ABSTRACT
The exoskeleton of insects and other arthropods is a very versatile material that is characterized by a complex multilayer structure. In Sobala and Adler (2016) we analyzed the process of wing cuticle deposition by RNAseq and electron microscopy. In this extra view we discuss the unique aspects of the envelope the first and most outermost layer and the gene expression program seen at the end of cuticle deposition. We discussed the role of undulae in the deposition of cuticle and how the hydrophobicity of wing cuticle arises.
KEYWORDS: Drosophila, wing, cuticle, morpogenesis, gene expression
Introduction
The cuticular exoskeleton of insects is a remarkable bio-material that1,2 displays great variation in physical properties from one body region to another. Three factors influence this. One is that the structure of the cuticle is variable with differences in the thickness of the 3 major cuticle layers and in the number of sublayers. The second is local modifications that strongly influence region behavior. For example, wing vein cuticle is thicker than general wing blade cuticle and the veins serve as stiff structural supports that influence the properties of the wing as a whole.2,3 The third is that different sets of cuticle proteins and degrees of sclerotization are found in different cuticles.
The 3 major cuticle layers (Fig. 1) are the envelope, which is synthesized first and is external.4 The epicuticle, which is synthesized after the envelope and is located between the envelope and the procuticle. The protcuticle is the last to be synthesized and is juxtaposed to the apical surface of the epidermal cells. The procuticle is generally the thickest layer and it is composed of a series of sublayers formed by arrays of chitin fibers and proteins.5 One complication of studying the molecular basis for cuticle formation is that other tissues and cell types such as muscle become closely juxtaposed to the cuticle during the process of deposition. We took advantage of the Drosophila pupal wing, which can be dissected in a rather pure form without attached other tissues to identify the pattern of gene expression associated with the deposition of wing cuticle. This study identified candidate genes for mediating the deposition of each of the major cuticle layers.5
Figure 1.
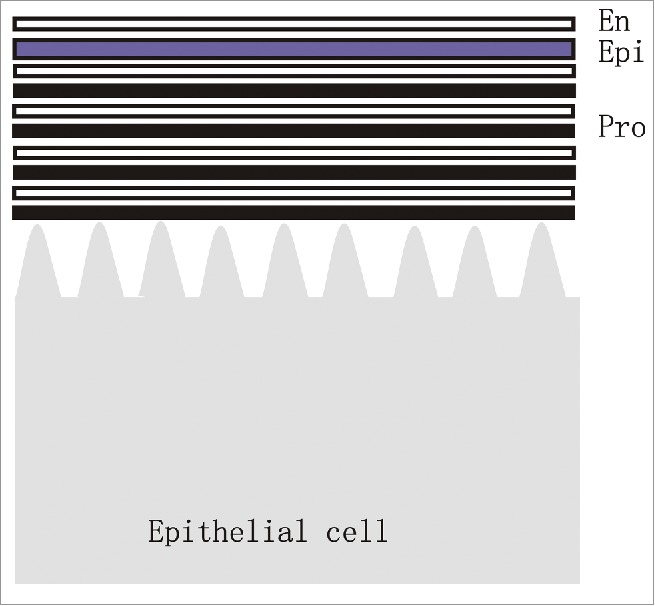
A diagram of cuticle structure. The outermost layer is the envelope (En) followed by the epicuticle (Epi). The multilayered procuticle (Pro) is juxtaposed to the epithelial cells.
The cuticular exoskeleton provides multiple functions for the animal including overall body shape, isolating the internal animal from the environment and the skeletal elements needed for locomotion.1,3 The envelope is lipid rich and the lipids are generally believed to function to minimize water loss and to boost the hydrophobicity of the cuticle.2,4,6,7 Since the envelope is the first layer one might expect that the pupal wing would become hydrophobic early in the process of cuticle deposition but that is not the case. Thus, if the waxy coat is responsible or important for the hydrophobicity than the lipids that comprise it will have to be transported across the cuticle. A likely explanation for this is that the pore canals that connect the epidermal cells to the surface4,8 are used to transport lipids to the surface late in cuticle deposition (Fig. 2). In the fly wing the pore canals ends are found in the center of cellular projections that remain in the adult wing after all of the epidermal cells have died (Fig. 2C). The role of the pore canals in lipid transport has usually been studied with respect to cuticular hydrocarbons that are used as pheromones or other signaling molecules but it also is likely for the general lipid coating. The lipids that are transported to the cuticular surface are thought to be synthesized in oenocytes and transported in the hemolymph to the epidermal cells.6,7,9 This model suggests that genes involved in lipid biosynthesis would not need to be expressed at a high level in epidermal cells before the time when the cuticle becomes hydrophobic. Our RNAseq analysis of wing cuticle deposition provides a test of this hypothesis.
Figure 2.
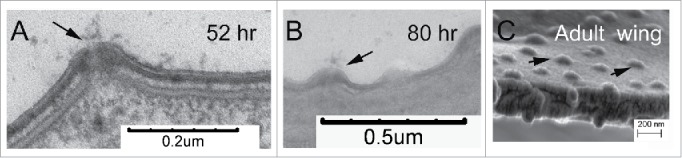
Pore canals connect the epithelial cells to the envelope and the outside. A. A pore canal in a 52 hr pupal wing during the deposition of the epicuticle. The arrow points to material that appears to be secreted from the pore. B. A pore canal in a relatively mature wing (80 hr). The arrow points to the pore canal. C. The remnant of a pore canal is seen as a bump on a fractured adult wing visualized by SEM. The arrows point to several such bumps. Since the epithelial cells have died back these pore canals are no longer functioning.
The deposition of the cuticle seems likely to be the principal activity of the wing epidermal cells synthesising it. These cells are not growing and indeed they undergo programmed cell death soon after eclosion of the adult.10,11 Our RNAseq data set also provides a test of these hypotheses. In this Extra View we review our RNAseq data set with regard to these predictions.5
Gene expression during the synthesis of the envelope
The 2 temporal transitions during the synthesis of wing cuticle that had the greatest changes in gene expression were also the 2 time points with the most dramatic developmental changes. These were the transitions from 42 to 52 hr after white prepupae (awp), which coincided with the expansion and flattening of the wing and from 88 to 96 hr awp when the wings became highly hydrophobic, more highly pigmented and the thickening of the cuticle was no longer obvious.
In transmission electron micrographs the first sign of wing envelope deposition is seen around 35 h awp but it is not complete until around 48 hr awp. The first sign of envelope deposition is the accumulation of patches of diffuse extracellular material on projections (undulae) of the apical surface of the wing cells (Fig. 3).5 The diffuse material disappears and is replaced by patches of the trilaminar envelope that eventually covers the entire apical surface (Fig. 1, Fig. 3). The envelope retains this morphology until late in the pupal period when the trilaminar structure becomes somewhat less distinct. We examined RNA from 42 hr awp pupal wings during the middle of the period of envelope deposition and from 52 hr awp during the formation of the epicuticle. Dramatic changes in gene expression were seen between the 42 and 52 hr time points. Significant changes in gene expression were seen for 1624 genes, and 334 genes were identified where their expression level changed more than 10-fold between 42 and 52 h awp. There were 67 genes where greater than 90% of their total FPKM (fragment per kilobase per million reads) values were from the 42 hr sample. We call these genes “42 hr genes” and we consider them to be candidates for mediating the formation of the envelope. Of the 40 most highly expressed 42 hr genes 37 encode proteins predicted to contain either a signal sequence or a transmembrane domain (or both) placing at least part of these proteins extracellularly as expected for a protein that is either a component of the envelope or a protein that in some other way mediates the formation of the envelope. Two families of 42 hr proteins stood out. One was the family of ZP domain proteins.12-14 Mutations in several of these are known to disrupt the formation of wing cuticle.13,15 The ZP domain is known to mediate polymerization and ZP domain proteins are usually thought of as organizers /components of the apical extracellular matrix.12,14,16 The second family that stood out was the Osirus family, which comprises a set of genes that are only found in insects.17 Little is known about the function of this gene family. Genetic experiments established that 13 out of 16 42 hr genes tested showed a mutant cuticle phenotype.5,13,15 Among these were 4 members of the ZP domain family. Although none have yet been tested we think it likely that members of the Osirus gene family will also have an important role in envelope deposition.
Figure 3.
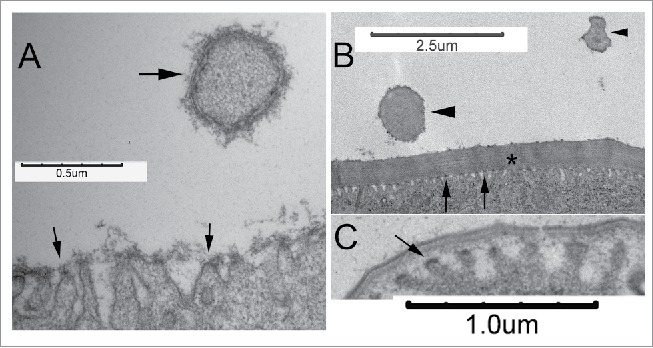
Undulae and the deposition of cuticle. A. In a 35 hr pupal wing undulae (arrows) are visible and a somewhat amporphous material accumulated above them. The large arrow points to a hair. The arrow also points to a region where trilaminar envelope is visible. Hair cuticle formation is somewhat advanced compared with the wing blade. Note that no undulae are visible. In a 72 hr wing the multilayered procuticle is obvious (asterisk) and many undulae are visible (arrows). A hair seen in a cross section in a proximal part of the hair is visible (large arrowhead). Note the lack of undulae. A cross section through the distal part of a hair is also seen (small arrowhead). Once again notice no undulae. C. Undulae (arrow) are prominent in 52 hr wing cells that are in the process of depositing the epicuticle.
Only 2 of the 40 most highly expressed genes in 42 hr wings encoded annotated cuticle proteins. These 2 genes represented less than 2.5% if the total FPKM value for the 42 hr time point. This suggests that the envelope cuticle is to a large extent composed of other types of proteins. In contrast in 62 hr pupal wings, during the deposition of the procuticle, 9 of the 40 most highly expressed genes including the first and third most highly expressed were annotated cuticle protein encoding genes. These 9 genes represented ∼26% of the total FPKM value for this time point. This is as expected as the procuticle is the thickest segment of the cuticle.
Gene expression during the last hours of pupal life
The second transition that showed the greatest changes in gene expression was between 88 and 96 hr awp. By the 88 hr time point the wing cuticle appeared largely complete by transmission electron microscopy. The 96 hr time point was just before the eclosion of the pupa. We found 1639 genes that showed a significant change in gene expression between 88 and 96 hr and 270 of these showed a 10-fold or greater change in expression. The 96 hr time point had the largest number of genes (87 genes) where >90% of their total FPKM was at that one time point. The 96 hr genes did not show the extreme bias in subcellular location that we saw in the 42 hr genes. Consistent with cuticle deposition being largely complete by this time only one of the 40 most highly expressed genes was an annotated cuticle gene (CG3402) and the expression of that gene peaked at 62 hr at a level more than 10 times higher than its 96 hr value. The FPKM value for CG3402 at 96 hr represented only about 0.5% of the total 96 hr FPKM value. There were no families of genes that were very highly represented and most the highly expressed genes did not contain a signal sequence or transmembrane domain suggesting that many of the 96 hr genes had internal cellular functions. Several notable features were detected among both 96 hr genes and genes that were primarily expressed at a high level in 96 hr wings even if they did not fit the strict >90% FPKM criteria. Most notable were several genes that based on mutant phenotype are known to play a role in pigmentation. Included among these are ebony (e), black (b), Punch (Pu), pale (ple) and dopa decarboxylase (Ddc).18 This makes sense as the darkening and sclerotization of the adult wing takes place both at the end of the pupal period and in the first few hours of adult life. There were also several genes involved in apoptosis and autophagy including hid, Dronc, Atg13, Drep4 and Drice.18 This also makes sense as the wing cells die soon after eclosion stimulated by Bursicon signaling.10,11
Chitin synthesis and undulae
During the synthesis of cuticle, epidermal cells contain cytoplasmic protrusions called undulae.19 The undulae are arranged as parallel units each of which extends over a long section of the apical surface of the cell. A popular model is that the undulae are sites of chitin deposition with cuticle proteins being secreted from the valleys between the undulae.19 We used transmission electron microscopy to assay the morphology of the epidermal cells and cuticle at various times during wing cuticle formation. Our observations support the idea that the undulae are sites for the secretion of cuticle contents but the results suggest at least a strict version of the popular model is incorrect. We observed undulae with closely associated amorphous material before the time when we could detect the trilaminar envelope (∼35 hr awp) (Fig. 3A). A few hours later we observed the amount of amorphous material was reduced and instead we detected trilaminar envelope directly over the undulae (∼42 h awp). It seems likely that the amorphous material was a precursor to the envelope but that remains to be determined. We also observed undulae closely juxtaposed to the cuticle during the deposition of the epicuticle (∼52 hr awp). However, in other experiments we failed to detect any chitin over the apical surface of wing cells until the deposition of the procuticle had started (∼62 hr awp).20 Thus, undulae are present and appear to be sites of deposition of cuticle material more than day before when chitin starts to be deposited. This suggests that undulae are sites of cuticular protein secretion. In contrast to the situation over the apical surface of wing cells we did not routinely see undulae on developing wing hairs (Fig. 3) even though they contain chitin. Thus, undulae are not likely to be essential for the deposition of chitin. We think the most likely explanation is that undulae are part of the general mechanism for the secretion of cuticle components over the apical surface of epidermal cells but that this mechanism is modified for the formation of cuticle over specialized cellular structures such as hairs.
The development of wing hydrophobicity
If one drops an anesthesized Drosophila into a dish of water it floats and indeed it does not wet. This is common among insects. This is also true for wings that have been removed from a Drosophila. During the dissection of pupal wings for both transmission electron microscopy and for isolating RNA for RNASeq we observed that 88 hr wings wet while 96 hr pupal wings (and the wings of freshly eclosed adults) did not. Thus, something about how this tissue interacts with water changed during this 8 hr period. Two factors have been implicated in producing highly hydrophobic surfaces. One is the microstructure of the surface.21-23 An array of projections has been found to be able to trap air and prevent the wetting of a surface. In the case of the wing this is the array of parallel cuticular hairs. In transmission electron microscopy the hairs are well formed in both 88 and 96 hr wings so a change in their shape is unlikely to be important for the change in hydrophobicity. The second factor found to be important is a hydrophobic waxy coat that covers the external surface of the cuticle.21 This coating is usually thought of as being important for both preventing desiccation and for pheromone signaling.7 The external surface of cuticle, the envelope is synthesized first but it remains accessible by epithelial cells as pore canals provide a channel for this. Indeed, in our transmission electron micrographs we often observed what appeared to be material being secreted from pore canals (Fig. 2). We suggest that the deposition of a lipid coating happens between 88 and 96 hr awp and this is essential for the strong hydrophobicity of the fly wing.
What is the source of the lipids?
A set of large cells (oenocytes) located below the epidermis is thought to be the source of the long chain alkanes, alkenes and branched alkanes that form the waxy coating that epidermal cells provide to the cuticular surface.6,9,24 The evidence for this is strong for hydrocarbons that are used for pheromone signaling but somewhat less so for the lipids that provide for hydrophobicity. In the case of the wing and a variety of other body regions the oenocytes are not located close to epidermal cells so long distance intercellular transport would need to play a major role in delivering the lipid. If the oenocytes provide the coating lipids then the wing epidermal cells would not need to express genes that encode enzymes that modify the structure of lipids although they would presumably need to express genes that are used for lipid transport. Since the change in hydrophobicity takes place in the short period between 88 and 96 hr we predict that if the epidermal cells express genes that encode lipid modifying/synthesising enzymes large increases in the expression of these genes would take place very late in pupal development. We assembled a list of 110 Drosophila genes that are either annotated as having a role in lipid metabolism/transport or that show sequence similarity to genes known to have such a role in other organisms. This list is likely incomplete but it serves as a useful starting point. Based on our RNASeq analysis at least 61 of these genes are expressed in pupal wings. Some of these genes show a decrease in expression late in pupal wing development. For example, CG30427, CG5065 and CG8306 all of which encode fatty-acyl-CoA reductases. Among these 61 genes were 15 that showed a dramatic increase in expression level late in pupal wing development (> 80 hr awp). These genes are good candidates for playing a role in forming the hydrophobic coating that likely plays a role in making the wing non-wetting. The cuticular lipids of several insects have been analyzed and they appear to be a complex mixture.6,7,24 Included are several waxes that contain long chain fatty acids. Interestingly, among the 12 candidate genes are 5 fatty acid elongases (CG33110, CG2781, baldspot, CG31523 and bond). The roles of these genes in the development of cuticular hydrophobicity will need to be directly tested in the future. Our data suggests that the wing epithelial cells are likely to modify fatty acids transported from the oenocytes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I acknowledge the key contributions of Lukasz Sobala who played a key and indispensable role in the original paper. He was unfortunately not available to participate in writing this Extra View. We thank FlyBase and the stock centers at Indiana University and the VDRC where we obtained many stocks used in the original paper. We also want to thank Anh Thu Nguyen in the University of Virginia Genomics Core Facility where the RNA-seq sequencing runs were performed and for her helpful suggestions and Stacey Guillot and Yalin Wang at the University of Virginia Advanced Microscopy facility where the electron microscopy was done.
Funding
This research was supported by a grant from the National Institutes of Health (GM037163), by funds from the Associate Provost for Research at the University of Virginia and by funds from the William R. Kenan Jr Professorship.
References
- [1].Moussian B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol 2010; 40(5):363-75; PMID:20347980; https://doi.org/ 10.1016/j.ibmb.2010.03.003 [DOI] [PubMed] [Google Scholar]
- [2].Vincent JF. Deconstructing the design of a biological material. J Theor Biol 2005; 236(1):73-8; PMID:15967184; https://doi.org/ 10.1016/j.jtbi.2005.02.017 [DOI] [PubMed] [Google Scholar]
- [3].Vincent JF, Wegst UG. Design and mechanical properties of insect cuticle. Arthropod Struct Dev 2004; 33(3):187-99; PMID:18089034; https://doi.org/ 10.1016/j.asd.2004.05.006 [DOI] [PubMed] [Google Scholar]
- [4].Locke M. The structure and formation of the cuticulin layer in the epicuticle of an insect, Calpodes ethlius (Lepidoptera, Hesperiidae). J Morphol 1966; 118(4):461-94; PMID:5956243; https://doi.org/ 10.1002/jmor.1051180403 [DOI] [PubMed] [Google Scholar]
- [5].Sobala LF, Adler PN. The Gene Expression Program for the Formation of Wing Cuticle in Drosophila. PLoS Genet 2016; 12(5):e1006100; PMID:27232182; https://doi.org/ 10.1371/journal.pgen.1006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wigglesworth VB. The source of lipids and polyphenols for the insect cuticle: The role of fat body, oenocytes and oenocytoids. Tissue Cell 1988; 20(6):919-32; PMID:18620248; https://doi.org/ 10.1016/0040-8166(88)90033-X [DOI] [PubMed] [Google Scholar]
- [7].Chung H, Carroll SB. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015; 37(7):822-30; PMID:25988392; https://doi.org/ 10.1002/bies.201500014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y. Cuticle formation and pigmentation in beetles. Curr Opin Insect Sci 2016; 17:1-9; PMID:27720067; https://doi.org/ 10.1016/j.cois.2016.05.004 [DOI] [PubMed] [Google Scholar]
- [9].Makki R, Cinnamon E, Gould AP. The development and functions of oenocytes. Annu Rev Entomol 2014; 59:405-25; PMID:24397521; https://doi.org/ 10.1146/annurev-ento-011613-162056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Natzle JE, Kiger JA Jr, Green MM. Bursicon signaling mutations separate the epithelial-mesenchymal transition from programmed cell death during Drosophila melanogaster wing maturation. Genetics 2008; 180(2):885-93; PMID:18780731; https://doi.org/ 10.1534/genetics.108.092908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peabody NC, Diao F, Luan H, Wang H, Dewey EM, Honegger HW, White BH. Bursicon functions within the Drosophila CNS to modulate wing expansion behavior, hormone secretion, and cell death. J Neurosci 2008; 28(53):14379-91; PMID:19118171; https://doi.org/ 10.1523/JNEUROSCI.2842-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jovine L, Darie CC, Litscher ES, Wassarman PM. Zona pellucida domain proteins. Annu Rev Biochem 2005; 74:83-114; PMID:15952882; https://doi.org/ 10.1146/annurev.biochem.74.082803.133039 [DOI] [PubMed] [Google Scholar]
- [13].Roch F, Alonso CR, Akam M. Drosophila miniature and dusky encode ZP proteins required for cytoskeletal reorganisation during wing morphogenesis. J Cell Sci 2003; 116(Pt 7):1199-207; PMID:12615963; https://doi.org/ 10.1242/jcs.00298 [DOI] [PubMed] [Google Scholar]
- [14].Fernandes I, Chanut-Delalande H, Ferrer P, Latapie Y, Waltzer L, Affolter M, Payre F, Plaza S. Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev Cell 2010; 18(1):64-76; PMID:20152178; https://doi.org/ 10.1016/j.devcel.2009.11.009 [DOI] [PubMed] [Google Scholar]
- [15].Adler PN, Sobala LF, Thom D, Nagaraj R. dusky-like is required to maintain the integrity and planar cell polarity of hairs during the development of the Drosophila wing. Dev Biol 2013; 379(1):76-91; PMID:23623898; https://doi.org/ 10.1016/j.ydbio.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol 2002; 4(6):457-61; PMID:12021773; https://doi.org/ 10.1038/ncb802 [DOI] [PubMed] [Google Scholar]
- [17].Shah N, Dorer DR, Moriyama EN, Christensen AC. Evolution of a large, conserved, and syntenic gene family in insects. G3 (Bethesda) 2012; 2(2):313-9; PMID:22384409; https://doi.org/ 10.1534/g3.111.001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dos Santos G, Schroeder AJ, Goodman JL, Strelets VB, Crosby MA, Thurmond J, Emmert DB, Gelbart WM, FlyBase C. FlyBase: introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic Acids Res 2015; 43(Database issue):D690-7; PMID:25398896; https://doi.org/ 10.1093/nar/gku1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moussian B, Seifarth C, Muller U, Berger J, Schwarz H. Cuticle differentiation during Drosophila embryogenesis. Arthropod Struct Dev 2006; 35(3):137-52; PMID:18089066; https://doi.org/ 10.1016/j.asd.2006.05.003 [DOI] [PubMed] [Google Scholar]
- [20].Sobala LF, Wang Y, Adler PN. ChtVis-Tomato, a genetic reporter for in vivo visualization of chitin deposition in Drosophila. Development 2015; 142:3974-81, in press; PMID:26395478; https://doi.org/ 10.1242/dev.126987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Watson GS, Cribb BW, Watson JA. The role of micro/nano channel structuring in repelling water on cuticle arrays of the lacewing. J Struct Biol 2010; 171(1):44-51; PMID:20347993; https://doi.org/ 10.1016/j.jsb.2010.03.008 [DOI] [PubMed] [Google Scholar]
- [22].Watson GS, Cribb BW, Watson JA. How micro/nanoarchitecture facilitates anti-wetting: an elegant hierarchical design on the termite wing. ACS Nano 2010; 4(1):129-36; PMID:20099910; https://doi.org/ 10.1021/nn900869b [DOI] [PubMed] [Google Scholar]
- [23].Goodwyn PP, De Souza E, Fujisaki K, Gorb S. Moulding technique demonstrates the contribution of surface geometry to the super-hydrophobic properties of the surface of a water strider. Acta Biomater 2008; 4(3):766-70; PMID:18296131; https://doi.org/ 10.1016/j.actbio.2008.01.002 [DOI] [PubMed] [Google Scholar]
- [24].Wicker-Thomas C, Garrido D, Bontonou G, Napal L, Mazuras N, Denis B, Rubin T, Parvy JP, Montagne J. Flexible origin of hydrocarbon/pheromone precursors in Drosophila melanogaster. J Lipid Res 2015; 56(11):2094-101; PMID:26353752; https://doi.org/ 10.1194/jlr.M060368 [DOI] [PMC free article] [PubMed] [Google Scholar]


