 25–48 Median: 32 |
34.6 ± 7.35 25–49 Median: 35.0 |
0.285a |
34.9 ± 6.34 26–49 Median: 35.0 |
34.1 ± 6.40 25–48 Median: 34.0 |
| LVEDV index (MRI) – baseline (ml) | 76, 109 ± 29.4 41–194 Median: 107.5 |
39, 106 ± 26.2 45–176 Median: 107 |
112 ± 32.5 41–194 Median: 109 |
0.432a | 107 ± 26.4 45–176 Median: 109 |
107 ± 32.6 41–194 Median: 104 |
0.941a | 101 ± 27.9 41–162 Median: 101 |
117 ± 29.1 76–194 Median: 110 |
0.033a | 110 ± 35.5 41–194 Median: 109 |
100 ± 29.6 41–194 Median: 101 |
| LVESV index (MRI) – baseline (ml) | 76, 71.3 ± 22.4 21–141 Median: 71 |
39, 68.7 ± 19.2 31–110 Median: 69 |
74.0 ± 25.4 21–141 Median: 75 |
0.308a | 71.2 ± 19.8 31–110 Median: 71.0 |
70.4 ± 25.3 21–141 Median: 73.0 |
0.893a | 67.0 ± 20.8 21–110 Median: 69 |
76.7 ± 24.0 40–141 Median: 77.0 |
0.109a | 72.9 ± 25.8 21–141 Median: 69.0 |
65.9 ± 23.2 21–141 Median: 69.0 |
| Stress Perfusion score (mean Segment 1–17) (MRI) | 58, 0.84 ± 0.39 0–1.6 Median 0.88 |
28, 0.83 ± 0.38 0–1.6 Median 0.84 |
29, 0.84 ± 0.4 0–1.6 Median 1.0 |
0.774b | 24, 0.81 ± 0.36 0.2–1.6 Median 0.81 |
27, 0.87 ± 0.40 0–1.6 Median 1 |
0.330b | 32, 0.78 ± 0.38 0–1.4 Median 0.84 |
19, 0.94 ± 0.38 0.4–1.6 Median 1 |
0.172b | 27, 0.72 ± 0.43 0–1.6 Median 0.72 |
39, 0.86 ± 0.39 0.2–1.6 Median 0.86 |
| Patient characteristics and randomisation analysis set III | ||||||||||||
| Safety (SAS) All |
Safety (SAS) Placebo |
CD133 + | P | Efficacy (PPS) Placebo/CD133 + |
CD133 + | P | Efficacy (PPS) Resp |
NonResp | p | MRI early/late Placebo/CD133 + |
Biomarker | |
| N | 77 | 40 | 37 | 30 | 28 | 35 | 23 | 29 | 39 | |||
| Operative procedure and postoperative course | ||||||||||||
| CD133 + BMSC treated infarct area (% LV Segments) | ||||||||||||
| Segment 1 (%) | 13 (16.9) | 7 (17.5) | 6 (16.2) | 0.542c | 5 (16.7) | 3 (10.7) | 0.707c | 6 (17.1) | 2 (8.7) | 0.458c | 4 (13.8) | 4 (10.3) |
| Segment 2 (%) | 1 (1.3) | 1 (2.5) | 0 (0) | 1.000c | 1 (3.3) | 0 (0) | 1.000c | 1 (2.9) | 0 (0) | 1.000c | 0 (0) | 1 (2.6) |
| Segment 3 (%) | 14 (18.2) | 9 (22.5) | 5 (13.5) | 0.382c | 8 (26.7) | 5 (17.9) | 0.534c | 9 (25.7) | 4 (17.4) | 0.534c | 8 (27.6) | 7 (17.9) |
| Segment 4 (%) | 44 (57.1) | 24 (60.0) | 20 (54.1) | 0.650c | 18 (60.0) | 15 (53.6) | 0.791c | 21 (60.0) | 12 (52.2) | 0.597c | 18 (62.1) | 21 (53.8) |
| Segment 5 (%) | 51 (66.2) | 27 (67.5) | 24 (64.9) | 0.815c | 19 (63.3) | 17 (60.7) | 1.000c | 24 (68.6) | 12 (52.2) | 0.272c | 20 (69.0) | 20 (51.3) |
| Segment 6 (%) | 34 (44.2) | 19 (47.5) | 15 (40.5) | 0.647c | 14 (46.7) | 10 (35.7) | 0.435c | 15 (42.9) | 9 (39.1) | 1.000c | 14 (48.3) | 12 (30.8) |
| Segment 7 (%) | 26 (33.8) | 14 (35.0) | 12 (32.4) | 1.000c | 10 (33.3) | 10 (35.7) | 1.000c | 12 (34.3) | 8 (34.8) | 1.000c | 11 (37.9) | 13 (33.3) |
| Segment 8 (%) | 6 (7.8) | 4 (10.0) | 2 (5.4) | 0.676c | 3 (10.0) | 2 (7.1) | 1.000c | 4 (11.4) | 1 (4.3) | 0.639c | 3 (10.3) | 5 (12.8) |
| Segment 9 (%) | 17 (22.1) | 9 (22.5) | 8 (21.6) | 1.000c | 6 (20.0) | 6 (21.4) | 1.000c | 9 (25.7) | 3 (13.0) | 0.329c | 6 (20.7) | 7 (17.9) |
| Segment 10 (%) | 59 (76.6) | 30 (75.0) | 29 (78.4) | 0.792c | 22 (73.3) | 25 (89.3) | 0.182c | 29 (82.9) | 18 (78.3) | 0.738c | 24 (82.8) | 33 (84.6) |
| Segment 11 (%) | 65 (84.4) | 31 (77.5) | 34 (91.9) | 0.117c | 22 (73.3) | 27 (96.4) | 0.026c | 32 (91.4) | 17 (73.9) | 0.135c | 26 (89.7) | 32 (82.1) |
| Segment 12 (%) | 54 (70.1) | 27 (67.5) | 27 (73.0) | 0.628c | 19 (63.3) | 21 (75.0) | 0.402c | 26 (74.3) | 14 (60.9) | 0.385c | 21 (/2.4) | 26 (66.7) |
| Segment 13 (%) | 37 (48.1) | 20 (50.0) | 17 (45.9) | 0.821c | 15 (50.0) | 12 (42.9) | 0.610c | 18 (51.4) | 9 (39.1) | 0.426c | 12 (41.4) | 19 (48.7) |
| Segment 14 (%) | 22 (28.6) | 12 (30.0) | 10 (27.0) | 0.806c | 10 (33.3) | 9 (32.1) | 1.000c | 13 (37.1) | 6 (26.1) | 0.410c | 8 (27.6) | 14 (35.9) |
| Segment 15 (%) | 56 (72.7) | 27 (67.5) | 29 (78.4) | 0.317c | 19 (63.3) | 25 (89.3) | 0.031c | 29 (82.9) | 15 (65.2) | 0.209c | 22 (75.9) | 29 (74.4) |
| Segment 16(%) | 67 (87.0) | 33 (82.5) | 34 (91.9) | 0.314c | 23 (76.7) | 26 (92.9) | 0.147c | 30 (85.7) | 19 (82.6) | 1.00c | 25 (86.2) | 33 (84.6) |
| Segment 17 (%) | 43 (55.8) | 22 (55.0) | 21 (56.8) | 1.000c | 18 (60.0) | 16 (57.1) | 1.000c | 21 (60.0) | 13 (56.5) | 1.000c | 13 (44.8) | 26 (66.7) |
| Distal CABG-anastomoses N |
3.44 ± 0.90 2–5 Median: 3 |
3.35 ± 0.95 2–5 Median: 3 |
3.54 ± 0.84 2–5 Median: 3 |
0.426b | 3.4 ± 0.97 2–5 Median: 3 |
3.64 ± 0.87 2–5 Median 4 |
0.351b | 3.57 ± 0.884 2–5 Median: 4 |
3.43 ± 0.992 2–5 Median: 3 |
0.542b | 3.48 ± 0.871 2–5 Median: 3 |
3.49 ± 0.914 2–5 Median: 3 |
| Aortic clamping time (min) | 65.9 ± 21.6 24–154 Median: 62 |
64.0 ± 18.4 24–110 Median: 63 |
68.0 ± 24.7 37–154 Median: 60 |
0.422a | 63.5 ± 18.8 24–110 Median: 63.0 |
68.4 ± 26.9 37–154 Median: 59.5 |
0.429a | 67.7 ± 23.4 37–154 Median: 65.0 |
63.1 ± 22.4 24–131 Median: 59 |
0.460a | 59.6 ± 17.7 24–97 Median: 55 |
61.8 ± 16.2 24–97 Median 60 |
| ECC time (min) | 106 ± 34.8 38–236 Median: 102 |
100 ± 27.5 38–161 Median: 102 |
112 ± 40.9 53–236 Median: 106 |
0.155a | 102 ± 23.9 38–161 Median: 102 |
113 ± 44.7 53–236 Median: 103 |
0.248a | 109 ± 39.0 53–236 Median: 102 |
105 ± 30.4 38–185 Median: 102 |
0.644a | 99.9 ± 37.6 38–236 Median: 93 |
106 ± 32.0 38–236 Median: 102 |
| Postoperative | ||||||||||||
| CK max (U/l) | 1299 ± 2525 192–16,584 Median: 583 |
1565 ± 2955 192–16,584 Median: 692 |
1012 ± 1959 200–12,062 Median: 547 |
0.119b | 1262 ± 1885 192–10,116 Median: 711 |
752 ± 664 200–3263 Median: 561 |
0.205b | 913 ± 805 203–4056 Median: 672 |
1171 ± 2086 192–10,116 Median: 547 |
0.369b | 701 ± 552 192–2800 Median: 562 |
1229 ± 1717 198–10,116 Median: 677 |
| CK-MB max (U/l) | 60.0 ± 118 4–892 Median: 37.0 |
57.6 ± 93.9 10–611 Median: 31 |
62.5 ± 141 4–892 Median: 41 |
0.642b | 60.6 ± 107 24–611 Median: 30 |
39.8 ± 15.7 4–79 Median: 41.5 |
0.575b | 60.1 ± 97.9 17–611 Median: 42 |
36.2 ± 20.5 4–107 Median: 28 |
0.028b | 36.1 ± 15.8 17–82 Median: 30.0 |
42.8 ± 23.1 21–115 Median: 31.0 |
Data are n (%), mean ± SD, minimum-maximum, median (interquartile range). BMSC bone marrow stem cell, CABG coronary artery bypass surgery, PCI, percutaneous coronary intervention; AMI, acute myocardial infarction; CAD, coronary artery disease; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; CK, creatine kinase; MRI, magnetic resonance imaging; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist. Perfusion score: 0- normal perfusion, 1 – hypoperfusion, 2 – strong reduced perfusion.
t-Test for independent samples.
U test Mann-Whitney.
Fisher's exact test.
Chi-square test.
2.3. Cell Preparation and Manufacturing
All patients enrolled in the study underwent bone marrow aspiration (mean 166 ± 20 ml) and withdrawal of 20 ml blood one to two days before CABG surgery. To ensure consistent quality and individual safety of the cell product, central manufacturing according to GMP standard was performed at Seracell GmbH, Rostock. CD133+ cells were selected from the bone marrow aspirate of each patient and individuals in the active group received autologous CD133+ cells suspended in physiological saline + 10% autologous serum. Patients of the control group received the placebo preparation with saline + 10% autologous serum; their CD133+ cells were stored by the cell product manufacturing site. In the CD133+ group the recovery percentage of CD133+ cells was 23.7 ± 10.4%, non-target cell depletion efficiency was > 99.2% and the final dose of CD133+ cells administered was 2.29 × 106 ± 1.42. Cell counts were determined by FACS using single platform analysis. The final preparation dose was 0.5 × 106–5 × 106 CD133+ cells suspended in 5 ml of saline supplemented with 10% autologous serum, drawn into 5 × 1 ml syringes.
2.4. Randomisation and Masking
Randomisation to study treatment was done after all screening procedures had been performed, eligibility for the study confirmed and after bone-marrow aspiration. We used permuted block randomisation, randomly varying block sizes, stratified by study site (Rosenberger and Lachin, 2003). Patients were randomised on a 1:1 basis to receive CD133+ cells or placebo (Fig. 1). The study was performed in a double blind manner up to final data closure in 4/2016. Only the cell preparation team at the contract GMP manufacturer was unblinded for production of placebo or CD133+. The appearance of the final placebo and cellular product was indistinguishable to the investigators. In the event of a medical emergency, and necessity for breaking the code, an emergency envelope was available 24 h a day, 7 days a week for a member of the treatment team responsible for patient recruitment and clinical assessment, bone marrow harvest and performing the treatment.
2.5. Magnetic Resonance Imaging
Cardiac MRI was performed in the participating study centres according to an identical standard protocol. Each centre provided test MRI scans to ensure image quality and adherence to the protocol before recruiting patients into the study. Patients were scanned in the supine position in 1·5 T scanners with dedicated cardiac software, using retrospective ECG gating and a phased array receiver coil. Standard imaging protocol included morphologic images of the whole thorax, functional measurements of the heart for LV-volumes and function, perfusion-MRI with adenosine for detection of ischemia, and gadolinium late enhancement measurement for the assessment of LV viability. LV volumes were measured based on a series of breath-hold SSFP-CINE sequences. An end-diastolic, four-chamber view of the left ventricle at end-expiration provided the reference image on which a series of contiguous short axis slices was positioned to cover the entire left ventricle. Infarct volume was assessed on late-gadolinium enhancement MRI images in short axis orientation and vertical long axis. All MRI analyses were performed in a core lab at the University Hospital Göttingen, Department of Diagnostic and Interventional Radiology, whose group members were unaware of treatment assignments. Core lab MRI readings were used to evaluate patient eligibility for the trial. Images were analysed with QMass MR 7.6 software (Medis Medical Imaging Systems).
2.6. Interventions
Placebo (5 ml saline + 10% autologous serum) or CD133+ stem cell (5 ml purified CD133+ BMSC in saline + 10% autologous serum) were administered intramyocardially into the infarction border zone (penumbra) during the cardiac surgical procedure. The procedure was performed with extracorporeal circulatory support, aortic cross clamping and cardioplegic arrest. The injections were done before cross-clamp release. The 5 ml suspensions were distributed in 15–20 injections applied within 3 min in the region of interest (infarct border zone) according to the affected left ventricular segments (see Supplement Fig. 1) at the end of bypass surgery. Not more than one injection per square centimetre was performed. During the whole duration of the study, patients were treated per the standards of the centres and the American Heart Association (AHA) guidelines.
2.7. Outcomes
2.7.1. Prespecified Primary Outcome
Delta (Δ) LVEF at 180 d postoperatively versus baseline (Δ 180 d vs. 0), measured by MRI at rest.
2.7.2. Prespecified Secondary Outcome
Objectives were (Δ 6 m vs. 0) left ventricular dimensions (LVEDV, LVESV), classification of heart failure (NYHA, CCS), NT-proBNP, scar and non-viable tissue, 6-minute-walk-test, adverse events (AE), serious adverse events (SAE), major adverse cardiac events (MACE), Serious Unexpected Serious Adverse Reactions (SUSAR), and Quality-of-Life (QoL). MACE outcome analysis was performed at 24 months.
2.7.3. Post Hoc Analysis
Kaplan-Meier survival (long term vigilance registry approved by the ethics committee of the University Medicine Rostock: A 2017-0031).
2.8. Biomarkers
2.8.1. Prespecified
Distinct hematopoetic and endothelial CD133+ EPC subpopulations and angiogenesis capacity were tested in a cohort of 39 patients in bone marrow (BM) and peripheral blood (PB) employing coexpression analysis using four-laser flow cytometric methods (LSR II, Becton Dickinson, Heidelberg, Germany) for costaining panel enumeration of EPC (Costaining panel CD133, 34, 117, 184, 309, 105, 45) and circulating endothelial cells (CEC) (Costaining panel: CD31, 146, 34, 45, 105, 184, 309) as well as in vitro CFU-EC, CFU-Hill and in vivo Matrigel plug assay. NT-proBNP as well as virus analysis were performed for EBV, CMV, and Parvovirus by IgG and antigen analysis in peripheral blood serum. Post hoc analysis before final data closure was performed for serum angiogenesis factors and cytokines.
2.8.2. Post Hoc Analysis
BM subpopulation analysis and SH2B3 mRNA RT-PCR in peripheral blood (PB): Methods and analysis of biomarkers studied in BM CD133+ and PBMNCs samples using cytometric bead array (CBA) and enzyme-linked immunosorbent assay (ELISA) and RT-PCR are depicted in Supplement Appendix 3. Samples were taken from informed study patients who gave their written consent according to the Declaration of Helsinki. (approval by the Ethical committee, Rostock University Medical Center 2009; No. HV-2009-0012). Analyses and examinations were performed before unblinding of the trial and under careful adherence to the protection of data privacy (pseudonyms).
2.9. Statistical Analysis
The stratification of the primary analysis by centre was neglected in the sample size calculation. Instead of the analysis of covariance (ANCOVA) used in the primary analysis, the two-sample t-test scenario with equal variances was considered. Sample size was determined with the assumption of a two-sided type I error (α) at 5% and a type II error (β) at 10% (i.e. a power at 90%). The scenario of a difference in LVEF at month 6 post-operatively between the two treatment arms of 4 to 5% was considered as a clinically relevant difference. With a difference of 4.5 and a standard deviation of 7.5, at least n = 60 patients per group were considered necessary and, with an additional 15% drop-out rate, a total of at least 142 patients were to be randomised. Sample size was calculated using the commercial program nQuery Advisor 5.0, section 8, Table MTT0-1 (Hofmann et al., 2002). Computation was realized using central and non-central t-distribution where the non-centrality parameter is √ n δ/√2 and δ is defined as effect size | μ1-μ2 |/σ (O'Brien et al., 1993). The two-sided hypothesis for the continuous primary efficacy variable LVEF at 6 months (180 days) postoperatively will be assessed using analysis of covariance (ANCOVA) adjusting for baseline LVEF. Statistical analyses, final data set calculation, and preparation were performed by Koehler GmbH, Freiburg, and G.K., who was not involved in patient recruitment and follow-up.
Multivariance analysis included the ANCOVA, MANCOVA comparison of Placebo vs. CD133 + and for LVEF responders vs. non-responders group using all single parameters of CRF outcome dataset specified in Table 1 and biomarker analysis listed in Appendix 3. Given the complexity of variables additionally machine learning was applied for validation of parameter correlations.
2.10. Data Analysis With Machine Learning
Identifying key features and classification of the comprehensive patient data was obtained by employing supervised and unsupervised machine learning (ML) algorithms (Kuhn, 2008). We preprocessed the data while removing features with low variance and high correlation for dimension reduction following best practices recommendations. Missing measurements were filled with zeros as frequently used in standard data imputation practices. We compared the following supervised algorithms: AdaBoost, Support Vector Machines (SVM) and Random Forest (RF) (Forman and Cohen, 2004). Small clinical datasets are often prone to overfitting. We employed classifiers that are suitable for training on small data sets for a comparison of features given little training and chose the most appropriate algorithm according to accuracy and robustness towards overfitting (Saeb and Al-Naqeb, 2016). Supervised ML models have been 10-fold cross-validated. We then applied feature selection from AdaBoost and RF to further reduce the number of features to < 20. We employed t-distributed stochastic neighbor embedding (t-SNE) for unsupervised machine learning classification and nonlinear dimensionality reduction (Maaten and Hinton, 2008).
2.11. Role of the Funding
The funding had no role in study design, in the collection, analysis, interpretation of data, in the writing of the report, and in the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Patient baseline characteristics analysed in SAS and PPS patient populations are depicted in Table 1. Analysis follows the description of prespecified cohort analyses SAS (n = 77) and PPS (n = 58) placebo vs. CD133+ (Fig. 1). Post hoc analysis was additionally performed to analyse factors influencing primary endpoint outcome. For this, patients were grouped as responders (increase in LVEF ≥ 5% at 180 days) or and non-responders (increase in LVEF < 5% at 180 d). According to this post hoc analysis 35/58 (60.3%) patients were responders and 23/58 (39.7%) did not improve in LVEF. This responder/non-responder (NR) ratio was similar in the placebo group 57/43% (R/NR: 17/13 pt.) and in the CD133+ group 64/36% (R/NR: 18/10 pt.) respectively (placebo vs. CD133+: p = 0.373).
3.1. Safety Outcome Analysis
Prespecified safety outcome (n = 77): Up to 180 d follow-up, two MACE-incidents occurred in 2.6% of the patients (n = 2), ventricular arrhythmias occurring in one patient in the placebo group and one in the CD133 + group (Supplement Table 2). During the main trial phase until 180 days 80 days there was a total of 49 SAE, 24 (15 subjects) in the placebo group and 25 (19 subjects) in the CD133+ group (Supplement Table 3). There were no statistical differences observed between the placebo and the CD133+ group neither overall nor in any of the system organ classes. The most common SAEs were cardiac disorders such as atrial fibrillation, ventricular arrhythmia and cardiac failure, as well as respiratory and wound infections (Supplement Table 3). Of these, 19 were classified as possibly related (placebo 13/68, CD133+ 6/67; p = 0.156) (Supplement Table 4). There were no signs of related classifications of adverse events (Supplement Table 5) or unwanted tissue formation (data not shown) for CD133+ treatment in the initial patient treatment follow-up to 180 days. Post hoc safety analysis in PPS (n = 58): NR revealed increase in lung infection (p = 0.021) (Supplement Table 6).
3.2. Efficacy Outcome Analysis
The PPS efficacy analysis group (n = 58) was characterized by reduced pump function post MI (measured in MRI at rest) with baseline LVEF 33.5%, SD ± 6.26% [Min-Max-25–49], n = 58.
3.2.1. Prespecified Primary Endpoint
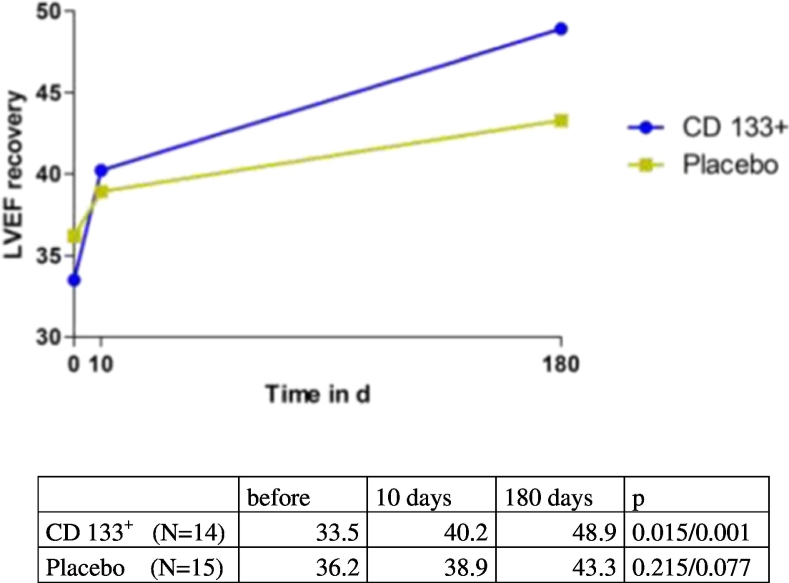
Six months post treatment the left ventricular function showed a considerable increase in LVEF of + 9.6% ± SD 11.3% [Min-Max-13–42], p < 0.001 (n = 58). To discriminate early improvement of left ventricular function by CABG revascularization and late myocardial reverse remodeling, additional intermediate MRI analysis at hospital discharge was available in a subgroup of patients (n = 29). This revealed mainly late (day 10–180) increase of ∆ LVEF by + 6.5%, SD ± 7.92% [Min-Max-11–23], p = 0.007 (n = 29). In ANCOVA analysis of the primary endpoint the placebo group improved from baseline LVEF 33.5% to 42.3% at 180 days (∆ LVEF + 8.8%, and the CD133+ group LVEF was raised from 33.5% to 43.9% (∆ LVEF + 10.4% (Table 2). Treatment group difference CD133+ versus placebo with + 2.58, p = 0.414 was not statistically significant in ANCOVA analysis (Table 2). CD133+ stem cell group displayed ∆ LVEF improvement mainly in the late phase (day 10–180 ∆ LVEF) with + 8.8%,SD ± 6.38% [Min-Max-4–10], p = 0.001 (n = 14) versus placebo controls (day 10–180 ∆ LVEF) + 4.3%, SD ± 8.8% [Min-Max-11–23], p = 0.077 (n = 15) (Fig. 2).
Table 2.
Overall results of the ANCOVAa for primary and secondary outcome parameters in Placebo vs. CD 133+ BMSC (PPS; n = 58).
| Estimated Baseline |
Estimate (at 180 days) | Standard-error | 95% CI | p-Value | |
|---|---|---|---|---|---|
| LVEF (%) | |||||
| Placebob (nevaluable = 30) | 33.52 | 42.30 | 2.17 | [38.0, 46.6] | < 0.001 |
| CD133 +b (nevaluable = 28) | 43.93 | 2.33 | [39.0, 48.5] | < 0.001 | |
| ∆ CD133 +-Placeboc | 2.58 | 3.13 | [− 3.7, 8.9] | 0.414 | |
| LVEDV (index) | |||||
| Placebob (nevaluable = 30) | 107.12 | 100.97 | 11.21 | [79.0, 122.9] | 0.113 |
| CD133 +b (nevaluable = 28) | 105.86 | 12.01 | [82.3, 129.4] | 0.882 | |
| ∆ CD133 +-Placeboc | 5.80 | 7.40 | [− 9.1, 20.7] | 0.437 | |
| LVESV (index) | |||||
| Placebob (nevaluable = 30) | 71.52 | 58.87 | 8.90 | [41.4, 76.3] | < 0.001 |
| CD133 +b (nevaluable = 28) | 61.54 | 9.53 | [42.8, 80.2] | 0.053 | |
| ∆ CD133 +-Placeboc | 2.51 | 6.04 | [− 9.6, 14.6] | 0.680 | |
| Scar size (g) | |||||
| Placebob (nevaluable = 27) | 31.48 | 34.52 | 3.36 | [27.9, 41.1] | 0.087 |
| CD133 +b (nevaluable = 23) | 28.13 | 3.94 | [20.4, 35.9] | 0.212 | |
| ∆ CD133 +-Placeboc | − 7.53 | 3.19 | [− 14.0, − 1.1] | 0.023 | |
| Non-viable tissue (g) | |||||
| Placebob (nevaluable = 27) | 25.20 | 27.78 | 3.73 | [20.5, 35.1] | 0.099 |
| CD133 +b (nevaluable = 23) | 21.57 | 4.38 | [13.0, 30.1] | 0.177 | |
| ∆ CD133 +-Placeboc | − 7.71 | 3.13 | [− 14.0, − 1.4] | 0.018 | |
| LV mass (g) | |||||
| Placebob (nevaluable = 30) | 183.93 | 173.87 | 15.78 | [142.9, 204.8] | 0.025 |
| CD133 +b (nevaluable = 28) | 171.00 | 16.91 | [137.9, 204.1] | 0.051 | |
| ∆ CD133 +-Placeboc | − 3.23 | 6.83 | [− 16.9, 10.5] | 0.638 | |
| 6 MWT (meter) | |||||
| Placebob (nevaluable = 25) | 384.73 | 434.80 | 21.14 | [393.4, 476.2] | 0.039 |
| ∆ CD133 +b (nevaluable = 17) | 441.74 | 31.10 | [380.8, 502.7] | 0.058 | |
| CD133 +-Placeboc | 20.19 | 29.72 | [− 40.1, 80.5] | 0.501 | |
| NT-proBNP | |||||
| Placebob (nevaluable = 28) | 1489.83 | 766.36 | 655.89 | [− 519.2, 2051.9] | 0.037 |
| CD133 +b (nevaluable = 26) | 1465.50 | 706.34 | [81.1, 2849.9] | 0.699 | |
| ∆ CD133 +-Placeboc | 996.82 | 324.15 | [344.7, 1648.9] | 0.004 | |
Source: P132_perfect - EFF02T.sas Data Extract: 15JUL2016 Generation Date: 10AUG2016 21:02.
Bold values indicate significance at p < 0.05.
ANCOVA in final analysis (GK) For primary endpoint analysis in SAP-CTR (Appendix 1) an additional analysis was made using a mixed model analysis for repeat measures approach (MMRM) in order to compensate possible artefacts due to incomplete data groups. This was the approach used for the interim analysis as well.
Average change from Baseline.
Difference in Treatment Groups.
Fig. 2.
Early and late recovery of LVEF in Placebo and CD133+ groups.
MRI analysis of LVEF (%) is depicted in 29 patients with intermediate MRI at day 10 postoperatively and at 180 days. *p value for delta LVEF at 10 days versus 0. #p value for delta LVEF at 6 months versus 10 days.
3.2.2. Prespecified Secondary Endpoint
The delta (∆) change of ventricular dimensions between the CD133+ versus placebo groups after 180 days was not significant in ANCOVA for LVESV index 2.51 ml/m2, p = 0.680 and for LVEDV index + 5.80 ml/m2, p = 0.437 (Table 2). Increased reductions in scar size by − 7.53 g, p = 0.023 and non-viable tissue by − 7 ⋅ 71 g, p = 0.018 (Table 2) were detected in CD133+ versus placebo.
Improvement (Δ) of segmental myocardial perfusion MRI at 180 days versus vs. baseline was observed for CD133+ (p = 0.006), but not in placebo group (p = 0.065) (Supplement Table 1). Improvement (Δ) of hypoperfused LV-segments after stem cell/placebo injections under adenosine stress induction was present in CD133+ group (p = 0.006) in comparison to non-injected segments (p = 0.057) as compared to placebo group (injected segments p = 0.045; non-injected segment p = 0.140) (Supplement Table 1). In contrast, the reduction (Δ) of NT-proBNP values was elevated in placebo versus CD133+ (p = 0.004) (Table 2).
3.2.3. Prespecified Survival
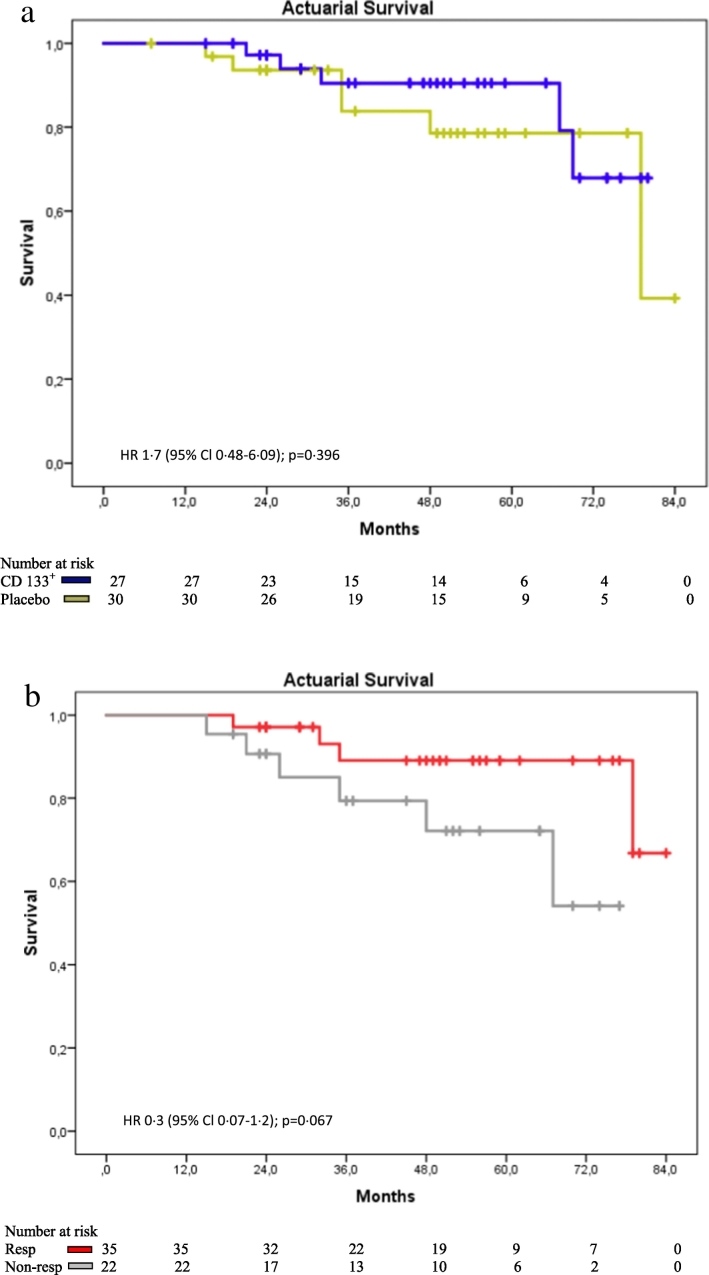
100% at 180 days. Post hoc actuarial computed mean survival time was 70.1 ± 4.75 months (CD133+) vs. 72.0 ± 3.46 months (placebo), and at 5 years follow-up 76.8% (CD133+)/88.1% survival (placebo), HR 1.7 (95% Cl 0.48–6.09); p = 0.396) (Fig. 3a).
Fig. 3.
a: Kaplan-Meier survival analysis in longterm follow-up: Placebo vs. CD133+. b: Kaplan-Meier survival analysis in longterm follow-up: Responder vs. Non-responder
3.3. Responder/Non-responder
In post hoc primary endpoint analysis treatment responders were defined as having a ∆ LVEF at 180 days versus baseline higher than 5%. This results in dissemination of 35 responders in a cohort of 58 patients were characterized by an overall increase in ∆ LVEF in ANCOVA at 180 d/0 of + 17.1% (Table 3). LVEF increase was + 19.1% in CD133+ vs. + 13.9% in placebo, p = 0.099, n = 35 (data not shown). In contrast, non-responders showed a ∆ LVEF at 180 d/0 by 0%, SE ± 5 ⋅ 73% [CI 22.3; 44.8] p = 0.287 (placebo/NR + 3 ⋅ 3%, CD133+/NR-2 ⋅ 4%).
Table 3.
Overall results of the ANCOVA for primary and secondary parameters in Responder vs. Non-responder (n = 58).
| Estimated Baseline |
Estimate (at 180 days) | Standard-error | 95% CI | p-Value | |
|---|---|---|---|---|---|
| LVEF (%) | |||||
| Respondera (nevaluable = 35) | 33.52 | 49.34 | 3.76 | [42.0; 56.7] | < 0.001 |
| Non-respondera (nevaluable = 23) | 33.57 | 5.73 | [22.3; 44.8] | 0.287 | |
| Responder - Non-responderb | 17.10 | 2.08 | [12.9; 21.3] | < 0.001 | |
| LVEDV (index) | |||||
| Respondera (nevaluable = 35) | 107.12 | 90.77 | 9.72 | [71.7; 109.8] | 0.009 |
| Non-respondera (nevaluable = 23) | 122.43 | 14.80 | [93.4; 151.4] | 0.483 | |
| Responder - Non-responderb | − 20.98 | 7.58 | [− 36.2; − 5.8] | 0.008 | |
| LVESV (index) | |||||
| Respondera (nevaluable = 35) | 71.52 | 46.66 | 8.99 | [29.0; 64.3] | < 0.001 |
| Non-respondera (nevaluable = 23) | 80.70 | 13.69 | [53.9; 107.5] | 0.376 | |
| Responder - Non-responderb | − 27.93 | 5.02 | [− 38.0; − 17.8] | < 0.001 | |
| Scar size (ml) | |||||
| Respondera (nevaluable = 31) | 31.48 | 27.48 | 2.86 | [21.9; 33.1] | 0.980 |
| Non-respondera (nevaluable = 19) | 38.26 | 4.67 | [29.1; 47.4] | 0.934 | |
| Responder - Non-responderb | − 8.19 | 3.50 | [− 15.2; − 1.1] | 0.024 | |
| Non-viable tissue (ml) | |||||
| Respondera (nevaluable = 31) | 25.1 | 20.81 | 3.12 | [14.7; 26.9] | 0.841 |
| Non-respondera (nevaluable = 19) | 31.63 | 5.09 | [21.7; 41.6] | 0.981 | |
| Responder - Non-responderb | − 8.55 | 3.56 | [− 15.7; − 1.4] | 0.021 | |
| LV mass (ml) | |||||
| Respondera (nevaluable = 35) | 183.93 | 168.71 | 12.89 | [143.5; 194.0] | 0.032 |
| Non-respondera (nevaluable = 23) | 178.22 | 19.61 | [139.8; 216.7] | 0.092 | |
| Responder - Non-responderb | − 6.01 | 7.01 | [− 20.1; 8.1] | 0.396 | |
| 6 Minute Walk Test (meter) | |||||
| Respondera (nevaluable = 27) | 384.73 | 430.57 | 18.23 | [394.8; 466.3] | 0.016 |
| Non-respondera (nevaluable = 15) | 450.27 | 32.81 | [386.0; 514.6] | 0.141 | |
| Responder - Non-responderb | − 7.19 | 29.82 | [− 67.7; 53.4] | 0.811 | |
| NT-proBNP | |||||
| Respondera (nevaluable = 32) | 1489.83 | 588.41 | 561.48 | [− 512.1; 1689] | 0.005 |
| Non-respondera (nevaluable = 22) | 1851.45 | 816.69 | [250.7; 3452] | 0.867 | |
| Responder - Non-responderb | − 1318.40 | 326.42 | [− 1975; − 661.7] | 0.002 | |
Source: P132_perfect - EFF02T.sas Data Extract: 15JUL2016 Generation Date: 10AUG2016 21:02.
Average change from Baseline.
Difference in Treatment Groups, CI = Confidence Interval.
Post hoc secondary endpoint: Responders showed a significant reduction in LV-dimensions (LVEDV p = 0.008, LVESV p = 0.0001) and reduction in NT-pro-BNP, p = 0.002 compared to non-responders (Table 3). This was not reflected by a similar improvement of 6 MWT (p = 0.811).
The intramyocardial tissue recovery was found in responders with improvement in scar size RvNR − 8.19 g, p = 0.0238 (Table 3). CD133+ treated NR also displayed reduction in scar size (CD133 + NR ∆ scar size 180 d/0: − 13 ⋅ 9 g, SD ± 20 ⋅ 9 g placebo NR + 11 ⋅ 9, SD ± 16 ⋅ 7 g, p = 0.008, n = 20) and non viable tissue (∆ non viable tissue 180 d/0: CD133+ NR -12.4 g, SD ± 19.3 g vs. placebo NR + 11.5 g, SD ± 12.0 g, p = 0.004, n = 19) (data not presented). This tendency was not observed in responders: scar size (CD133+ NR vs. placebo NR -1.9, SD ± 16.0 g vs. placebo + 2.5, SD ± 13.2 g, p = 0.398, n = 33) and non viable tissue (CD133+ NR vs. placebo NR − 1.4, SD ± 16.7 g vs. placebo + 1.8, SD ± 12.3 g, p = 0.544, n = 32). Improvement (Δ) of segmental myocardial perfusion MRI at 180 days versus vs. baseline was observed for R (p = 0.004), but not in NR group (p = 0.101) (Supplement Table 1). Improvement (Δ180 d/0) of hypoperfused LV-segments under adenosine stress induction was present in R group in injected segments (p = 0.009) as well as in non-injected segments (p = 0.017), whereas in NR only injected segments were improved (injected segments p = 0.034; non-injected segment p = 0.383) (Supplement Table 1). Long term survival: Actuarial computed mean survival time was 76.9 ± 3.32 months (R) vs. + 72.3 ± 5.0 months (NR), HR 0.3 [Cl 0.07–1.2]; p = 0.067 (Fig. 3b).
3.4. Peripheral Blood Biomarker Profile
Circulating EPC (CD133+/CD34+/CD117+) in peripheral blood were found to be reduced by a factor of two in NR versus R before treatment. For CD34+ MNC subpopulations preoperative blood levels were (R): CD34+ 0.072%, SD ± 0.05% vs. (NR) 0.039%, SD ± 0.017, RvsNR p = 0.027. Similar difference was found preoperatively for CD133+ and CD133+ CD117+ subpopulations (Table 4). This difference was not found for the comparison of placebo and CD133+ (Table 4). In contrast, CD146+ CEC showed higher preoperative levels in non-responders versus responders (p = 0.053) (Table 4).
Table 4.
Analysis of angiogenesis related biomarkers in blood.
| Responder versus non-responder | ||||||
|---|---|---|---|---|---|---|
| Biomarker (peripheral blood, unit) |
Time point | Responder (n = 15) | P 10 days vs 0 |
Non-responder (n = 8) | P 10 days vs 0 |
PA R vs NR |
| SH2B3 mRNA (ΔCT %) | 0 | − 1.17 ± 0.28 | … | − 1.56 ± 0.51 | … | 0.073 |
| CD34 (% MNC) -EPC |
0. | 0.072 ± 0.05 | 0.197 | 0.039 ± 0.017 | 0.116 | 0.027 |
| 10 d | 0.059 ± 0.048 | 0.027 ± 0.01 | 0.026 | |||
| CD133 (% MNC) – EPC |
0 | 0.048 ± 0.031 | 0.245 | 0.021 ± 0.011 | 0.932 | 0.005 |
| 10 d | 0.041 ± 0.039 | 0.021 ± − 0.013 | 0.105 | |||
| CD133,117 (% MNC) EPC |
0 | 0.019 ± − 0.016 | 0.421 | 0.007 ± 0.008 | 0.765 | 0.024 |
| 10 d | 0.022 ± 0.024 | 0.006 ± 0.004 | 0.024 | |||
| CD146 (% MNC) -CEC |
0 | 1.1 ± 0,57 | … | 2.2 ± 1.3 | … | 0.053 |
| 10 d | 1.72 ± 1.73 | 1.86 ± 1.53 | 0.853 | |||
| IGFBP-3 (ng/ml) | 0 | 2121.9 ± 487.1 | 0.115 | 1623.7 ± 651.4 | 0.257 | 0.089 |
| 10 d | 1753.6 ± 830.8 | 1378.4 ± 518.7 | 0.261 | |||
| VEGF (pg/ml) | 0 | 24.6 ± − 36.6 | 0.015 | 39.6 ± 33.4 | 0.913 | 0.056 |
| 10 d | 51.2 ± 55.8 | 40.8 ± − 44.5 | 0.528 | |||
| IP-10 (pg/ml) | 0 | 96.7 ± 42.6 | 0.04 | 157.6 ± 94.5 | 0.01 | 0.076 |
| 10 d | 63.3 ± 28.3 | 95.8 ± 85.2 | 0.324 | |||
| EPO (mlU/ml) | 0 | 5.9 ± 3.7 | 0.001 | 16.9 ± 14.1 | 0.006 | 0.023 |
| 10 | 60.1 ± 27.7 | 42.1 ± 23.9 | 0.180 | |||
| Placebo versus CD133 + | ||||||
| Biomarker (peripheral blood, unit) |
Time point | Stem cell (n = 11) | P | Control (n = 13) | P | PA |
| SH2B3 mRNA (ΔCT %) | 0 | − 1.35 ± 0.45 | … | − 1.29 ± 0.41 | … | 0.756 |
| CD34 (% MNC) -EPC | 0. | 0.062 ± 0.037 | 0.128 | 0.064 ± 0.053 | 0.250 | 0.975 |
| 10 d | 0.041 ± 0.038 | 0.058 ± 0.047 | 0.363 | |||
| CD133 (% MNC) – EPC | 0 | 0.04 ± 0.03 | 0.338 | 0.04 ± 0.029 | 0.619 | 0.995 |
| 10 d | 0.032 ± 0.026 | 0.038 ± 0.032 | 0.637 | |||
| CD133,117 (% MNC) – EPC | 0 | 0.014 ± 0.013 | 0.902 | 0.016 ± 0.017 | 0.265 | 0.892 |
| 10 d | 0.015 ± 0.02 | 0.019 ± 0.022 | 0.626 | |||
| CD146 (% MNC) -CEC | 0 | 1.53 ± 1.33 | … | 1.48 ± 0.67 | … | 0.919 |
| 10 d | 1.64 ± 1.55 | 1.87 ± 1.74 | 0.750 | |||
| IGFBP-3 (ng/ml) | 0 | 1950.6 ± 689.9 | 0.139 | 1946.8 ± 507 | 0.231 | 0.972 |
| 10 d | 1561.6 ± 783.2 | 1679.4 ± 742.6 | 0.715 | |||
| VEGF (pg/ml) | 0 | 30.2 ± 29.1 | 0.142 | 29.6 ± 39.1 | 0.124 | 0.961 |
| 10 d | 55.8 ± − 58.5 | 38.5 ± 44.7 | 0.293 | |||
| IP-10 (pg/ml) | 0 | 129.2 ± 96.7 | 0.011 | 102.9 ± 34.6 | 0.001 … |
0.275 |
| 10 d | 83.2 ± 77.9 | 64.5 ± 22.7 | 0.457 | |||
| EPO (mlU/ml) | 0 | 7.7 ± 3.1 | 0.001 | 10.3 ± 12.6 | 0.001 | 0.561 |
| 10 d | 53.5 ± − 30.6 | 56.4 ± 25.5 | 0.814 | |||
Responder versus non-responder and placebo versus CD133+ groups were analysed for change in biomarkers of peripheral blood samples between preoperative (Assessment I) and day 10 postoperative (discharge). The data are derived from the Rostock cohort with complete analysis (per protocol clinical dataset and biomarker). In this cohort all samples were immediately processed to avoid any change of the samples due to storage or transport. Data are expressed as mean values ± Standard deviation, P-value between time point 0 and 10 days, PA -value between responder/non-responder, stem cell/control in each time point, PB – peripheral blood, EPO - Erythropoeitin.
Postoperatively, reduction of EPC in NR remained significant until discharge: peripheral blood CD34+ (NR vs. R p = 0.026 preop and day 10) and CD133+ CD117+ (NR vs. R p = 0.024 preop and day 10) despite postoperative increased levels of EPO (NR: preop. 16.9 U/ml, SD ± 14.1 U/ml; NR day 10: 42.1 U/ml, SD ± 23.9 U/ml; p = 0.006 preop/day 10) and reduction of IP10/CXCL10 (NR preop: 157.6 pg/ml, SD ± 94.5 pg/ml; NRday 10: 95.8 pg/ml, SD ± 85.2 pg/ml; p = 0.01 preop/day 10).
Treatment responders were characterized preoperatively by lower serum levels of pro-angiogenic factors such as VEGF (p = 0.056 R/NR), EPO (p = 0.023 R/NR), CXCL10/IP10 (p = 0.076 R/NR), higher levels of IGFBP-3 (p = 0.089 R/NR) (Table 4), as well as strong induction of VEGF (+ 26.6 pg/ml, p = 0.015 preop/day 10) at day 10 after intervention versus non-responders (+ 1.2 pg/ml, p = 0.913 preop/day 10) (Table 4). The CFU-EC capacity of purified CD 133 + bone marrow cells was positive in all tested patients without difference between responders (n = 13; mean 63.133 ± SD 13.6) and non-responders (n = 9; mean 77,833 ± SD 15.81) p = 0.177. Matrigel plug assay in vivo was positive in responders and non-responders (Supplement Table 7).
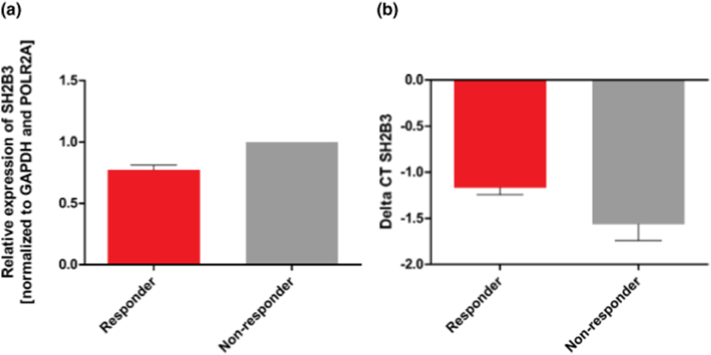
Thrombocyte counts were preoperatively reduced in NR (208 × 109/L, SD ± 51.2 109/L [CI 73–311], n = 23) versus R (257 × 109/L, SD ± 81.5109/L [CI 123–620] n = 35) (NR vs R: p = 0.004, n = 58) before treatment. Suspecting bone marrow stem cell suppression by finding reduced PB thrombocyte and CD133+ CD34+ EPC count, we tested RT-PCR gene expression analysis of SH2B3 mRNA coding for the lnk adaptor protein SH2B3 which is associated with inhibition of hematopoietic stem cell response for EPC and megakaryocytes in immediately frozen blood samples. First analysis in 21 patients revealed a tendency of increased mRNA expression in peripheral blood with non-responders (p = 0 ⋅ 073) (Fig. 4, Table 4).
Fig. 4.
SH2B3 expression analysis in peripheral blood of responder and non-responder.
Whole blood samples were obtained from 21 patients before coronary artery bypass graft (CABG) revascularization. Relative expression of SH2B3 (a) and corresponding ΔCT values (b) were calculated using the 2− ΔΔCT method. All values are presented as mean ± SEM and normalized to GAPDH and POLR2A. n = 13 (responder); n = 8 (non-responder). ΔCT values: p = 0.073.
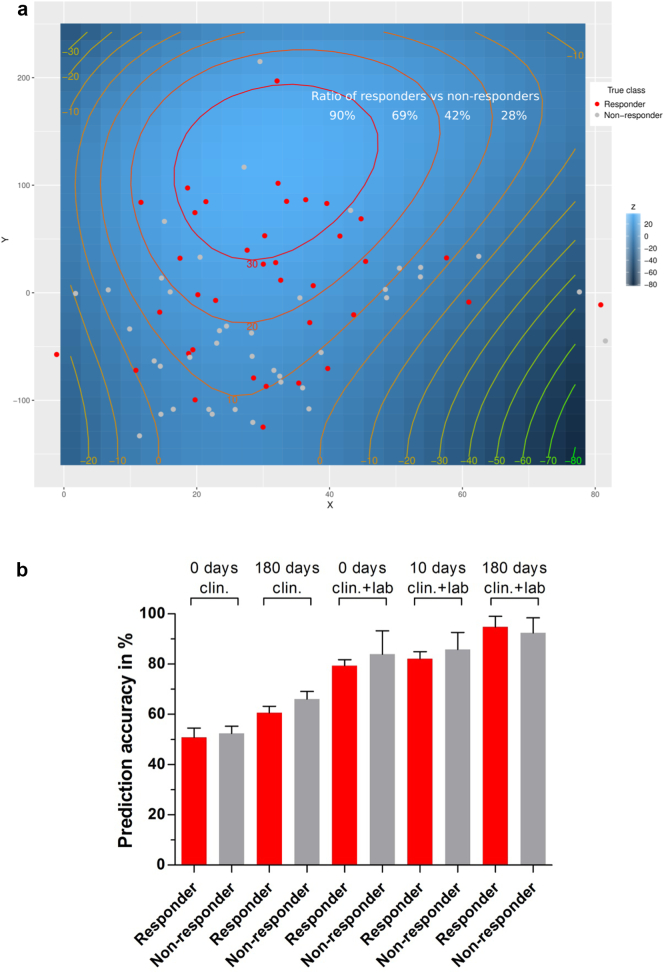
To identify a diagnostic response signature for R/NR we used machine learning methods as a tool for the prediction of functional improvement after CD133 + BMDC therapy and CABG surgery. First analyses were performed to particularly exclude overfitting in small populations. Then, blinded patient data from the PERFECT clinical database (Table 1) was investigated by t-SNE unsupervised ML, which is able to cluster similar patients in close proximity and reveals distinct groups (Fig. 5). Investigating the underlying segmentation, the firstline supervised ML analysis was made for all time points to place patient characteristics into two distinct groups (Fig. 5). The calculation independently assigned patient characteristics according to ∆ LVEF at 180 days confirming the preselection criteria of > 5% (Table 5). Then we used machine learning algorithms to investigate the decisive parameters to a response signature. For this the underlying PERFECT clinical dataset and biomarker laboratory measurements (Table 1, Appendix 3) were combined and analysed to validate classification specificity of parameter profiles for responders and non-responders before and after the CABG procedure. In particular, we used discriminative primary and secondary endpoint parameters as well as thrombocyte and leukocyte counts. Using only the clinical parameters (n = 160) classification resulted in a specificity of responders assuming mean accuracy of 63.35% (180 days) (Table 5). Combination of preoperative clinical data (n = 49) and biomarker laboratory parameters (n = 142), however, revealed higher sensitivity of angiogenesis/EPC/CEC related parameters in peripheral blood already preoperative with respective assuming max. Accuracy of 81.64% ± SE 0.51% [CI 80.65–82.65] (n = 31) (Table 5). Interestingly, 17/20 relevant parameters were related to angiogenesis parameters, bone marrow EPC/CEC responses, NT-proBNP, and SH2B3 gene expression in peripheral blood (Table 5). Using both clinical and biomarker parameters preoperative prediction accuracy for responders was 79.35% ± SE 0.24% [CI 78.87–79.84] (n = 31) and for non-responders 83.95% ± SE 0.93% [CI 82.10–85.80] (n = 31). Postoperative evaluation at day 10 (n = 382) revealed a prediction accuracy of 82.12% ± SE 0.28% [CI 81.56–82.67] (n = 31) (R) and 85.89% ± SE 0.67% [CI 84.56–87.22] (n = 31) (NR) (Fig. 5b), while day 0–180 combined clinical and biomarker analysis (n = 522) allowed a prediction accuracy of 94.77% ± SE 0.43% [CI 93.92–95.63] (n = 31) (R) and 92.44% ± SE 0.60% [CI 91.24–93.64] (n = 31) (NR) (Fig. 5).
Fig. 5.
a Three-dimensional t-SNE calculation of the Rostock subgroup. The variables x and y refer to the newly calculated features that are used to classify the patients into distinct groups. The model was subsequently fitted by a polynomial (n3) equation to visualize the z-axis as a geographic profile. The respective colors for the responder (red dot) and non-responder (grey dot) patients have been added afterwards. The classified groups have been roughly summarized by a red and grey dashed line. Results are obtained after 3000 iterations. The calculation of the ratio between responder and non-responder is indicated for each circle. It is more likely for the non-responder group to be located at smaller z-values (z < 20, ratio < 42%). The responders tend to be enriched within the light blue areas (z > 20) including a ration > 69%. b Obtained supervised ML prediction results for pre- and postoperative time points (0 days to 180 days) of the clinical and clinical & laboratory dataset to distinguish between responder and non-responder. The graph shows the true positive prediction results of five independent feature selected ML models (AdaBoost for feature selection and RF for final prediction).The error bars indicate the respective accuracy standard deviation for the constructed models that have been obtained after 100 iterations. The 100 model iterations are significant different according to one-way ANOVA (p < 0.001).
Table 5.
Machine-learning selected parameters for diagnostic discrimination of responders and non-responders.
| Computationally selected features for the multi-centric clinical trial data subset (0–180 days) N = 58 |
Weights for the selected features | Computationally selected features for the clinical trial data and laboratory biomarker subset of the Rostock group (day 0 - preoperative) N = 31 |
Weights for the selected features |
|---|---|---|---|
| DeltaViable tissue 6 m/0 | 2.554 | NT proBNP 0 | 9.718 |
| Triglycerides 0 |
2.260 | VEGF_I | 7.810 |
| Scarsize 6 months |
2.159 | Erythropoietin_I | 4.262 |
| DeltaScarsize 6 m/0 |
2.063 | Vitronectin_I | 3.898 |
| Nonviable tissue 6 months |
1.999 | CFU_Hill_I | 2.871 |
| Body mass index 0 |
1.982 | CD45Neg_EPC_I | 2.186 |
| 6MWT 0 |
1.974 | CD117_184_PB_EPC_IHG_I | 2.146 |
| DeltaEF 6 m/0 |
1.967 | CD45_117_184_EPC_I | 2.118 |
| 6MWT 10 days |
1.920 | CD45_133_146_PB_CEC_I | 1.969 |
| LVEF 0 |
1.890 | Thrombocytes I | 1.951 |
| Bypasstime min | 1.883 | IGFBP-3_I | 1.922 |
| Euroscore 0 |
1.874 | CD133 pro ml PB_I IHG | 1.910 |
| CKmax | 1.857 | CD146_PB_CEC_I | 1.799 |
| Scarsize 0 |
1.771 | CD105_PB_CEC_I | 1.793 |
| NTproBNP 0 |
1.771 | CD45_133_34_105_PB_CEC_I | 1.489 |
| Crossclamptime | 1.675 | MatrigelPlug_PB_31_I | 1.475 |
| Delta6MWT 6 m/0 |
1.673 | CD45_133_34_117_309_EPC_I | 1.420 |
| Creatinine 0 |
1.645 | Delta_CT_SH2B3_I | 1.393 |
| LVESV 0 |
1.604 | Weight | 1.363 |
| Weight 0 |
1.389 | LVESV I 0 | 1.352 |
| Accuracy | 63.35% | Accuracy | 81.64% |
Selected features of the AdaBoost ML algorithm showing the most informative selection criteria for the subsequently created ML models. The features are ordered due to their calculated weights in a decreasing manner. Accuracies are based on 100 independent predictions of 10-fold cross-validation calculations (Model has been built after AdaBoost feature selection and random forest feature learning).
4. Discussion
4.1. Baseline Characteristics of Treatment Responders vs. Non-responders
Induction of cardiac repair in patients with heart failure after myocardial infarction and ischemic cardiomyopathy has been targeted using numerous approaches including cardiac stem cell therapy (Fisher et al., 2016). However, the lack of efficacy and the lack of response predictability have been the main obstacles for treatment standardization and success (Tian et al., 2014). Our approach employing CD133+ autologous bone marrow derived cells intramyocardially in conjunction with CABG revascularization was promising in previous Phase I and Phase IIa trials and led to the multicentric placebo controlled phase III PERFECT trial investigation, which again confirmed the induction of cardiac repair (Stamm et al., 2003, Tse et al., 2003, Stamm et al., 2007, Nasseri et al., 2014). In the meantime, however, similar trials involving placebo-treated controls undergoing bone marrow harvest showed almost the same improvement of LVEF in the CD133 + group as in the placebo group (Nasseri et al., 2014, Bartunek et al., 2016). Similarly, the Chart-1 trial demonstrated a relevant functional recovery in only 60% of the patients, whereas 40% of both cell-treated and placebo-treated patients were non-responsive for unkown reasons (Bartunek et al., 2017). This significant non-responder rate was recently corroborated in CABG surgery for patients with reduced pump function (Vakil et al., 2016). In the clinical setting of the PERFECT trial, a nearly identical percentage of patients were non-responders to induction of cardiac repair, irrespective of their treatment with placebo or CD133+ cells.
The underlying mechanism for a lack of response to induction of cardiac repair may be a failure of vascular repair by reduced circulating EPC. This mechanism was shown already 12 years ago to be associated with progression in atherosclerosis and coronary artery disease (Werner et al., 2005). Recently, the investigation of responders to cardiac repair in the CCTRN-trials obtained similar findings in bone marrow of BMDC treated non-responsive chronic ischemic heart failure patients (Bhatnagar et al., 2016, Contreras et al., 2017). In the PERFECT trial we found a striking difference in cardiac recovery between responders and non-responders. This was found for the first time to be associated with a specific signature composition of angiogenesis related biomarkers in peripheral blood. This was accompagnied by improved microvascular perfusion in the myocardium. Non-responsive patients did not exhibit any change in deteriorated left ventricular pump function both in placebo and CD133+ groups. Only a minor effect on scar size and non-viable tissue repair was found in intramyocardial treated CD133+ NR. In addition to numerous local tissue processes that have been shown to influence myocardial repair, such as fibrosis, inflammation, apoptosis, and potential endogenous cardiac stem cell niches, our data support the notion that blood and bone marrow components regeneration also play a key role.
4.2. Mechanism of Action for Cardiac Repair and Diagnostic Access
The typical blood components in non-responders are lowered CD133+ CD34+ CD117+ EPC and thrombocytes counts in the peripheral blood and elevated angiogenesis stimulating factors as VEGF and EPO. In contrast, responders display basically elevated EPC and thrombocytes also in the absence of angiogenesis stimulating factors. We propose that the mechanism of impaired angiogenesis is caused by a dysfunctional bone marrow response. Potential mechanisms of impaired angiogenesis response may be either the anti-angiogenic interference of inflammatory cytokines, such as IP10, or NT-proBNP that may influence EPC proliferation or release mechanisms (Strieter et al., 1995, Stamm et al., 2003, Cesari et al., 2008). In this context, the first description of upregulated SH2B3 gene expression enhancement in the peripheral blood of non-responders associated with reduced EPC and thrombocyte counts suggests a potential regulatory role of SH2B3 with respect to suppression of the bone marrow response (Cesari et al., 2008, Kwon et al., 2009, Lee et al., 2016). Experimental models have depicted the potential importance and diagnostic or therapeutic relevance of SH2B3 gene expression and lnk adaptor protein SH2B3 for regulation of bone marrow responses and impairment of angiogenesic capacity (Ishige-Wada et al., 2016, Takizawa et al., 2008). Moreover, associations with hematological traits, coronary artery disease, and arteriosclerosis have been found for point mutations of SH2B3 promotor regions as well as influence of SH2B3 SNP on human longevity (Auer et al., 2014, McPherson and Tybjaerg-Hansen, 2016, Fortney et al., 2015). However, further clinical evaluation of SH2B3 expression is needed to unravel the precise mechanism in humans.
Feature selection based on our machine learning approach led to the identification of decisive factors for lack of response and the induction of cardiac repair, which can be used for diagnostic R/NR selection before and monitoring of during treatment. The core factors for laboratory diagnosis in peripheral blood were NT-proBNP, VEGF, Erythropoeitin, vitronectin, circulating EPC/CEC/Thrombocytes, SH2B3 mRNA expression, the CFU-Hill assay/Matrigel plug for peripheral blood, as well as weight and LVESV index. We found a statistical correlation of the identified factors and calculated their diagnostic use for the selection of responder and non-responder patients using repeated cross-validation (Fig. 6).
Fig. 6.
Outcome results of the PERFECT trial.
4.3. Relevance of LVEF Endpoint for Longterm Survival
The current analysis of longterm survival benefit in patients with induction of LVEF recovery after CABG/CD133+ treatment suggests a clinical conversion of progressive heart failure by restitution of ventricular function. Moreover, considering the proposed underlying mechanism of impaired angiogenesis and vascular repair capacity of bone marrow, cardiac functional restitution may be dependent on bone marrow function. The current example of peripheral blood analysis focusing on angiogenesis factors and bone marrow derived cell subpopulations allows the definition of signature constellations defining normal or pathological stimulation/response patterns. The machine learning tool independently confirmed the response state as well as the angiogenesis factors involved in deficient response.
Long term deficit in vascular repair may result in progressive heart failure. CABG surgery can be considered as a potent intervention for the induction of cardiac repair most likely stimulated by bone marrow harvest prior to surgery as a preconditioning signal in responders (Blatt et al., 2016). Of utmost importance, however, is the further analysis of factors downregulating blood repair mechanisms in non-responders.
5. Conclusion
The PERFECT trial shows that cardiac tissue repair and restitution of left ventricular function can be successfully installed in ischemic heart disease by CABG surgery associated with presence of enhanced peripheral circulating CD133 + EPC level. In addition, dysfunctional left ventricular post-infarct tissue may be recruited by the local injection of purified CD133 + BMDC. The induction of cardiac repair, however, is correlated to CD133 + EPC release from bone marrow. Resistence of HSC/EPC to growth factor induction may be caused by elevated SH2B3 gene expression in non-responders. The diagnostic sensitivity of the responder vs. non-responder signature may be useful for diagnosis of deficient repair capacity in cardiovascular disease and for the preselection of patients for inductive stem cell therapy.
Limitations of the Study
Main limitations of the study are: 1. Preterm closure of recruitment resulting in limited patient number for efficacy analysis. 2. Non-significant CD133+ effect on primary endpoint despite positive intermediate analysis. 3. Unknown mechanism of treatment unresponsiveness interfering with treatment intervention. 4. Need for further clinical evaluation of suspected blood/bone marrow suppression by SH2B3/lnk activator. 5. Predictive value of response signature in larger patient populations.
Declaration of Interests
All authors declare no competing interests.
Acknowledgments
Funding
German Ministry of Research and Education (BMBF): FKZ0312138A, EU ESF/IV-WM-B34-0011/08, ESF/IV-WM-B34-0030/10 and Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany.
Acknowledgments
Acknowledgements
This study was funded by the German Ministry of Research and Education, Berlin, Germany, and Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany. We would like to thank the PERFECT study group for their dedicated performance and support of the trial. We thank Guilio Pompilio (Milano, Italy), Francesco Siclari (Zuerich, Switzerland), Warren Sherman (New York City, USA), and Johannes Waltenberger (Münster, Germany) who served as members of the Data and Safety Monitoring Board. We thank the medical writers Dr. Claudia Frumento for the careful preparation of data in the CTR and James Hewlett for careful correction of the manuscript. The statisticians Uta Mehdorn and Horst Lorenz for careful control of data analysis.
Acknowledgments
Contributors
G Steinhoff contributed to study design, trial organization, medical controlling, enrolment and clinical follow-up of patients, research plan, analysis of clinical data, analysis of research data, data collection, data control, and drafted the manuscript.
A Haverich, S Sarikouch, FW Mohr, J Garbade, C Stamm, J Börgermann, F M Wagner, A Kaminski contributed to enrolment and clinical follow-up of patients, data collection and interpretation.
G Tiedemann contributed to study design, management and GxP control of the trial.
J Lotz analysed MRI data.
G Kundt performed statistical analyses.
J Nesteruk contributed to laboratory study design, follow-up analysis of patients, data collection, and statistical analyses.
P Oostendorp examined and interpreted data for final analysis.
P Mueller, J Große, A Skorska, U Ruch, R David performed laboratory analysis and examined data collection.
M Wolfien, H Hennig and O Wolkenhauer applied and investigated machine learning data analysis.
All authors contributed to final data interpretation, critically revised the manuscript, and approved the final version for submission.
PERFECT Study Group: Jana Große, Ulrike Ruch, Alexander Kaminski, Christian Klopsch, Peter Donndorf, Julia Nesteruk, Anna Skorska, Paula Müller, Robert David, Ralf Gaebel, Cornelia Lux, Peter Mark, Andreas Martens, Axel Haverich, Andreas-Matthaeus Bader, Michael Dandel, Christoph Knosalla, Elke Wenzel, T. Deuse, Anne-K. Funkat, Florian Wagner, Hermann Reichenspurner, Dirk Balshüsemann, Jens Brickwedel, Bernd Schröder, Dagmar Hartung, Günther Kundt, Heike Kurzidim, Heike Windhagen, Ilona Maeding, Ina Wagner, IPiroze Minoo Davierwala, J. Börgermann, Jan Kormann, Jan Martin Sohns, Jens Garbade, Joachim Lotz, Katharina Gawenda, Katharina-Wiebke Felke, Katja Schönefeld, Liane Preußner, Mandy Ludwig, Marcel Vollroth, Martin Fasshauer, Melanie Wittenberg-Marangione, Michiel Morshuis, Monika Teichmann, Murat Aktas, Nicole Schütz, Nicole Sprathof, Pascal Dohmen, Friedrich-Wilhelm Mohr, Roland Hetzer, Christof Stamm, S. Helms, S. Huebler, Samir Sarikouch, Sandra Bubritzki, Sebastian Rojas, Silke Holtkamp, Tanja Otto, Tatjana Alberg, Ulrike Hess, Ingo Kutschka, and Gustav Steinhoff.
Nomenclature
- AE
Adverse Event
- AESI
Adverse Event of Special Interest
- AHA
American Heart Association
- ANCOVA
Analysis of covariance
- BM
Bone marrow
- BMSC
Bone marrow stem cells
- BMDC
Bone marrow derived cells
- CABG
Coronary Artery Bypass Graft
- CAP-EPC
Concentrated Ambient Particels – Endothelial Progenitor Cells
- CBA
Cytometric Bead Array
- CCS
Canadian Cardiovascular Society
- CCTRN
Cardiovascular Cell Therapy Research Network
- CD
Cluster of Differentiation
- CEC
Circulating endothelial cells, CEC panel, CDs measured in PB
- CFU
Colony-forming unit
- CI
Confidence interval
- CMV
Cytomegalovirus
- EA
Early Antigen
- EBNA1
EBV-Nuclear Antigen 1
- EBV
Epstein-Barr-Virus
- EC
Endothelial Cells
- ECG
Echocardiography
- ELISA
Enzyme-Linked Immunosorbend Assay
- EPC
Endothelial Progenitor Cells, EPC panel, CDs measured in PB
- EPO
Erythropoietin
- GMP
Good Manufacturing Practice
- HR
Hazard ratio
- HIF
Hypoxia-Inducible Factor, transcription factor
- ICH GCP
Tripartite Guidelines Guideline for Good Clinical Practice
- IGF-1
Insulin-like Growth Factor 1
- IGFBP2/3
Insulin-like Growth Factor-Binding Protein 2/3
- IHG
Analysis performed in accordance with ISHAGE guidelines
- IL
Interleukin
- IP-10
Interferon Gamma-induced Protein 10 also known as C-X-C motif chemokine 10 (CXCL10)
- LMCA
Left Main Coronary Artery
- LVEDV
Left Ventricular End Diastolic Volume
- LVEF
Left Ventricular Ejection Fraction
- LVESD
Left Ventricular End Systolic Dimension
- MACE
Major Adverse Cardiovascular Events
- ML
Machine learning
- MNC
Mononuclear cells
- MRI
Magentic Resonance Imaging
- 6MWT
6-Minute Walk Test
- NT-proBNP
B-type Brain Natriuretic Peptide
- PB
Peripheral blood
- PBMNC
mononuclear cells isolated from peripheral blood
- PCI
Percutaneous Coronary Intervention
- PEI
Paul-Ehrlich Institute
- PPS
Group of patients for per-protocol set
- SAE
Serious adverse event
- SAS
Group of patients for safety set
- SDF-1
Stromal Cell-derived Factor 1
- SH2B3
Lnk [Src homology 2-B3 (SH2B3)] belongs to a family of SH2-containing proteins with important adaptor functions
- SCF
Stem Cell Factor
- STEMI
ST- segment Elevation Infarction
- SUSAR
Suspected Unexpected Serious Adverse Reaction
- TNF
Tumor Necrosis Factor
- t-SNE
t-distributed neighbor embedding
- VCA
Virus-Capsid-Antigen
- VEGF
Vascular Endothelial Growth Factor
- VEGF rec
Vascular Endothelial Growth Factor Receptor
- VEGFR2/KDR
Vascular Endothelial Growth Factor Receptor 2/Kinase Insert Domain Receptor
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.07.022.
Contributor Information
Gustav Steinhoff, Email: gustav.steinhoff@med.uni-rostock.de.
Julia Nesteruk, Email: iuliia.nesteruk@med.uni-rostock.de.
Markus Wolfien, Email: markus.wolfien@uni-rostock.de.
Günther Kundt, Email: guenther.kundt@med.uni-rostock.de.
Jochen Börgermann, Email: jboergermann@hdz-nrw.de.
Robert David, Email: robert.david@med.uni-rostock.de.
Jens Garbade, Email: jens.garbade@helios-kliniken.de.
Jana Große, Email: jana.grosse@med.uni-rostock.de.
Axel Haverich, Email: haverich.axel@mh-hannover.de.
Holger Hennig, Email: holger.hennig@uni-rostock.de, holgerh@broadinstitute.org.
Alexander Kaminski, Email: alexander.kaminski@med.uni-rostock.de.
Joachim Lotz, Email: joachim.lotz@med.uni-goettingen.de.
Friedrich-Wilhelm Mohr, Email: chir@herzzentrum-leipzig.de.
Paula Müller, Email: paula.mueller@med.uni-rostock.de.
Robert Oostendorp, Email: robert.oostendorp@tum.de.
Ulrike Ruch, Email: ulrike.ruch@med.uni-rostock.de.
Anna Skorska, Email: anna.skorska@med.uni-rostock.de, sarikouch.samir@mh-hannover.de.
Christof Stamm, Email: stamm@DHZB.de.
Gudrun Tiedemann, Email: gudrun.tiedemann@web.de.
Florian Mathias Wagner, Email: fl.wagner@uke.de.
Olaf Wolkenhauer, Email: olaf.wolkenhauer@uni-rostock.de.
Appendix A. Supplementary data
Supplementary material
Supplementary material
References
- Auer P.L., Teumer A., Schick U. Rare and low-frequency coding variants in CXCR2 and other genes are associated with hematological traits. Nat. GenetNat. Genet. 2014;46(6):629–634. doi: 10.1038/ng.2962. (Epub 2014 Apr 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartunek J., Terzic A., Davison B.A. Cardiopoietic cell therapy for advanced ischemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2016 doi: 10.1093/eurheartj/ehw543. http://dx.doi.org/10.1093/eurheartj/ehw543 Dec 23. pii: ehw543. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartunek J., Terzic A., Davison B.A. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2017;38(9):648–660. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A., Bolli R., Johnstone B.H. Cardiovascular cell therapy research network (CCTRN). Bone marrow cell characteristics associated with patient profile and cardiac performance outcomes in the LateTIME-cardiovascular cell therapy research network (CCTRN) trial. Am. Heart J. 2016;179:142–150. doi: 10.1016/j.ahj.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt A., Elbaz-Greener G.A., Tuby H., Maltz L. Low-level laser therapy to the bone marrow reduces scarring and improves heart function post-acute myocardial infarction in the pig. Photomed. Laser Surg. 2016;34(11):516–524. doi: 10.1089/pho.2015.3988. [DOI] [PubMed] [Google Scholar]
- Cesari F., Caporale R., Marcucci R. NT-proBNP and the anti-inflammatory cytokines are correlated with endothelial progenitor cells' response to cardiac surgery. Atherosclerosis. 2008;199(1):138–146. doi: 10.1016/j.atherosclerosis.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Contreras A., Orozco A.F., Resende M. Identification of cardiovascular risk factors associated with bone marrow cell subsets in patients with STEMI: a biorepository evaluation from the CCTRN TIME and LateTIME clinical trials. Basic Res. Cardiol. 2017;112(1):3. doi: 10.1007/s00395-016-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donndorf P., Kaminski A., Tiedemann G., Kundt G., Steinhoff G. Validating intramyocardial bone marrow stem cell therapy in combination with coronary artery bypass grafting, the PERFECT phase III randomized multicenter trial: study protocol for a randomized controlled trial. Trials. 2012;13:99. doi: 10.1186/1745-6215-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S.A., Mathur A., Taggart D.P., Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev. 2016;12 doi: 10.1002/14651858.CD007888.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman G., Cohen I. 2004. Learning From Little: Comparison of Classifiers Given Little Training. [Google Scholar]
- Fortney K., Dobriban E., Garagnani P. Genome-wide scan informed by age-related disease identifies loci for exceptional human longevity. PLoS Genet. 2015;11(12):e1005728. doi: 10.1371/journal.pgen.1005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Timothy D., Moyé Lem, Traverse Jay H. Consistently inconsistent—bone marrow mononuclear stem cell therapy following acute myocardial infarction - a decade later. Circ. Res. 2016;119:404–406. doi: 10.1161/CIRCRESAHA.116.309231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W.K., de Vos S., Elashoff D. Relation between resistance of Philadelphia-chromosome-positive acute lymphoblastic leukaemia to the tyrosine kinase inhibitor STI571 and gene-expression profiles: a gene-expression study. Lancet. 2002;359(9305):481–486. doi: 10.1016/S0140-6736(02)07678-X. [DOI] [PubMed] [Google Scholar]
- Ishige-Wada M., Kwon S.M., Eguchi M. Jagged-1 signaling in the bone marrow microenvironment promotes endothelial progenitor cell expansion and commitment of CD133 + human cord blood cells for postnatal Vasculogenesis. PLoS One. 2016;11(11):e0166660. doi: 10.1371/journal.pone.0166660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M. Building Predictive Models in R using the caret package. J. Stat. Softw. 2008;28(5):1–26. [Google Scholar]
- Kwon S.M., Suzuki T., Kawamoto A., Ii M., Eguchi M., Akimaru H., Wada M., Matsumoto T., Masuda H., Nakagawa Y., Nishimura H., Kawai K., Takaki S., Asahara T. Pivotal role of lnk adaptor protein in endothelial progenitor cell biology for vascular regeneration. Circ. Res. 2009;104(8):969–977. doi: 10.1161/CIRCRESAHA.108.192856. [DOI] [PubMed] [Google Scholar]
- Lee Jun Hee, Ji Seung Taek, Kim Jaeho. Specific disruption of Lnk in murine endothelial progenitor cells promotes dermal wound healing via enhanced vasculogenesis, activation of myofibroblasts, and suppression of inflammatory cell recruitment. Stem Cell Res Ther. 2016;7(1):158. doi: 10.1186/s13287-016-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaten L.V.D., Hinton G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008;9:2579–2605. http://jmlr.org/papers/v9/vandermaaten08a.html [Google Scholar]
- McPherson R., Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ. Res. 2016;118(4):564–578. doi: 10.1161/CIRCRESAHA.115.306566. Feb 19. [DOI] [PubMed] [Google Scholar]
- Nasseri B.A., Ebell W., Dandel M. Autologous CD133 + bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur. Heart J. 2014;35(19):1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- O'Brien R.G., Muller K.E., Edward L.K., editors. Applied Analysis of Variance in Behavioral Science. Marcel Dekker; New York: 1993. Signified power analysis for t-tests through multivariate hypotheses. [Google Scholar]
- Rosenberger William F., Lachin John M. Wiley publ; 2003. Randomization in Clinical Trials: Theory and Practice; pp. 1–14. [Google Scholar]
- Saeb A.T., Al-Naqeb D. The impact of evolutionary driving forces on human complex diseases: a population genetics approach. Scientifica (Cairo) 2016;2016 doi: 10.1155/2016/2079704. (Epub 2016 May 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm C., Westphal B., Kleine H.D. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361(9351):45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- Stamm C., Kleine H.D., Choi Y.H. Intramyocardial delivery of CD133 + bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J. Thorac. Cardiovasc. Surg. 2007;133(3):717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Strieter R.M., Kunkel S.L., Arenberg D.A., Burdick M.D., Polverini P.J. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem. Biophys. Res. Commun. 1995;210(1):51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- Takizawa H., Eto K., Yoshikawa A., Nakauchi H., Takatsu K., Takaki S. Growth and maturation of megakaryocytes is regulated by Lnk/Sh2b3 adaptor protein through crosstalk between cytokine- and integrin-mediated signals. Exp. Hematol. 2008 Jul;36(7):897–906. doi: 10.1016/j.exphem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Taylor D.A., Perin E.C., Willerson J.T. Cardiovascular cell therapy research network (CCTRN). Identification of bone marrow cell subpopulations associated with improved functional outcomes in patients with chronic left ventricular dysfunction: an embedded cohort evaluation of the FOCUS-CCTRN trial. Cell Transplant. 2016;25(9):1675–1687. doi: 10.3727/096368915X689901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Chen B., Xiao Y., Yang K., Zhou X., Zhou X. Intramyocardial autologous bone marrow cell transplantation for ischemic heart disease: a systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. 2014;233(2):485–492. doi: 10.1016/j.atherosclerosis.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Tse H.F., Kwong Y.L., Chan J.K., Lo G., Ho C.L., Lau C.P. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361(9351):47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- Vakil K., Florea V., Koene R. Effect of coronary artery bypass grafting on left ventricular ejection fraction in men eligible for implantable cardioverter-defibrillator. Am. J. Cardiol. 2016;117:957–960. doi: 10.1016/j.amjcard.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Werner N., Kosiol S., Schiegl T. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material