ABSTRACT
The germline stem cells (GSCs) are critical for gametogenesis throughout the adult life. Stem cell identity is maintained by local signals from a specialized microenvironment called the niche. However, it is unclear how systemic signals regulate stem cell activity in response to environmental cues. In our previous article, we reported that mating stimulates GSC proliferation in female Drosophila. The mating-induced GSC proliferation is mediated by ovarian ecdysteroids, whose biosynthesis is positively controlled by Sex peptide signaling. Here, we characterized the post-eclosion and post-mating expression pattern of the genes encoding the ecdysteroidogenic enzymes in the ovary. We further investigated the biosynthetic functions of the ovarian ecdysteroid in GSC maintenance in the mated females. We also briefly discuss the regulation of the ecdysteroidogenic enzyme-encoding genes and the subsequent ecdysteroid biosynthesis in the ovary of the adult Drosophila.
KEYWORDS: Drosophila, ecdysone, Halloween gene, mating, steroid hormone, sex peptide
Introduction
In many animals, sperm and egg production requires a robust stem cell system that balances self-renewal with differentiation.1 Germline stem cells (GSCs) produce progeny germ cells that differentiate into gametes and replicate themselves to maintain the generative cell population. The balance between self-renewal and differentiation of GSCs is important because perturbation of this balance causes germ cell depletion, infertility or tumorigenesis.1,2 GSCs are maintained by a specialized microenvironment called the niche.3 The niche provides local signals to maintain stem cell identity.4 Furthermore, GSC number is also controlled by systemic signals, including the insect steroid hormone ecdysteroids,5-11 which are also known as “molting hormones.” In the larval stage, ecdysteroids are biosynthesized from dietary cholesterol through several catalyzed steps in the specialized endocrine organ called the prothoracic gland. Recently, molecular studies have identified several ecdysteroidogenic enzymes such as Noppera-bo,12-14 Neverland,15-17 Non-molting glossy/Shroud,18 CYP307A1/Spook,19,20 CYP307A2/Spookier,19 CYP306A1/Phantom,21,22 CYP302A1/Disembodied,23-25 CYP315A1/Shadow,24 and CYP314A1/Shade26 (Fig. 1A). Molecular genetics has revealed that ecdysteroid signaling is indeed active in adult insects, and is involved in controlling multiple steps during adult oogenesis, including egg chamber development and vitellogenesis,27,28 follicle growth and survival,9,29 and stem cell niche formation.9 In addition, certain genes encoding the ecdysteroidogenic enzymes are required for egg development after stage 8,19,26 egg production,30 and border cell migration.31
Figure 1.
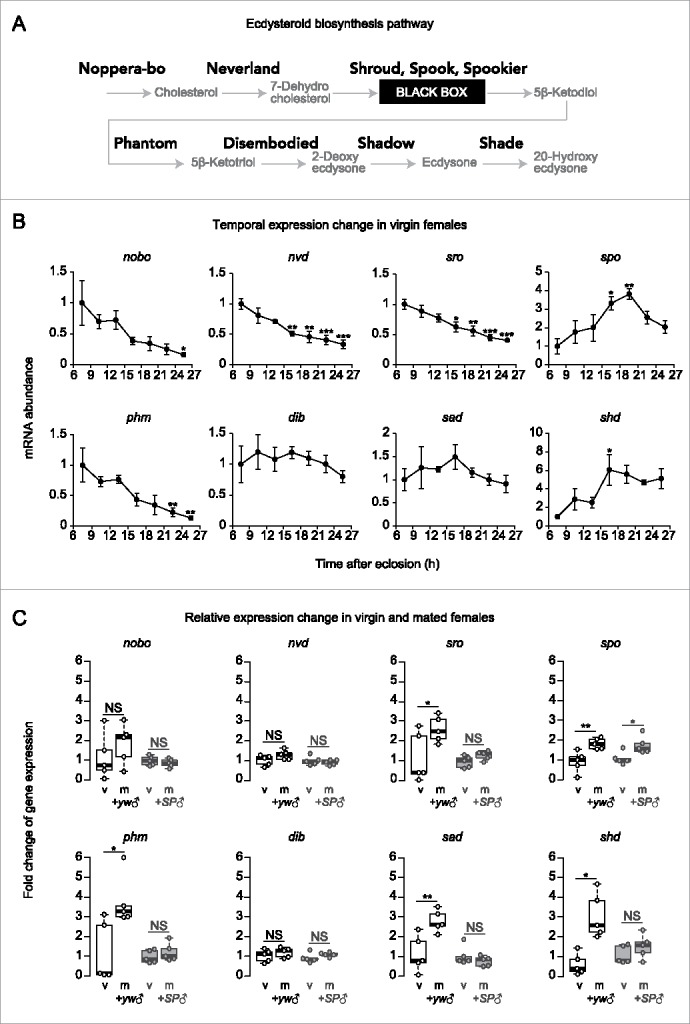
Transcriptional regulation of ecdysteroidogenic enzyme genes in the ovary. (A) The ecdysteroid biosynthesis pathway. Cholesterol is converted into 20-hydroxyecdysone (active form of ecdysone) by several ecdysteroidogenic enzymes (Shown in bold). (B) Temporal changes in ecdysteroidogenic enzyme genes in virgin female flies in post-eclosion period (n = 4). Most of the genes showed higher expression levels at 6 hours or 15–21 hours post-eclosion (nobo, nvd, sro, spo, phm, and shd). (C) Relative changes in ecdysteroidogenic enzyme gene expression in ovary. Ovaries were dissected from age-matched virgin and mated females at 16 hours post-mating. Some genes showed significant increase in mated female flies compared to virgin female flies (sro, spo, phm, sad, and shd). These increased expressions after mating were suppressed when female flies mated with SP null male flies (except for spo). Values are presented as the mean with standard error of the mean in B. For statistical analysis, t-test with Holm's correction was used for B, Student's t-test was used for C. ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05, NS, non-significant (P > 0.05).
It is well-documented that, for the regulation of molting and metamorphosis, the biosynthesis and signaling of ecdysteroids are coordinately modulated in response to various environmental cues such as nutrition, photoperiod, and temperature.32,33 Therefore, it is possible that the environmental cues are also reflected in egg production processes such as in the control of GSC number. However, the mechanism by which ecdysteroids regulate GSCs in response to environmental cues is unclear.
One of the major environmental cues that affect egg production is the mating stimulus. In the Drosophila female, mating induces dramatic changes in reproductive behavior such as increased egg laying and decreased mating behavior.34,35 The post-mating response is triggered by the male's Sex peptide (SP), which is present in the seminal fluid and transferred to the female during copulation.36-38 Because mating functions as a switch for reproductive activation, the demand for gametes increases during mating to generate more offspring. Therefore, it is possible that mating modulates GSC activity to activate gametogenesis and increase the supply of eggs. This is indeed the case, as our previous study has demonstrated that GSC number increases in response to mating.30 Moreover, we also found that the mating-induced GSC increase is mediated by ovarian ecdysteroids.30 In contrast, the underlying mechanisms that control ovarian ecdysteroid biosynthesis in virgin and mated females are still unknown.
In this Extra View, we extend our previous findings by characterizing the expression pattern of the ecdysteroidogenic enzyme-encoding genes in the ovary in the post-eclosed and post-mated periods. In addition, we show that ovarian ecdysteroid biosynthesis has a long-term effect on GSC maintenance in the mated female flies.
Results and discussion
Expression of ecdysteroidogenic enzyme-encoding genes in the ovary of virgin females
In our original paper, we have demonstrated that ovarian ecdysteroid biosynthesis is activated by mating stimuli, and the level of the ovarian ecdysteroid in the mated females is significantly higher than that in the virgin females.30 In addition, our data suggest that this activation, at least in part, results from transcriptional upregulation of ecdysteroidogenic enzyme-encoding genes.30 On the other hand, several previous studies have reported that the ovarian ecdysteroid is detected in virgin females.39,40 Specifically, Tu et al. describe the changes in ecdysteroid level in the wild type ovary 0–48 hours after eclosion without mating. Interestingly, a peak in the levels of the ovarian ecdysteroid is observed in virgin female flies approximately 18 hours after eclosion, which may be required for initiating oogenesis.40 This observation suggests that the expression of the genes encoding the ecdysteroidogenic enzymes fluctuate temporally even in virgin females. However, this scenario has not yet been tested.
We therefore investigated the transcription pattern of the ecdysteroidogenic enzyme-encoding genes, including noppera-bo (nobo), neverland (nvd), shroud (sro), spook (spo), phantom (phm), disembodied (dib), shadow (sad), and shade (shd) (Fig. 1A) in virgin females. The ovaries of the virgin females were dissected at 3-hour intervals within the first 6 to 27 hours post-eclosion. We observed that the expression levels of certain genes, namely, nobo, nvd, sro, and phm, gradually decreased after eclosion, while there was no significant change in the temporal expression of dib and sad (Fig. 1B). However, we observed a significant increase in the spo and shd mRNA abundance at 15–21 hours post-eclosion (Fig. 1B). Taken together, most of the genes were highly expressed until 18 hours after eclosion. We speculate that the expression of the ecdysteroidogenic enzyme-encoding genes might be a preparation to achieve the highest level of ovarian ecdysteroids at 18 hours post-eclosion.40 In contrast, most of the genes involved in biosynthesis showed lower expression levels 18–21 hours after eclosion and later, when the virgin females had lower ecdysteroid levels in the ovary.40 These results suggest that the ecdysteroidogenic enzyme-encoding gene expression is regulated not only in the mated females but also in the virgin females, implying that unknown tropic stimuli, other than the mating stimuli, may be involved in controlling the ovarian ecdysteroid biosynthesis in the post-eclosion period. However, it should be noted that the physiological relevance of the temporal change in individual ecdysteroidogenic enzyme-coding genes is unclear so far.
Sex peptide and its receptor up-regulate the expression of the ecdysteroidogenic enzyme-encoding genes differently
We have previously found that the mating-induced ecdysteroid biosynthesis is mediated by the male-derived SP and its receptor SPR, the components of a canonical neuronal pathway that induces a post-mating behavioral switch in females.30,37,41,42 Moreover, we have described that flies with a loss of SPR function exhibit significant reduction in nvd and phm expression.30 To further investigate how SP signaling affects the expression of the ecdysteroidogenic enzyme-encoding genes in the ovary, we examined their expression levels in wild-type female flies that were mated with the SP null mutant males (the ligand mutant).41 We found that the female flies that mated with the control males showed increased expression of certain ecdysteroidogenic enzyme-encoding genes, including sro, spo, phm, sad, and shd, compared to those in the virgin female flies (Fig. 1C). However, mating with the SP null mutant males did not induce any increase in transcript levels of most of the genes except for spo (Fig. 1C). These results suggest that mating up-regulates the transcription of the ecdysteroidogenic enzyme-encoding genes in the ovary via SP from the male's seminal fluid. In addition, it is noteworthy that SP appears to influence the expression of more ecdysteroidgenic enzyme-encoding genes than SPR.30 These results imply that SP might affect the expression of the ecdysteroidogenic enzyme-encoding genes in the ovary via both the SPR-dependent pathway and an unknown SPR-independent pathway.
Ovarian ecdysteroid biosynthesis and GSC maintenance
While our previous study has revealed an indispensable role of ecdysteroids in GSC proliferation within 24 hours after mating, other studies have demonstrated that the ovarian ecdysteroid signaling and its downstream cascade are essential for many aspects of oogenesis,9,27-29,43 particularly GSC maintenance,5-10 over a week and more after mating. Therefore, we examined the effect of ecdysteroid biosynthesis on oogenesis, including stem cell regulation, over a longer period after mating. We have previously reported that mating increases GSC number and this increase is maintained after 6 days from the first mating.30 To confirm the role of the ovarian ecdysteroid in GSC maintenance for over a week, we dissected ovaries from 2-week-old females at 1 week after mating (Fig. 2A).
Figure 2.

The role of ecdysteroid biosynthesis on the regulation of GSC maintenance. (A) Protocol for all experiments in this figure. One-week-old females were mated with males and used for the assay 1 week after mating. (B) Drosophila germarium. Germline stem cell (GSC) resides in a niche, comprising somatic cells called cap cells, terminal filament, and escort stem cells. GSCs are identifiable by their typical spectrosome morphology and their location (adjacent to the niche cells). GSC produces one self-renewing daughter and one cystoblast (CB) that differentiates into a germline cyst. The cystoblast divides four times with incomplete cytokinesis (2 cc: 2-cell cyst, 4 cc: 4-cell cyst, 8cc: 8-cell cyst and 16 cc: 16-cell cyst). (C) Drosophila ovary is composed of 15–20 ovarioles. The continuous developing egg chamber is divided into 14 stages. (D) Left: Frequencies of germaria containing zero, one, two and three GSCs (left y-axis), and average number of GSCs per germarium (right y-axis) in mated females. Ovarian neverland (nvd) knockdown in ovarian somatic cells (escort cells and follicle cells, using c587-GAL4) reduced average GSC number as compared to the control (P = 0.006145). Right: Temporal change in GSC number in virgin females (1-day-old and 1-week-old) and mated females (2-week-old), (n ≥ 94). (E and F) The average number of germline cyst (E) and egg chamber in each stage (F) was not changed in ovarian nvd RNAi female flies (c587>nvd RNAi). (G) UAS-nvd-Bm [wt] and UAS-nvd-Bm [H190A] were used for overexpressing the wild-type form and enzymatic inactive form of Bombyx mori nvd transgenes, respectively. Ovarian ecdysteroid decreased in c587>nvd RNAi female flies as compared to the control flies (P = 0.0233). This reduction was restored by overexpressing UAS-nvd-Bm [wt] but not UAS-nvd-Bm [H190A]. (H) GSC phenotype in c587>nvd RNAi animals was restored by overexpressing UAS-nvd-Bm [wt] but not UAS-nvd-Bm [H190A]. (I) GSC phenotype in c587>nvd RNAi flies was rescued by oral administration of 20E or 7dC. Values are presented as the mean with standard error of the mean in G. The numbers of samples examined are indicated in parentheses in D, E, F, H and I. For statistical analysis, Wilcoxon rank sum test was used for D, E and F. t-test with Holm's correction was used for G. Steel-Dwass test was used for H and I. ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05, NS, non-significant (P > 0.05).
To generate the ovary in which ecdysteroid biosynthesis is impaired, we knocked down nvd, which encodes the ecdysteroidogenic enzyme responsible for catalyzing the first step of the ecdysteroid biosynthesis pathway,16,17 by transgenic RNA interference (RNAi) with the c587-GAL4 driver. While the c587-GAL4 driver is known to be active in adult ovarian somatic cells, including escort cells and follicle cells, but not in nurse cells, we have previously found that the c587-GAL4-driven nvd RNAi (c587>nvd RNAi) efficiently leads to a significant reduction of NVD protein levels in both follicle and nurse cells for unknown reasons.30 In addition, we have reported that c587>nvd RNAi leads to reduction in ovarian ecdysteroid levels compared to those in control animals.30
In the experimental flies that underwent the mating protocol shown in Figure 2A, we found that the c587>nvd RNAi female flies had significantly less GSCs (1.90 GSCs on average) compared to that in the control female flies (2.13 GSCs on average) (Fig. 2D, left). To eliminate the possibility of developmental defects in oogenesis caused by this genotype (c587-GAL4 is already active in somatic cells at the larval stage),44 we confirmed that the number of GSCs in the female flies were not affected by the c587>nvd RNAi condition 1 day after eclosion compared to those in the control flies (Fig. 2D, right). Second, there were no differences in GSC number between the c587>nvd RNAi and control pre-mating flies, which were at 1 week after eclosion (Fig. 2D, right). These results suggest that c587>nvd RNAi does not affect GSC establishment during either pre-adult oogenesis or pre-mating ovarian maturation. In other words, our data strongly support our hypothesis that ovarian ecdysteroid biosynthesis in the adult stage is required for GSC maintenance in the mated females. We also tested whether ecdysteroid biosynthesis affects the process of germ cell differentiation in the germarium. However, we did not observe any changes in the number of germ cells in the cystoblast, 2-cell cyst, 4-cell cyst, 8-cell cyst, and 16-cell cyst (Fig. 2B) in the c587>nvd RNAi female flies (Fig. 2E). In addition, the number of egg chambers in each stage (Fig. 2C) was not affected by the downregulation of nvd in the ovary (Fig. 2F). Taken together, ovarian ecdysteroid biosynthesis controls the number of GSC, but not the number of differentiating germ cells and stage of the egg chamber.
We next examined whether the GSC maintenance phenotype in the c587>nvd RNAi is caused by a reduction in ovarian ecdysteroid levels. We measured the ovarian ecdysteroid levels in the c587>nvd RNAi female flies. We found that knocking down of nvd resulted in reduced ovarian ecdysteroid levels compared to that in the control female flies (Fig. 2G). To confirm whether this reduction is caused by a decrease of NVD enzymatic activity, we performed a transgenic rescue experiment in the c587>nvd RNAi background. As expected, the levels of the ovarian ecdysteroid were restored upon overexpression of the wild-type nvd ortholog of the silkworm Bombyx mori (nvd-Bm[wt]), but not its enzymatically dead form (nvd-Bm[H109A]) (Fig. 2G), suggesting that the reduction in ecdysteroid level is not caused by any off-target effects of the transgenic RNAi. Consistent with this data, the GSC phenotype in the c587>nvd RNAi flies was also rescued by co-expression of the wild-type nvd-Bm, but not the enzyme-dead form (Fig. 2H). To further investigate the role of ecdysteroid on the regulation of GSC maintenance, we performed a feeding rescue experiment using 7-dehydrocholesterol (7dC), the downstream metabolite generated by NVD. We found that the c587>nvd RNAi females fed with 7dC did not show a significant decrease in GSC number than the control female flies. (Fig. 2I). In addition, the GSC number in c587>nvd RNAi flies was rescued by the oral administration of 20-hydroxyecdysone (20E), the biologically active ecdysteroid. These data suggest that ovarian ecdysteroid biosynthesis plays an important role in controlling GSC proliferation and long-term GSC maintenance in the mated female flies (Fig. 3).
Figure 3.
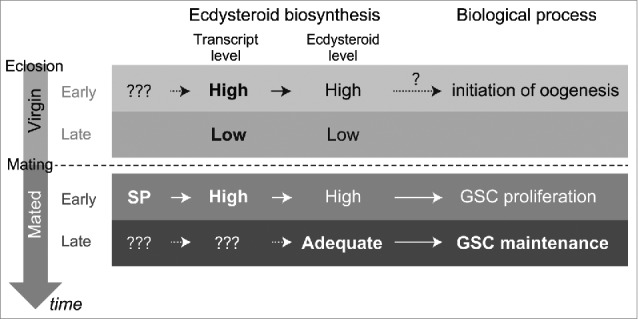
Model for this study. Ecdysteroid biosynthesis in the ovary is differentially regulated in different adult life stages, including the post-eclosion and pre-mated stage (upper column), the post-mating early stage, and the post-mating late stage (lower column). In post-eclosion stage, ecdysteroidogenic enzyme gene expression is regulated by unknown tropic stimuli and may be involved in controlling the ovarian ecdysteroid biosynthesis to initiate oogenesis. In post-mated early stage, SP stimulates ecdysteroid biosynthesis via upregulation of biosynthesis enzyme gene expression, which control GSC proliferation. Ovarian ecdysteroid biosynthesis is also required for GSC maintenance in post-mated late stages.
Outlook
In conjunction with our previous study,30 our data suggest that ecdysteroid biosynthesis in the ovary is differentially regulated in the different life-stages of the female adult fly, including the post-eclosion and pre-mating stage, the post-mating early stage, and the post-mating late stage (Fig. 3). In every stage, ecdysteroid biosynthesis plays essential roles in controlling oogenesis, especially GSC proliferation and/or maintenance. We have confirmed that ecdysteroid-dependent GSC proliferation in the post-mating stage is controlled by the SP-SPR signaling pathway, which stimulates the ovarian ecdysteroid biosynthesis via regulation of the expression of the ecdysteroidogenic enzyme-encoding genes. In contrast, the identity of the genes and signaling pathways that influence the expression of the enzyme-encoding genes and the subsequent ecdysteroid biosynthesis in the ovary are unclear. Moreover, the cause of the fluctuation in ovarian ecdysteroid biosynthesis during the female adult lifespan is not yet clear. This is in contrast to the fluctuation of ecdysteroid titer that is observed during the embryonic, larval, and pupal development.32,33,45 It should be remembered that studies on the role of steroid hormone biosynthesis in sexual maturation and gametogenesis in the postnatal stage of mammals has received more attention.46 In this sense, further studies on ovarian ecdysteroid biosynthesis in Drosophila and other insects would be intriguing to comprehensively understand the roles of steroid hormone biosynthesis across the animal phyla in the future.
Materials and methods
The flies were raised on cornmeal-agar-yeast media at 25°C. yw was used as the control strain. SP0 and SPΔ (41) were gifts from Nobuaki Tanaka (Hokkaido University, Japan). c587-GAL447,48 was a gift from Hiroko Sano (Kurume University, Japan). Other strains used were UAS-nvd-IR, UAS-nvd-Bm [wt], UAS-nvd-Bm [H190A] (16). Staining of GSCs with the 1B1 antibody,49 quantitative reverse transcription-polymerase chain reaction, and ecdysteroid measurements were performed as previously described.30
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Reiko Kise, Maki Kashikawa-Yoshida, and Yuko Shimada-Niwa for their technical support and Katsuo Furukubo-Tokunaga for allowing us to use his microscope. We are also grateful to Toshiro Aigaki, Nobuaki Tanaka, Hiroko Sano, and the Developmental Studies Hybridoma Bank for stocks and reagents.
Funding
TA is a recipient of the research fellowship for young scientists from the Japan Society for the Promotion of Science. This work was supported by a grant to TA from the JSPS KAKENHI grant number 15J00652 and by grants to RN from the MEXT KAKENHI grant number 23116701 (on Innovative Areas ‘Regulatory Mechanism of Gamete Stem Cells’) and 16H04792 as well as by the JST/PRESTO.
References
- [1].Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harb Perspect Biol 2011; 3:a002642; PMID:21791699; https://doi.org/ 10.1101/cshperspect.a002642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001; 414:98-104; PMID:11689954; https://doi.org/ 10.1038/35102160 [DOI] [PubMed] [Google Scholar]
- [3].Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132:598-611; PMID:18295578; https://doi.org/ 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 1998; 94:251-60; PMID:9695953; https://doi.org/ 10.1016/S0092-8674(00)81424-5 [DOI] [PubMed] [Google Scholar]
- [5].Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 2010; 7:581-92; PMID:21040900; https://doi.org/ 10.1016/j.stem.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].König A, Yatsenko AS, Weiss M, Shcherbata HR. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J 2011; 30:1549-62; PMID:21423150; https://doi.org/ 10.1038/emboj.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morris LX, Spradling AC. Steroid signaling within Drosophila ovarian epithelial cells sex-specifically modulates early germ cell development and meiotic entry. PLoS One 2012; 7:e46109; PMID:23056242; https://doi.org/ 10.1371/journal.pone.0046109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].König A, Shcherbata HR. Soma influences GSC progeny differentiation via the cell adhesion-mediated steroid-let-7-Wingless signaling cascade that regulates chromatin dynamics. Biol Open 2015; 4:285-300; PMID:25661868; https://doi.org/ 10.1242/bio.201410553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ables ET, Bois KE, Garcia CA, Drummond-Barbosa D. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev Biol 2015; 400:33-42; PMID:25624267; https://doi.org/ 10.1016/j.ydbio.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ables ET, Hwang GH, Finger DS, Hinnant TD, Drummond-Barbosa D. A genetic mosaic screen reveals ecdysone-responsive genes regulating Drosophila Oogenesis. G3 (Bethesda) 2016; 6:2629-42; PMID:27226164; https://doi.org/ 10.1534/g3.116.028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Uryu O, Ameku T, Niwa R. Recent progress in understanding the role of ecdysteroids in adult insects: Germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool Lett 2015; 1:32; PMID:26605077; https://doi.org/ 10.1186/s40851-015-0031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Enya S, Ameku T, Igarashi F, Iga M, Kataoka H, Shinoda T, Niwa R. A Halloween gene noppera-bo encodes a glutathione S-transferase essential for ecdysteroid biosynthesis via regulating the behaviour of cholesterol in Drosophila. Sci Rep 2014; 4:6586; PMID:25300303; https://doi.org/ 10.1038/srep06586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chanut-Delalande H, Hashimoto Y, Pelissier-Monier A, Spokony R, Dib A, Kondo T, Bohère J, Niimi K, Latapie Y, Inagaki S, et al.. Pri peptides are mediators of ecdysone for the temporal control of development. Nat Cell Biol 2014; 16:1035-44; PMID:25300303; https://doi.org/ 10.1038/srep06586 [DOI] [PubMed] [Google Scholar]
- [14].Niwa R, Niwa YS. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci Biotechnol Biochem 2014; 78:1283-92; PMID:25130728; https://doi.org/ 10.1080/09168451.2014.942250 [DOI] [PubMed] [Google Scholar]
- [15].Lang M, Murat S, Clark AG, Gouppil G, Blais C, Matzkin LM, Guittard É, Yoshiyama-Yanagawa T, Kataoka H, Niwa R, et al.. Mutations in the neverland Gene Turned Drosophila pachea into an Obligate specialist species. Science 2012; 337:1658-61; PMID:23347514; https://doi.org/ 10.1016/B978-0-12-385979-2.00001-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yoshiyama-Yanagawa T, Enya S, Shimada-Niwa Y, Yaguchi S, Haramoto Y, Matsuya T, Shiomi K, Sasakura Y, Takahashi S, Asashima M, et al.. The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J Biol Chem 2011; 286:25756-62; PMID:21632547; https://doi.org/ 10.1074/jbc.M111.244384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yoshiyama T, Namiki T, Mita K, Kataoka H, Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development 2006; 133:2565-74; PMID:16763204; https://doi.org/ 10.1242/dev.02428 [DOI] [PubMed] [Google Scholar]
- [18].Niwa R, Namiki T, Ito K, Shimada-Niwa Y, Kiuchi M, Kawaoka S, Kayukawa T, Banno Y, Fujimoto Y, Shigenobu S, et al.. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the “Black Box” of the ecdysteroid biosynthesis pathway. Development 2010; 137:1991-9; PMID:20501590; https://doi.org/ 10.1242/dev.045641 [DOI] [PubMed] [Google Scholar]
- [19].Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, Jarcho M, Warren JT, Marqués G, Shimell MJ, et al.. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol 2006; 298:555-70; PMID:16949568; https://doi.org/ 10.1016/j.ydbio.2006.07.023 [DOI] [PubMed] [Google Scholar]
- [20].Namiki T, Niwa R, Sakudoh T, Shirai KI, Takeuchi H, Kataoka H. Cytochrome P450 CYP307A1/Spook: A regulator for ecdysone synthesis in insects. Biochem Biophys Res Commun 2005; 337:367-74; PMID:16188237; https://doi.org/ 10.1016/j.bbrc.2005.09.043 [DOI] [PubMed] [Google Scholar]
- [21].Niwa R, Matsuda T, Yoshiyama T, Namiki T, Mita K, Fujimoto Y, Kataoka H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem 2004; 279:35942-9; PMID:15197185; https://doi.org/ 10.1074/jbc.M404514200 [DOI] [PubMed] [Google Scholar]
- [22].Warren JT, Petryk A, Marqués G, Parvy J-P, Shinoda T, Itoyama K, Kobayashi J, Jarcho M, Li Y, O'Connor MB, et al.. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol 2004; 34:991-1010; PMID:15350618; https://doi.org/ 10.1016/j.ibmb.2004.06.009 [DOI] [PubMed] [Google Scholar]
- [23].Niwa R, Sakudoh T, Namiki T, Saida K, Fujimoto Y, Kataoka H. The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by prothoracicotropic hormone. Insect Mol Biol 2005; 14:563-71; PMID:16164612; https://doi.org/ 10.1111/j.1365-2583.2005.00587.x [DOI] [PubMed] [Google Scholar]
- [24].Warren JT, Petryk A, Marque G, Jarcho M, Parvy J-P, Dauphin-villemant C, Connor MBO, Gilbert LI. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A 2002; 99:11043-8; PMID:12177427; https://doi.org/ 10.1073/pnas.162375799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chávez VM, Marqués G, Delbecque JP, Kobayashi K, Hollingsworth M, Burr J, Natzle JE, O'Connor MB. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 2000; 127:4115-26; PMID:10976044 [DOI] [PubMed] [Google Scholar]
- [26].Petryk A, Warren JT, Marqués G, Jarcho MP, Gilbert LI, Kahler J, Parvy JP, Li Y, Dauphin-Villemant C, O'Connor MB. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A 2003; 100:13773-8; PMID:14610274; https://doi.org/ 10.1073/pnas.2336088100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics 2000; 154:1203-11; PMID:10757764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 1999; 126:4581-9; PMID:10498692 [DOI] [PubMed] [Google Scholar]
- [29].Romani P, Bernardi F, Hackney J, Dobens L, Gargiulo G, Cavaliere V. Cell survival and polarity of Drosophila follicle cells require the activity of ecdysone receptor B1 isoform. Genetics 2009; 181:165-75; PMID:19015542; https://doi.org/ 10.1534/genetics.108.096008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ameku T, Niwa R. Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet 2016; 12:e1006123; PMID:27310920; https://doi.org/ 10.1371/journal.pgen.1006123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Domanitskaya E, Anllo L, Schüpbach T. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev Biol 2014; 386:408-18; PMID:24373956; https://doi.org/ 10.1016/j.ydbio.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Niwa YS, Niwa R. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster. Genes Genet Syst 2014; 89:27-34; PMID:24817759; https://doi.org/ 10.1266/ggs.89.27 [DOI] [PubMed] [Google Scholar]
- [33].Yamanaka N, Rewitz KF, O'Connor MB. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol 2013; 58:497-516; PMID:23072462; https://doi.org/ 10.1146/annurev-ento-120811-153608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wolfner MF. Battle and ballet: molecular interactions between the sexes in Drosophila. J Hered 2009; 100:399-410; PMID:19349638; https://doi.org/ 10.1093/jhered/esp013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carmel I, Tram U, Heifetz Y. Mating induces developmental changes in the insect female reproductive tract. Curr Opin Insect Sci 2016; 13:106-13; PMID:27436559; https://doi.org/ 10.1016/j.cois.2016.03.002 [DOI] [PubMed] [Google Scholar]
- [36].Apger-McGlaughon J, Wolfner MF. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J Insect Physiol 2013; 59:1024-30; PMID:23891750; https://doi.org/ 10.1016/j.jinsphys.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci 2003; 60:1689-704; PMID:14504657; https://doi.org/ 10.1007/s00018-003-3052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A 2003; 100:9923-8; PMID:12893873; https://doi.org/ 10.1073/pnas.1631635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Harshman LG, Loeb AM, Johnson BA. Ecdysteroid titers in mated and unmated Drosophila melanogaster females. J Insect Physiol 1999; 45:571-7; PMID:12770342; https://doi.org/ 10.1016/S0022-1910(99)00038-4 [DOI] [PubMed] [Google Scholar]
- [40].Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell 2002; 1:158-60; PMID:12882346; https://doi.org/ 10.1046/j.1474-9728.2002.00016.x [DOI] [PubMed] [Google Scholar]
- [41].Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A 2003; 100:9929-33; PMID:12897240; https://doi.org/ 10.1073/pnas.1631700100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008; 451:33-7; PMID:18066048; https://doi.org/ 10.1038/nature06483 [DOI] [PubMed] [Google Scholar]
- [43].Belles X, Piulachs MD. Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation. Biochim Biophys Acta - Gene Regul Mech 2014; 1849:181-6; PMID:24939835; https://doi.org/ 10.1016/j.bbagrm.2014.05.025 [DOI] [PubMed] [Google Scholar]
- [44].Gilboa L, Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature 2006; 443:97-100; PMID:16936717; https://doi.org/ 10.1038/nature05068 [DOI] [PubMed] [Google Scholar]
- [45].Rewitz KF, Yamanaka N, O'Connor MB. Developmental checkpoints and feedback circuits time insect maturation. Curr Top Dev Biol 2013; 103:1-33; PMID:23347514; https://doi.org/ 10.1016/B978-0-12-385979-2.00001-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morohashi K, Baba T, Tanaka M. Steroid hormones and the development of reproductive organs. Sex Dev 2013; 7:61-79; PMID:22986257; https://doi.org/ 10.1159/000342272 [DOI] [PubMed] [Google Scholar]
- [47].Zhu CH, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development 2003; 130:2579-88; PMID:12736203; https://doi.org/ 10.1242/dev.00499 [DOI] [PubMed] [Google Scholar]
- [48].Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 2004; 428:564-9; PMID:15024390; https://doi.org/ 10.1038/nature02436 [DOI] [PubMed] [Google Scholar]
- [49].Zaccai M, Lipshitz HD. Differential distributions of two adducin-like protein isoforms in the Drosophila ovary and early embryo. Zygote 1996; 4:159-66; PMID:8913030 [DOI] [PubMed] [Google Scholar]


