ABSTRACT
Diet profoundly influences the behavior of animals across many phyla. Despite this, most laboratories using model organisms, such as Drosophila, use multiple, different, commercial or custom-made media for rearing their animals. In addition to measuring growth, fecundity and longevity, we used several behavioral and physiological assays to determine if and how altering food media influence wild-type (Canton S) Drosophila melanogaster, at larval, pupal, and adult stages. Comparing 2 commonly used commercial food media we observed several key developmental and morphological differences. Third-instar larvae and pupae developmental timing, body weight and size, and even lifespan significantly differed between the 2 diets, and some of these differences persisted into adulthood. Diet was also found to produce significantly different thermal preference, locomotory capacity for geotaxis, feeding rates, and lower muscle response to hormonal stimulation. There were no differences, however, in adult thermal preferences, in the number or viability of eggs laid, or in olfactory learning and memory between the diets. We characterized the composition of the 2 diets and found particularly significant differences in cholesterol and (phospho)lipids between them. Notably, diacylglycerol (DAG) concentrations vary substantially between the 2 diets, and may contribute to key phenotypic differences, including lifespan. Overall, the data confirm that 2 different diets can profoundly influence the behavior, physiology, morphology and development of wild-type Drosophila, with greater behavioral and physiologic differences occurring during the larval stages.
KEYWORDS: bioactive peptides, diet, D. melanogaster, geotaxis, learning, locomotion, muscle, memory, morphology, thermal preference
Introduction
The fruit fly, Drosophila melanogaster, is a focal model organism for investigations of almost all aspects of organismal biology. Since the origins of Drosophila research, investigators have used varied media upon which to rear flies; the ability of flies to adapt and survive on a wide-variety of food sources has likely promoted Drosophila divergence and is at least one of their attractive features as a model organism. Since the beginning of the 20th century, a great number of investigations have sought to determine the optimum rearing conditions and media for maximizing the growth and development of Drosophila.1,2 Subsequently, it has become common practice to alter one or 2 constituents of commonly used media to ascertain their role in various physiological, developmental or behavioral paradigms.3-6 However, few investigations have directly addressed potential confounding effects of using different rearing media and what this might mean in terms of phenotypic differences.
Here we assessed 2 commercially available Drosophila diets to test for dietary derived differences in development, physiology, morphology or behavior. Numerous experimental approaches exist for the examination, differentiation and characterization of dietary effects. Timing of development to landmark stages such as instars, pupation and eclosion, is perhaps the simplest measure of effect.7 Measures of fitness, reproductive rates and longevity are also key indicators.8-12 It is also common to examine the effects of diet or other factors on internal and external structural development, and general morphology.1,13-15 A number of metrics can be used to examine morphological differences that persist across all stages of development; the 2 most heavily investigated stages of Drosophila development are the larval and adult forms. Most commonly, weight, length and width are used when examining larvae.16 In adults, the number of phenotypic interest points is substantially higher due to their use as correlates to genotypic markers (i.e. genetics / functional genomics). However, when examining changes in gross morphology, previous research has typically focused on body size, wing length, color, and weight.16-18
Behavioral assays have been developed to the point that some assessments can begin as early as the embryo (e.g. peristalsis19 ). Numerous assays have been designed to study larval behavior including gustation,20 olfaction,21 vision,22 foraging,23 locomotion,24-27 as well as thermotactic-28,29 and phototactic-behavior.30,31 For adults, assays include thermotatic behavior,32,33 geotaxis,34 learning and memory,35 social interactions,36 aggression,37 courtship,38 vision,39 acoustic activity,40 olfaction,41 taste,42 and others.43
Whereas many diet related studies tend to focus on only a single phenotypic criterion for evaluation, here we took a broader yet more integrative approach, making use of several well-established Drosophila research ‘tools’ to test the hypothesis that development, morphology, physiology, and/or behavior, at larval, pupal, and adult stages, differ depending upon the rearing media used. The breadth of analyses was chosen so as to capture as wide a range of potential phenotypic changes associated with diet as possible. We reared D. melanogaster Canton S, in parallel, on either of 2 commercial diets: Formula 4–24® media or Jazz mix ®. We examined the following: (i) developmental timing of larva, pupae and adults, (ii) weight and size of third-instar larvae and size of adults, (iii) effects of a stress hormone on third-instar body-wall muscle force production, and (iv) several larval and adult behaviors including thermal preference, response to a temperature extreme, velocity of larval crawling, locomotory response to gravity (negative geotaxis), and capacity for learning and memory in adults. We sought to initially relate observed differences and similarities to the composition of both diets by assessing the fat, carbohydrate, protein, ash, and moisture content of each medium. More detailed analyses revealed significant differences in phospholipid concentrations in the starting dietary material which translated to substantive differences in the larval and adult tissues. Our results confirm significant physical and behavioral phenotypic differences that occur as a consequence of using different dietary materials.
Materials and methods
Fly stocks
All flies used were D. melanogaster Canton S, obtained from the Bloomington Drosophila stock center (Bloomington, IN, USA). Flies were kept at constant temperature (21°C) and humidity (65–70%), under a 12:12 h light:dark cycle. Fly vials were randomized in the stock trays across all experiments and treatments. All flies we maintained on their respective dietary media for a minimum of 4 generations before testing. Flies were reared using either Formula 4–24 ® media (Carolina Biological) or Jazz mix ® (Fisher Scientific), in VWR ® polypropylene 25 mm × 95 mm Drosophila vials. The composition of these diets, listed by the manufacturer is as follows: Formula 4–24 – Oat flour, soy flour, wheat flour, other starches, dibasic calcium phosphate, calcium carbonate, citric acid, niocinamide, riboflavin, sodium chloride, sodium iron pyrophosphate, sucrose, thiamine, mononitrate, brewer's yeast, emulsifier preservatives, mold inhibitor, food coloring; Jazz-mix – sugar, maize meal, yeast, agar, benzoic acid, methyl paraben, propionic acid. The Formula 4–24 diet required separate application of yeast pellets (Saccharomyces cerevisiae) and saturation of this dry media mixture with water. The Jazz-mix did not require yeast pellets as yeast is contained within the media; however, each final vial of media required that the stock food be mixed with 10 mL of water, and boiled for 10 min. Dietary effects on rearing for the 2 groups were assessed contemporaneously under all conditions. Other than the rearing media, all other conditions were identical for all flies used. A control diet was used for comparison in some experiments. The control diet is the media used at Massachusetts Institute of Technology and is composed of: distilled water, agar, brewer's yeast, maize meal, granulated sugar, and methyl 4 hydroxybenzoate dissolved in ethyl alcohol.
Growth and development
To assess if either diet had an effect on the timing of landmark developmental stages (i.e., third-instar, and pupation), we placed 5 females 5 d post eclosion from each diet and placed them for 2 h on grape agar dishes with yeast paste added. 25 eggs were then transferred to vials containing fresh media from each of the diets. Timing to each of the developmental stages was then assessed in each of the 10 replicate vials.
In a separate experiment, we determined the longevity on each of the diets by putting 50 flies of each sex (the day of eclosion) reared on the separate diets into separate vials containing the same diet they were reared on (Jazz-mix or Formula 4–24). Each day we counted and removed any dead adults. We transferred the adults onto fresh media every 7 d. We repeated these procedures until all flies had died in each of the 8–10 replicate vials tested for each diet.
To determine if diet has an effect on the total number of eggs laid by females, 5 adult females 5 d post-eclosion were obtained from each of the respective rearing media and transferred onto a grape-agar plate with fresh yeast paste added. The females were kept at 25 °Celsius for 3 d in an incubator. Each day at the 24 h point, fresh grape-agar plates with yeast paste were exchanged for the day old plates, and the total number of eggs laid was counted. This was repeated 10 times for each of the 3 diets. We transferred 100 eggs from each of plates to a fresh grape-agar plate with yeast paste kept at 25 °Celsius and subsequently counted the number of eggs that progressed to first-instar larvae over 5-days. This was repeated 10 times for each of the 3 diets. Next, one virgin male and one virgin female were transferred to a fresh vial of food from each of the diets. After 4 d, the 2 adults were transferred to fresh grape-agar with yeast paste for 24 h and kept at 25 °Celsius. 50 eggs were isolated the following day, transferred to a fresh agar plate, and the total number of eggs that progressed to first-instar larvae was assessed. This procedure was repeated 7 times for each of the diets.
To examine if either of the commercial rearing media affected the morphology of Drosophila, the weight and size of wandering third-instar larvae and the size of adults reared on each of the diets were measured. To assess the ‘wet’ larval weight we pooled 10 randomly selected larvae from each diet and weighed them collectively on a Mettler Toledo mx5 scale; these larvae were then kept in a 60°C oven overnight, and the resulting ‘dry’ weight measured. This procedure was repeated for 10 sets (i.e., separate stock vials) of larvae on each diet. To examine if these diets affected body size, the length and width of third-instar larvae (N = 40 for length, N = 50 for width) reared on either diet were measured. As the length of third-instar larvae can vary depending on the contractile state of the bodywall muscles, the larvae were pinned at one end and stretched during size measurements. For metrics of adult Drosophila, it has been previously established that wing-length is strongly correlated with adult size; to test for an effect of diet, the length of the 4th longitudinal vein was measured.17
Muscle contractions
To determine if changes in size as a consequence of differential dietary rearing had physiologic implications, we measured changes in octopamine-induced muscle contractions in larvae reared on both diets. Octopamine is a neurotransmitter and neuromodulator in invertebrates (Roeder, 2005) and is present in the Drosophila nervous system.44 Octopamine induces slow contractions (lasting up to 5 min) in Drosophila larval body wall muscles which are easily detected as an increase in muscle tonus.45 Detailed descriptions of the larval third-instar dissection and HL6 composition are reported elsewhere.46 Briefly, wandering third-instar larvae were pinned longitudinally on a magnetic dissection dish. A superficial incision was made along the dorsal mid-line, the larva eviscerated and the CNS removed.47 After the dissection, the anterior pin was removed, and the larva was hooked to a Grass FT03 tension transducer (Grass Instruments, Quincy, MA) so that contractions of the longitudinal muscles were parallel to the movement of the force transducer spring. The longitudinal muscles were stretched slightly by pulling the body in a dorsal and anterior direction. Contractions were amplified using a MOD CP 122A amplifier (Grass Telefactor, W. Warwick, RI) and were recorded using a computerized data acquisition system. The recording dish had a volume of approximately 0.2–0.4 mL and was perfused continuously at a rate of 0.7 mL/min. Excess fluid was removed by continuous suction.
Larval thermal preference
To assess thermal preference, a linear thermal gradient of 15°C to 30°C was generated using an aluminum dish machined into a rectangular lane (48 mm × 20 mm × 9 mm), which was placed on top of 2 Peltier units, each positioned at either end of the lane. Each Peltier unit had an independent digitally controlled power supply box (Brock University Electronics Division), ensuring accurate and constant temperature extremes. The temperature range and linear nature of the gradient were confirmed using a thermal camera (FLIR SC660, FLIR Systems, Inc.), connected to a computerized data acquisition system (Examine-R, FLIR Systems, Inc.). During experiments, the thermal gradient was monitored using 2 thermocouples, one at each end of the lane. Third-instar larvae were removed from stock vials (maintained at 21°C) without anesthesia, washed in dH20 (i.e., to avoid contaminating the testing surface with food) and placed in the center of the lane. Testing was in the dark, and images were captured under IR light using a Vario-Sonnar Super Steady Shot© camcorder (Sony Carl Zeiss); the video stream was captured to still images every second using HandyAvi software (AZcendant Software, Tempe, AZ). ImageJ (NIH) software was used to assess the final positional coordinates and the corresponding temperature for each larva after 10 min, and this was scored as a thermal preference. The sample size for these experiments was 20 trials per diet (each trial containing 20 larvae). Prior to experimentation any potential side or wall bias was assessed by examining larval distribution in the lane set ubiquitously at 22°C. We arbitrarily divided the lane into 4 equal quadrants and observed no statistical differences between them, nor were there any side or wall biases.
Larval heat avoidance response
A modified thermal gradient (22–38°C) was established by setting the Peltier units to extreme temperatures. Using the same approach as described above, we placed larvae (N = 4 per trial) at the hot end (38°C) of the lane and assessed their movement within the gradient for 2 min, acquiring still images every second. Using Image J (NIH), larval position after 2 min was assessed as total displacement, measured in centimeters. The sample size for warm avoidance was 54 trials per diet. In an alternate set of experiments, larvae were placed on the cold side (22°C) of the lane to test for any preference for the warmer temperatures. The sample size when placing larvae on the cool side was 10 trials per diet.
Larval velocity
Drosophila larvae are well known to have negative phototaxis,31 so we placed larvae at one end of a rectangular lane (to mitigate escape and to encourage locomotion in a linear direction) set to a constant 21°C, then using fiber-optic cables, cast light directly onto the larvae, which caused them to crawl away. We recorded their behavior on a video camcorder (see above) and calculated their velocity over a distance of 4 mm. Calculations of larval velocity were determined for 10 individual larvae from each of the 2 diets.
Adult negative geotaxis
Adult flies were subjected to a negative geotaxis assay in an apparatus described previously34 to assess speed and propensity to climb. Flies used for this study were between one and 8 d post-eclosion. Using CO2 anesthesia, flies were removed from their stock vials one day before testing, and placed in groups of 25 in separate clean vials containing the food on which they were reared. The following day, we placed the flies into each of 6 testing chambers and performed the negative geotaxis assay. The apparatus containing the 6 vials was rapidly descended vertically from a height of 73 mm. We subsequently counted the number of flies that ascended more than 45 mm (50% of the vial height) after 4 s, and after 55 s. The assay was repeated 3 times for each group at 1 min intervals, to establish the mean per experiment. For each diet, 72 vials (each containing 25 flies) were assessed (i.e., 1800 flies/diet).
Adult thermotaxis
To test for thermal preference in adult flies we followed a previously established protocol from Hong et al. (2006).48 Briefly, we placed flies in a glass tube (0.6 cm × 42 cm) fitted with a copper jacket. Either end of the copper jacket was connected to a digitally controlled Peltier device (Thermal Cycler; Brock University Electronics Division). The temperature gradient along the glass tube was linear and ranged from 14°C to 36°C, with a slope of (1.9°C/cm). The gradient was confirmed using a thermal camera (FLIR SC660, FLIR Systems, Inc.) connected to an acquisition program (Examine-R, FLIR Systems, Inc.). All flies used during these experiments were less than 12 d post-eclosion. Thirty flies were placed within the tube at a time and were allowed to distribute within the gradient for 25 min in the dark before a photograph was taken with a digital camera. We assigned each fly a thermal preference value based upon its location in the tube and the temperature value generated by the thermal camera. Sample size for these experiments was 19 trials for Formula 4–24 and 17 trials for Jazz mix.
Adult learning and memory (olfactory operant conditioning assay)
To assess if the 2 commercial rearing media influenced Drosophila learning and memory, we subjected adults to a modified version of the Tully and Quinn (1985)35 T-Maze, which examines olfactory-based associative learning and memory. Flies (75–100 at a time), between one and 8 d post-eclosion, were placed within a copper-grid wire lined acrylic chamber through which air and odors could flow. Flies were classically conditioned to associate one of 2 odors, 4-Methylcyclohexanol (MCH; Aldrich – 101123210) or 3-Octanol (OCT; Sigma-Aldrich – W358126), with a 90V shock as the negative stimulus. The odors were produced by dissolving these chemicals in mineral oil at a 10−6 dilution. Compressed air was forced through the mineral oil at a constant flow rate (500 mL/min), and the odor stream was diverted to appropriate test chambers. A no odor condition was produced simply by bubbling air through mineral oil. Half of the flies were trained to associate the shock with MCH, and the other half were trained to associate the shock with OCT. Each training session consisted of 90 s with air, 60 s with shock and odor #1, 30 s with air, 60 s with odor #2, and 30 s with air. After training, the flies were transferred down an elevator to a point where they were able to choose between 2 collection tubes, one containing odor #1 and the other with odor #2. The performance of the flies was calculated as in Tully and Quinn (1985).35 A learning index was determined as the fraction of flies that avoided the shock-associated odor minus the fraction of flies that preferred the shocked-associated odor. Since we trained flies to associate shock with either odor, we averaged the 2 odor-shock groups for each of the diets. If all avoided the shock-associated odor (perfect learning), the index value would be 1; if all preferred the shock-associated odor, the index value would be −1. A learning index value was computed for each group 2, 5 and 10 min after training. Ten independent groups of 75–100 flies were assessed for each diet.
Adult feeding assay
The capillary feeding (CAFE) assay was used to assay rates of food consumption between adults reared on different diets. The CAFE assay consists of a 50 mL conical centrifuge tube with 5 mL of distilled water to maintain humidity. A clean, empty Drosophila vial with a sponge top was placed inside the conical tube. A small hole is melted into the top of the conical tube and the underlying sponge. A 5μL calibrated glass tube (Accu-fill 90, micropet) was inserted into the hole, roughly 1 cm past the sponge. The glass tube was filled with 5μL of a solution of 5% sucrose and 5% yeast extract. Four males or 4 females were transferred from their respective rearing diets, into the assay for 8 h and kept in an incubator at 25 °Celsius. The total amount of food consumed over the 8 h was assessed by directly examining the calibrated glass. This was repeated 12 times for each of the diets.
Composition of media and fly tissue
Initially, to examine the compositional differences between the 2 commercially available diets, we submitted samples of each to a regulatory agency laboratory for the analysis of moisture, protein, ash, fat and carbohydrate (Agri-Food laboratories, Guelph, Ontario, Canada). Next, we assessed the lipid composition of each diet, as well as whole Drosophila tissue(5 animals per replicate, snap frozen in liquid nitrogen at the beginning of each developmental stage assessed: 3rd instar, pupal and adult). Drosophila tissue samples were manually homogenized on ice in 1x phosphate buffered saline. Samples were then subjected to lipid/protein extraction using the Bligh and Dyer method49 with some modifications50; the organic phase was subsequently dried under nitrogen and stored at −30°C before lipid analysis, and the combined interphase and aqueous phase was used to estimate protein concentration. Protein concentration was quantified using the EZQ kit as described previously51; resulting data were used to normalize the amount of lipid used for HPTLC analysis. Diet samples (Jazz-mix and Formula 4–24) were powdered and lipids were extracted from an equal amount of each diet, and dissolved in chloroform:methanol (2:1, v/v) for lipid analysis; 1.5 mg of yeast (Saccharomyces cerevisiae) was added per gram of Formula 4–24 before lipid extraction. For automated HPTLC analysis, lipid extracts were dissolved in choloroform:methanol (2:1, v/v) and loaded onto silica gel 60 HPTLC plates (EMD Chemicals, Darmstadt, Germany) and resolved using the CAMAG AMD 2 system. Neutral and phospholipids were resolved using solvent systems that have been described previously.52 Lipid standards (Avanta Polar Lipids, Alabaster, Alabama, USA) were resolved in parallel to enable identification of resolved lipid species. During the assessment of the phospholipids PE, PI, and PC, values were quantified and then standardized by diving all values from each dietary stage as a percentage of the value obtained for adults from the formula 4–24 diet.
Data analysis
To determine statistical differences between the diets, t-tests were used unless otherwise noted. The unit of replication was normally the pooled group of flies tested for behavioral or morphological characteristics, unless otherwise noted. Larval and pupal emergence and adult survival differences between diets were tested using the non-parametric log-rank test for survivorship (e.g., Kaplan-Meir survival analysis). An α value of 0.05 was used for rejecting the null hypothesis of no influence of diet on characteristics of interest. Unless otherwise noted (i.e., where mean values from multiple trials on the same replicate would warrant presenting a standard error of the mean), results are expressed as mean ± SD.
Effect size assessment
To summarize an overall effect of diet (i.e., across the numerous phenotypic measurements) with objective statistical measures and to qualify the biologic significance, effect sizes were calculated using Hedge's g, an unbiased estimate of Cohen's D, obtained from the pooled standard deviations and differences in the average values for each diet.53 Hedge's g was also converted into the point biserial correlation coefficient (r) for ease of comparison to the more familiar Pearson correlation coefficient. These effect sizes were assessed and compared using 95% confidence intervals against a null expected value of zero. An overall effect size across all experiments in this study was assessed using a technique common in meta-analyses of data sets to provide an assessment of the potential importance of diet on biologic function; this was possible since the groups of flies in each of these tests were independent replicates (Field, 2001).
Results
Development and longevity
Tracking the number of third-instar larvae that emerged each day for 20 d revealed a significant difference in the average developmental time required to reach the wandering phase: 177.2 ± 2.9 h vs. 223.6 ± 1.65 h, for Jazz-mix and Formula 4–24, respectively (Fig. 1, 1-way ANOVA, F = 40.17, P < 0.0001, Tukey post hoc P < 0.05).). There was also a significant difference between the time required to reach the wandering stage between Formula 4–24 and control larvae (170.6 ± 2.8, P < 0.05), but there was no difference between Jazz-mix and the control diet. There was no significant difference in the percentage of fertilized eggs that survived to wandering third-instar larvae in these experiments (Formula 4–24: 94.8 ± 4.3%, Jazz mix 91.6 ± 3.0%, control 92.4 ± 4.4%, 1-way ANOVA, F = 0.95, P = 0.39). Tracking the number of pupae that emerged each day for 20 d also revealed a significant difference between in the developmental time required to reach pupation: 276.1 ± 2.2 h vs. 237.9 ± 2.0 h, for Formula 4–24 and Jazz-mix respectively (Fig. 1B, 1-way ANOVA, F = 49.24, P = <0.0001, Tukey Post hoc P < 0.05). There was also a significant difference between the time required to reach pupation between the Formula 4–24 and a control diet (231.7 ± 2.3, P < 0.05) but not between the control and Jazz-mix diets. There was no significant difference in the percentage of fertilized eggs that survived to pupation in these experiments (Formula 4–24: 90.8 ± 5.4%, Jazz mix 89.2 ± 2.7%, control 91.6 ± 4.0%, 1-way ANOVA, F = 0.87, P=0.43). Subsequently, the number of the pupae that eclosed into adults was tracked in each of these vials. There was no significant difference between the 3 diets with respect to the percentage of eggs that survived to adulthood (Formula 4–24: 88.4 ± 3.5%, Jazz-mix: 87.2 ± 3.7%, Control: 90.0 ± 3.9%, 1-way ANOVA, F = 1.45, P = 0.25). Finally, adult flies, both males and females had greater survivorship on Jazz-mix compared with the Formula 4–24 diet (Fig. 1C, D). The time to 50% death was significantly different between the 2 diets, for both males and females, taking on average 20 d (Formula 4–24 29.1 ± 7.1 d, Jazz-mix 49.7 ± 12.3 d, 1-way ANOVA, F = 37.83, P < 0.0001, Tukey Post Hoc P < 0.05) longer to reach the 50% value for males, and 16 d (Formula 4–24 47.1 ± 11.1 d, 63.6 ± 8.1 d, 1-way ANOVA, F = 91.0, P < 0.0001, Tukey Post hoc, P < 0.05) for females. There was also a significant difference between male and female survivorship reared on Formula 4–24 compared with a control diet (males: 50.4 ± 11.3, females: 60.0 ± 12.6, P < 0.05).
Figure 1.

Diet-dependent changes in the average developmental times of: A. larvae, B. pupae, C. Female Adults, D. Male adults. Horizontal dotted lines represent the 50% development and survivorship values (A-C, N = 12 vial replicates per diet). 50% values in days (Formula 4–24 vs. Jazz-mix vs. Control): Third-instar – 223.6 ± 1.65 vs. *177.2 ± 2.9 vs. *170.6 ± 2.8. Pupae – 276.1 ± 2.2 vs. *237.9 ± 2.0 vs. *231.7 ± 2.3. Female adults – 47.1 ± 11.1 vs. *63.6 ± vs. 60.0 ± 12.6. Male adults – 29.1 ± 7.1 vs. 49.7 ± 12.3 vs. 50.4 ± 11.3. *indicates a significant effect of diet compared with Formula 4–24 (Mean ± SD, P < 0.05).
Fecundity
To assess if there were differences in the total number of eggs laid by females reared on each of the diets, an egg-laying assay was conducted. Five-day old females removed from communal vials on each of the respective diets were transferred to agar-plates for 3 d and the total number of eggs laid was assessed each day. There was no significant difference in the total number of eggs laid each day between the Formula 4–24 diet and Jazz-mix diet (234.6 ± 58.7 eggs and 238.0 ± 59.5eggs, 1-way ANOVA, F = 0.32, P = 0.73). There was also no significant difference between either diet and the control diet (241.2 ± 60.3eggs).
100 eggs from the egg-laying assay were transferred to a fresh grape-agar plate and the total number of eggs that progressed to first-instar was assessed. There was no significant difference in the percentage of eggs that progressed to first-instar larvae between any of the 3 diets (Formula 4–24: 93.4 ± 1.3%, Jazz-mix: 95.3 ± 2.0%, 94.6 ± 1.6%,1-way ANOVA, F = 3.33, P = 0.051).
A single virgin female and single virgin male were obtained from each of the diets, and after 4 d they were transferred to a grape-agar plate. Subsequently, 50 eggs were transferred to a fresh agar plate and the total number of eggs that progressed to first-instar larvae was assessed. There was no significant difference in the percentage of eggs that progressed to first-instar larvae between any of the diets (Formula 4–24: 96.6 ± 2.2, Jazz-mix: 96.9 ± 2.0, control: 97. 4 ± 1.0, 1-way ANOVA, F = 0.42, P = 0.67).
Body size parameters
Since there were differences in the rate of larval development between the 2 diets, morphometric measures and weight were also assessed. Third-instar larvae reared on Jazz-mix were significantly longer (Table 1, 3.688 ± 0.561 mm and 3.887 ± 0.371 mm, Formula 4–24 and Jazz-mix, respectively; Mann-Whitney, P = 0.039, t49 = 2225.5) and wider (0.661 ± 0.09 mm and 0.998 ± 0.10 mm, Formula 4–24 and Jazz-mix, respectively; P < 0.001, T49 = −17.77) than those reared on Formula 4–24. Larvae reared on Jazz-mix were nearly twice the weight of those fed Formula 4–24 (19.415 ± 0.236 mg vs.11.171 ± 0.247 mg, N = 10, t18 = −76.26, P < 0.001), and the mass of dried tissue was also significantly greater for Jazz-mix (5.506 ± 0.279 mg vs. 2.598 ± 0.245mg, N = 10, t18 = −24.75, P < 0.001). To test whether these differences in larval size and weight were manifested in differences in adult size, the length of the 4th wing vein was measured. Indeed, the size differences in larvae were found to persist into adulthood with the average wing size significantly larger for flies reared on Jazz-mix than those on Formula 4–24 (2.025 ± 0.148 mm and 1.823 ± 0.16 mm, respectively; Mann-Whitney Rank Sum, P < 0.001, T = 1122.0, N = 40). Additionally, the weight of newly eclosed virgin females was significantly different between those reared on Formula 4–24 and Jazz-mix (10 females, Formula 4–24 7.7 ± 0.6, Jazz-mix 9.3 ± 0.4, 1-way ANOVA, F = 39.96, P < 0.0001, Tukey Post Hoc P < 0.05). A significant difference was also observed between adults reared on Formula 4–24 and control diet (9.0 ± 0.3, P < 0.05); however no significant difference was observed between adults on Jazz-mix and the control diet.
Table 1.
Morphological features of Drosophila reared on 2 commercially available diets.
| Feature | Formula 4–24 | Jazz-mix | P-value |
|---|---|---|---|
| Larval wet mass (mg) | 11.17 ± 0.25 | 19.42 ± 0.24 | P < 0.001 |
| Larval dry mass (mg) | 2.60 ± 0.25 | 5.51 ± 0.28 | P < 0.001 |
| Larval length (mm) | 3.69 ± 0.56 | 3.89 ± 0.37 | N/S |
| Larval width (mm) | 0.66 ± 0.09 | 1.00 ± 0.10 | P < 0.001 |
| Wing measurement (mm) (4th vein – reference) | 1.82 ± 0.16 | 2.03 ± 0.15 | P < 0.001 |
| Wing length males (mm) | 1.77 ± 0.04 | 1.90 ± 0.06 | P < 0.001 |
| Wing length females (mm) | 1.88 ± 0.04 | 2.16 ± 0.08 | P < 0.001 |
| Adult wet mass (mg) | 7.75 ± 0.57 | 9.3 ± 0.35 | P < 0.001 |
Larval locomotory speed
A modified light-avoidance assay was used to evoke escape locomotion in larvae (Fig. 2C). Those reared on Formula 4–24 moved significantly faster, with an average speed of 1.19 ± 0.21 mm/s compared with those fed Jazz-mix 0.98 ± 0.15 mm/s (P = 0.019, t19 = 2.59).
Figure 2.

(A) 10−6 M octopamine-induced changes in basal tonus (*P < 0.01, N = 10 per diet); (B) the force of maximal contraction (elicited by 300 mM KCl) for larvae reared on both diets (N = 10 per diet); and (C) respective crawling speeds (Mean ± SD, *P < 0.05, N = 10 per diet).
Muscle contraction
The possibility that diet alters the physiology of larval body-wall muscles, either by altering the maximal force output of these muscles or by altering their responsiveness to hormonal stimulation, was assessed (Fig. 2). Third-instar larvae reared on Jazz-mix generated larger contractions in response to 10−6 M octopamine than did larvae reared on Formula 4–24 (Fig. 2A) (6.34 ± 0.09 and 4.10 ± 0.82 mN, P < 0.001, t19 = −5.86). In contrast, no difference was found in the maximal force generated by the larval body-wall muscles in response to KCl (Fig. 2B) (P = 0.6, t18 = −0.534). Thus, larvae reared on either diet are able to generate the same total force from muscle contractions but do not respond equally well to hormonal stimulation.
Assays of larval behavior
Third-instar larvae reared on either Formula 4–24 or Jazz-mix were tested for thermal preference (Fig. 3). Within a linear thermal gradient (15–30°C), larvae reared on Formula 4–24 had a significantly higher preferred temperature (17.3 ± 0.4°C) than those reared on Jazz-mix (16.0 ± 0.3°C; Fig. 3A; F = 6.587, P = 0.014, N = 20). Larvae reared on Formula 4–24 also traveled significantly further from a heat source (3.5 ± 0.1 cm, to 22.5°C from 38°C) than those reared on Jazz-mix (3.1 ± 0.1 cm, to 24.2°C; Fig. 4A, Kruskal-Wallis ANOVA on ranks, P < 0.01, H = 6.743, DF = 1, N = 54, total larvae = 1080 per group). In the heat-avoidance trials, significantly fewer larvae reared on Jazz-mix passed the half-way point (2.4 cm or 30°C) of the lane (65.6 ± 3.7 vs. 79.9 ± 2.9 reared on Formula 4–24; Mann-Whitney U, P < 0.01, T = 3433.0, N = 54) (Fig. 4B). In the reciprocal experiment, initiated with larvae on the cool side (22°C), larvae reared on Formula 4–24 migrated on average 0.9 ± 0.8 cm or 3.1 ± 2.8°C, away from the ‘cool’ side (N = 10 trials, 40 larvae total). It is of note that only 4 of 40 animals passed the half-way point. Larvae reared on Jazz-mix migrated on average 0.5 ± 0.6 cm or 1.9 ± 1.9°C (N = 10 trials, 40 larvae total). Here zero of the 40 animals passed the half-way point.
Figure 3.

(A) Thermotaxis of larval Drosophila melanogaster reared on 2 different commercially available media, tested on a thermal gradient. (B) Image of thermal gradient using a thermal camera. (C) Image of thermal gradient under ambient light. Larvae reared on Formula 4–24 had a significantly higher preferred temperature compared with those reared on the Jazz-mix (Mean ± SD; 17.32 ± 0.43°C and 15.99 ± 0.29°C; * P < 0.05; N = 20 per diet).
Figure 4.

(A) Larval Drosophila reared on the Formula 4–24 retreated significantly further than those reared on Jazz-mix when placed on the hot side (38°C) of a thermal gradient ranging from 22–38°C (Mean ± SD; P < 0.01, N = 54). (B) A greater percentage of larvae reared on Formula 4–24 retreated beyond the 50% or 30°C point than those reared on Jazz-mix (N = 54, *P < 0.01).
Assays of adult behavior
The negative geotactic response of flies was assessed at 4 and 55 s after an abrupt drop. More adults reared on Jazz-mix climbed past the halfway point of the vial (74.0 ± 0.6) than those on Formula 4–24 (60.6 ± 0.8) 4 s after the drop (N = 12, ANOVA: F statistic – 31.87, P < 0.001) (Fig. 5A). However, 55 s after the drop, the flies performed equally well (74.7 ± 0.7 and 75.0 ± 0.8, ANOVA: F statistic – 31.87, P>0.001, N = 72, total flies = 1800) irrespective of diet (Fig. 5A).
Figure 5.
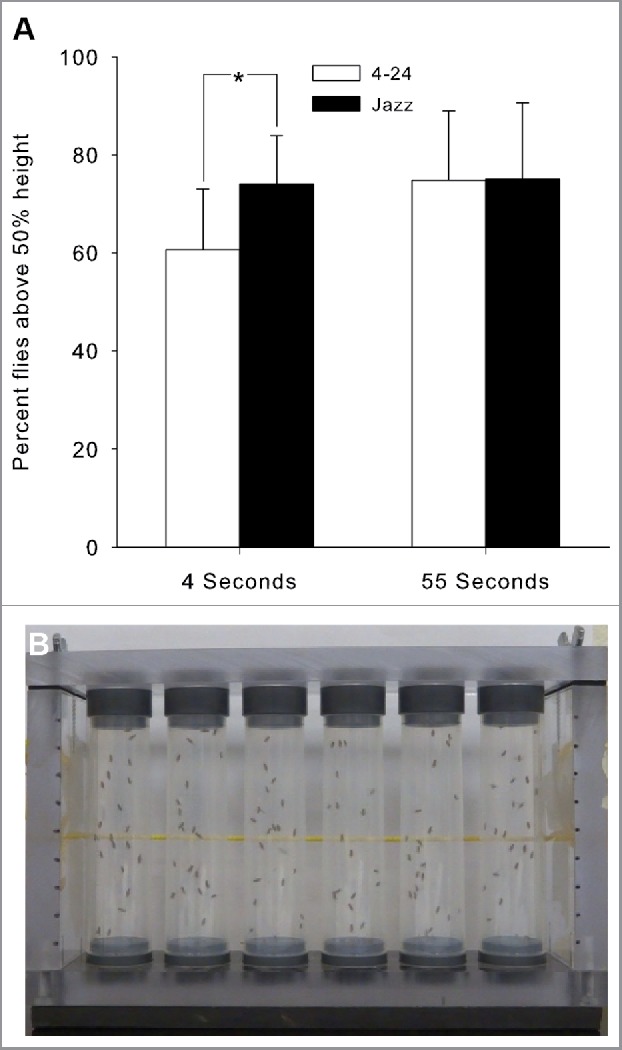
A significantly greater percentage of adult Drosophila climbed (A) beyond the 50% point when reared on jazz mix than those reared on Formula 4–24 (Mean ± SD; *P < 0.01, N = 12 per diet). (B) Depicts the apparatus used to assess negative geotaxis.
In the thermotaxis assay, flies reared on Formula 4–24 had a preferred temperature of 21.6 ± 1.5, which was not significantly different from those fed Jazz-mix: 22.2 ± 1.6 (P = 0.25).
Figure 6.

Thermal preference in adult Drosophila was not influenced by diet (Mean ± SD; P=0.25, Formula 4–24, N = 19; Jazz mix, N = 17).
The CAFE assay was used to assess rates of food consumption between the diets. Adult males reared on Jazz-mix consumed significantly more food than adult males reared on Formula 4–24 (2.6 ± 0.2 ul/fly vs. 2.0 ± 0.2 ul/fly, respectively, 1-way ANOVA, F = 56.72, P < 0.0001, Tukey Post hoc P < 0.05). Adult females reared on Jazz-mix also consumed significantly more food than adult females reared on Formula 4–24 (3.3 ± 0.2 ul/fly vs. 2.5 ± 0.2 ul/fly, respectively, 1-way ANOVA, F = 79.00, P < 0.0001, Tukey Post hoc, P < 0.05). Both male and female adults consumed significantly more food when reared on the control diet compared with Formula 4–24 (P < 0.05), however, there was no significant difference in the amount of food consumed between males or females reared on Jazz-mix median and those reared on a control diet.
As a final behavioral assay, adult olfactory learning and memory was assessed using a modified version of the Tully and Quinn T-maze.35 Adults reared on either diet performed equally well even when tested 10 min after training (2 min: Formula 4–24 0.7 ± 0.0 Jazz-mix 0.70 ± 0.0; ANOVA: P = 0.699, F = 0.601, N = 10, total flies>1000). We also assessed learning and memory 5 and 10 min after training (5 min Formula 4–24 0.7 ± 0.0, Jazz-mix 0.7 ± 0.0; 10 min Formula 4–24 0.6 ± 0.0, Jazz-mix 0.7 ± 0.0).
Figure 7.

Learning and memory assay reveals that adult Drosophila melanogaster learn equally well irrespective of the diet they are reared upon (Mean ± SD; *P>0.05, N = 10).
Diet composition
Both diets were initially chemically assayed for total moisture, protein, ash, fat and carbohydrate content (Table 2). There were apparent differences in all properties examined. The Formula 4–24 diet contained proportionally more protein, ash, fat and carbohydrates than did Jazz-mix, whereas Jazz-mix contained proportionally more moisture. Next, the raw starting material for each diet was assessed using HPTLC for to test for differences in: cholesterol, monoacylglycerol (MAG), diacylglycerol (DAG), triacylglycerol (TAG) phosphatidylcholine (PC), phosphatidylinositol (PI), and phosphatidylethanolamine (PE; Table 3). Significant differences were observed for all 7 components that were assessed. Cholesterol, and MAG were significantly higher in the Jazz-mix diet, however, TAG, DAG, PE, PI, and PC were all significantly higher in the Formula 4–24 diet.
Table 2.
General composition of Formula 4–24 and Jazz-mix.
| Breakdown | Formula 4–24 | Jazz-mix | Ratio of F4–24:Jazz |
|---|---|---|---|
| Moisture | 75.73 | 81.69 | 0.9 |
| Protein % | 1.91 | 0.80 | 2.4 |
| Ash % | 1.05 | 0.49 | 2.1 |
| Fat % | 0.12 | 0.01 | 12 |
| Carbohydrates % (calc) | 21.19 | 17.01 | 1.2 |
Table 3.
Major lipidic species in Formula 4–24 and Jazz-mix. Values expressed as arbitrary units.
| Formula 4–24 | Jazz-mix | Ratio F424:Jazz | |
|---|---|---|---|
| Cholesterol (AU) | 1 747 204 ± 37 086 | 4 382 774 ± 36 759 | 0.4*** |
| TAG (AU) | 2 813 326 ± 27 282 | 2 291 746 ± 85 901 | 1.2*** |
| DAG (AU) | 1 263 798 ± 15 115 | 722 640 ± 9 970 | 1.7*** |
| MAG (AU) | 1 434 098 ± 58 165 | 2 512 646 ± 43 899 | 0.6** |
| PE | 1 269 962 ± 22983 | 159 647 ± 9979 | 8.0*** |
| PI | 1 151 838 ± 26 744 | 310 915 ± 38 668 | 3.7*** |
| PC | 1 126 151 ± 44 230 | 325 646 ± 34 991 | 3.5*** |
Note.
P<0.05,
P<0.01,
P<0.001
Tissue from larval, pupae, and adults were then compared with determine if the differences in starting material manifested itself as a significant difference in the Drosophila tissue (Table 4). There was no significant difference in cholesterol concentrations between the 2 diets during any of the developmental stages assessed (Table 4). We also assessed the tissue for differences in other critical membrane lipids (Table 4). There were no significant differences in either MAG or TAG levels during any of the developmental stages assessed. DAG levels were higher in larvae reared on Formula 4–24 which correlates with higher DAG in their diet. However, DAG levels were comparable in pupae reared on both diets, but higher in adults reared on Jazz-mix compared with Formula 4–24. Both PE and PC were significantly higher in tissue isolated from animals reared on the Formula 4–24 diet during all 3 life stages assessed, consistent with the differences in dietary composition. There was however no differences in PI concentrations in tissue from any of the life stages assessed.
Table 4.
Body composition of animals reared on Formula 4–24 and Jazz-mix (Units: Cholesterol, TAG, DAG, MAG - ug/mg protein; PE, PI, PC – % of Adult formula 4–24 AU).
| Formula 4–24 | Jazz-mix | Ratio | |
|---|---|---|---|
| Larvae | |||
| Cholesterol | 4.6 ± 0.3 | 6.1 ± 0.5 | 0.8 |
| TAG | 90.7 ± 2.7 | 80.8 ± 8.3 | 1.1 |
| DAG | 1.6 ± 0.1 | 0.8 ± 0.2 | 2.0 * |
| MAG | 85.0 ± 6.5 | 66.9 ± 12.2 | 1.2 |
| PE | 108.5 ± 55.5 | 44.6 ± 35.9 | 2.4 * |
| PI | 230.8 ± 135.5 | 126.7 ± 128.1 | 1.8 |
| PC | 113.6 ± 49.4 | 59.8 ± 46.5 | 1.9 * |
| Pupae | |||
| Cholesterol | 2.3 ± 0.5 | 2.9 ± 0.4 | 0.8 |
| TAG | 71.1 ± 10.3 | 63.2 ± 1.7 | 1.1 |
| DAG | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.8 |
| MAG | 53.0 ± 7.1 | 57.6 ± 19.24 | 0.9 |
| PE | 46.1 ± 28.0 | 11.5 ± 7.8 | 4.0 * |
| PI | 92.7 ± 55.0 | 24.2 ± 20 | 3.8 |
| PC | 48.8 ± 28.2 | 15.8 ± 11.5 | 3.1 * |
| Adults | |||
| Cholesterol | 3.5 ± 0.4 | 3.1 ± 0.5 | 1.1 |
| TAG | 61.0 ± 12.4 | 68.4 ± 16.0 | 0.9 |
| DAG | 0.4 ± 0.0 | 1.3 ± 0.1 | 0.3 * |
| MAG | 70.3 ± 16.5 | 49.9 ± 14.5 | 1.4 |
| PE | 44.6 ± 36.0 | 49.7 ± 10.5 | 0.9 |
| PI | 100.0 ± 91.7 | 54.1 ± 12.9 | 1.8 |
| PC | 100.0 ± 76.6 | 63.7 ± 14.7 | 1.6 * |
Note.
P<0.05,
P<0.01,
P<0.001
Overall diet effect: Effect size estimates
Diet influenced multiple phenotypes (summarized in Table 5). The overall z score from a meta-analysis of effect size was 15.76, supporting a strong overall diet effect. Indeed, in 13 out of the 17 primary experimental tests, the biologic effect of diet (Hedge's g) was significantly different from zero.
Table 5.
Effect sizes of the influence of diet on developmental, behavioral, and physiologic parameters.
| Comparison | Phenotype | Hedge's g | r | Significant |
|---|---|---|---|---|
| Larval Wet Mass | Morphology | 32.66 | 1.00 | Yes |
| Larval Dry Mass | Morphology | 10.60 | 0.98 | Yes |
| Larval Width | Morphology | 3.53 | 0.87 | Yes |
| Adult Wing Length | Morphology | 1.30 | 0.55 | Yes |
| Adult Wet Mass | Morphology | 3.12 | 0.85 | Yes |
| Egg Laying | Fecundity | 0.05 | 0.03 | No |
| Larva Emergence | Development | 3.28 | 0.86 | Yes |
| Pupa Emergence | Development | 4.39 | 0.92 | Yes |
| Adult survivorship | Lifespan | 1.95 | 0.71 | Yes |
| Larva Thermal Preference | Sensory | 0.80 | 0.36 | Yes |
| Adult Thermal Preference | Sensory | 0.38 | 0.18 | No |
| Larval Thermal Retreat | Neuromuscular | `0.73 | 0.34 | Yes |
| Adult Geotaxis | Neuromuscular | 2.49 | 0.77 | Yes |
| Muscle Basal Tonus | Neuromuscular | 2.51 | 0.77 | Yes |
| Muscle Maximum | Neuromuscular | 0.23 | 0.11 | No |
| Feeding Rate | Feeding | 1.87 | 0.69 | Yes |
| Learning Index | Neurological | 0.34 | 0.16 | No |
Discussion
Here we have tested the hypothesis that rearing D. melanogaster on commercial media of differing chemical composition can have profound effects on a multitude of phenotypic characteristics. Our data demonstrate that these 2 media yielded significant differences in development, morphology, physiology, and behavior. In almost 80% of the phenotypic comparisons we observed significant biologic effects (Table 5). Notably, while learning and memory were not affected in adult flies, morphological parameters as well as developmental time as larvae and longevity as adults were significantly different, favoring flies reared on Jazz-mix as opposed to Formula 4–24.
We have demonstrated that diet can have a profound effect on several metrics for both the larval and adult forms of Drosophila melanogaster. Some of the more important characteristics in terms of fitness and survivorship are speed of development, fecundity and longevity. Other researchers have demonstrated an inverse relationship between longevity and reproduction rate for Drosophila, with lower nutritional diets favoring longevity at the expense of fecundity.8,11,54 Based on such findings, we might expect the longer lifespan in flies raised on Jazz-mix to reflect dietary restriction compared with Formula 4–24, and, indeed, Jazz-mix had lower levels of protein, carbohydrate and fat than did Formula 4–24 (Table 2). On the other hand, nutrient availability, rather than total protein, carbohydrate or lipid levels, must also be considered. Increased nutrient availability triggers a rapid elevation in reproductive activity in Drosophila, increasing both re-mating frequency and the number of eggs laid by females.8,11 Both our larval and pupal emergence data indicate that Jazz-mix larvae emerge faster, but there was no significant difference between the number of larvae or pupae produced on the 2 diets after about 30 d (Fig. 1). The total number of eggs laid, as well as the percentage of eggs that progressed from eggs to first-instars did not differ between the 2 diets. Thus, fecundity does not appear to be altered as a consequence of these diets. It is noteworthy that larvae on Jazz-mix are nearly twice the mass of those on Formula 4–24, and they do not require more time to achieve this size. It is therefore likely that Jazz-mix provides greater nutrient availability, potentially attributable to differences in media preparation; larvae would thus spend less time converting the substrates into metabolized nutrient stores than when raised on Formula 4–24. Previous work has also demonstrated that nutrient-poor diets can result in smaller sized adults55; the lower larval mass and smaller adult size of Drosophila reared on Formula 4–24 may thus represent poorer nutrition. It is also noteworthy that as adults the Jazz-mix flies consume food at a significantly greater rate. This added food intake may directly contribute to their improved health or it may simply be a product of their significantly greater size, as observed in previous reports.56 Taken together, our findings indicate that in spite of lower fat, carbohydrate and protein concentrations, the Jazz-mix food contains a nutrient balance that optimizes reproduction rates without negatively influencing lifespan, or conversely that Formula 4–24 hinders reproduction rates and lifespan due to malnutrition rather than dietary restriction.54
To elucidate whether rearing flies on the 2 diets resulted in physiologic differences in muscle function, we examined contractions of larval body-wall muscles in response to exogenous application of a well-known hormone and neuromodulator, octopamine (Fig. 2). Octopamine has been implicated as the ‘fight-or-flight’ hormone in Drosophila, and induces a very large response in larval body-wall muscle.45,57 Additionally, octopamine has been implicated in: coordinating thermoprotection in insects,58 associative olfactory memory in Drosophila,59 locomotion,60 and other physiologically and behaviorally relevant parameters.61-64 Application of 10−6 M octopamine (previously demonstrated to be the EC50 for inducing contractions in Drosophila body wall muscles45) induced a significantly greater change in tonus in larvae reared on Jazz-mix than in those reared on Formula 4–24. In contrast, rearing flies in the different media did not alter the maximal force of contraction induced by direct depolarization with KCl, indicating that diet did not alter the overall capacity of the muscles for force generation. These results suggest that diet may influence the responsiveness of muscles to internal signaling molecules and, potentially, that diet can affect relevant cellular signaling pathways. Diet is known to influence hormonal signaling in Drosophila. One critical dietary requirement is cholesterol, required for synthesis of numerous hormonal and signaling molecules, such as the production of ecdysone and 20-hydroxyecdysone associated with molting and pupation.65 Interestingly, Formula 4–24 has previously been used as a low-cholesterol diet, and is reported to contain low levels of other plant sterols.66 Consequently, cholesterol and other plant derived sterols are an area of dietary interest, particularly in insects, which require a dietary source for development and survival.66,67 Plant sterols have also been of interest in human research as potential treatments of hypercholesterolemia.68
As muscle responsiveness to exogenous application of octopamine was altered as a consequence of different dietary media, we postulated that overall muscle performance, i.e., locomotion or crawling rate, might be affected by differences in diet (Fig. 2). However, even though third-instar body wall muscles produced stronger contractions in response to octopamine in those larvae reared on Jazz-mix, those reared on Formula 4–24 had faster crawling rates. Nevertheless, these velocity values are comparable to what has previously been reported (∼1mm/s69). Thus, differences in crawling rates may be attributable to the dramatic size difference between larvae reared on the 2 diets, with Jazz-mix larvae being nearly twice the weight. It is also of note that because light-avoidance (Fig. 2) is mediated by a cellular signaling pathway, the differences we observed in mobility may not only be influenced by mass but also by differences in intra- and inter-cellular signaling.70
Although the thermal preference values for third-instar larvae measured here for both diets were similar to previous determinations, we observed that larvae reared on Jazz-mix had a slightly lower, yet significantly different preferred temperature value relative to those maintained on Formula 4–24 (Fig. 3).28,29 Subsequent to the larval preferred temperature assay, we subjected larvae to a thermal retreat assay and found that animals reared on Formula 4–24 traveled significantly further away from a high temperature extreme. Thus, despite a significantly lower preferred temperature value for larvae reared on Jazz-mix, these did not exhibit greater thermal avoidance behavior, although there was a statistical difference in crawling rates which might be interpreted as a potential confounding influence of the thermal avoidance assay. However, the length of time larvae were subjected to the thermal avoidance assay (2 min) was more than sufficient to mitigate these slight alterations in locomotory capacity, and thus this result is more likely indicative of a greater tolerance or reduced detection of warmer temperatures in Jazz-mix larvae.
As adults, differential rearing media does not appear to alter thermal preference as both groups preferred a value of ∼22°C, which is similar to previous research.32 We did not dissociate between males and females, however, the preferred temperature value established here has previously been demonstrated to be similar for males and females of D. melanogaster.32,33 Our adult geotaxis results revealed a significant reduction in the fast (4s) climbing ability of adults reared on the Formula 4–24 diet, however, this effect was not long lasting (i.e., after 55 s the distribution of flies in the tube was identical between both groups). Although we did not examine muscle in adults, third instar larvae raised on Formula 4–24 showed no signs of muscle damage, as maximal force generation was not impaired, and crawling speed was greater in this group than in the Jazz-mix group. Interestingly, a progressive diminishment in negative geotactic behavior is known to occur with age in adult Drosophila, even during time periods of minimal mortality.34 Since our data do not indicate any diet-induced differences in the capacity for learning and memory, the reduction in initial climbing speed in adults from the Formula 4–24 may be related to the accelerated mortality rate observed in the Formula 4–24 reared flies. Lastly, because adults reared on Formula 4–24 are significantly smaller than those reared on Jazz-mix, the impaired geotactic response cannot be attributed to greater body mass. Taken together, our results indicate that diet can influence the behavior of adult Drosophila, and these differences are at least initially attributable to differences in morphology or development of the animal.
The data on diet composition represent an initial effort to identify dietary factors that influence development and physiologic functions, using the Drosophila model system. Notably, Jazz-mix contained proportionally greater moisture than Formula 4–24; larvae prefer moist over dry substrates,71 and moisture can affect the number of pupae and rate of mortality72 along with many other factors.73-75 Interestingly, all other macro-nutritional components were proportionally greater in the Formula 4–24 diet, at least in terms of traditional chemical analyses (Table 2). Of the 4 remaining nutritional components, fat content is probably the most extensively investigated. Birse et al. (2010)76 demonstrated that Drosophila fed with a high-fat diet exhibit increased triglycerides and altered insulin/glucose homeostasis that resulted in cardiac lipid accumulation, reduced cardiac contractility, conduction blocks, and structural pathologies. Increasing fat content has also been shown to decrease lifespan,15 and alter stress responses77 in Drosophila. Consequently, we decided to focus on some of the critical components within the fat profile, and examine how they differ in both the raw material as well as how these changes translate to the animal. In terms of composition, cholesterol and MAG were significantly higher in the Jazz-mix diet, while TAG, DAG, PE, PI, and PC were significantly higher in the Formula 4–24 diet. However, only a few of these molecules were significantly different when we assessed their concentrations in whole bodies at each of the 3 developmental stages assessed.
Of the 7 phospholipid, glyceride, or sterol molecules investigated, 3 differed significantly in the different developmental stages. Both PE and PC were significantly more abundant in the larvae and pupal stages of development in animals reared on Formula 4–24. This is anticipated as both these molecules were in greater abundance in the Formula 4–24 diet. PC and PE are both very abundant in biologic membranes, with PC being the principle phospholipid in animals and PE comprising roughly 25% of the phospholipids in membranes. They are both synthesized via the CDP-ethanolamine pathway, using ethanolamine as their substrate.78 They serve a variety of biologic and biophysical roles for cells, including regulating membrane fluidity, permeability and fusion. While cholesterol was not significantly different during any of the developmental stages, there was a large difference in the larval tissue. This lack of difference might be attributable to an inability of the technique used to differentiate between closely related sterols, including ergosterol.
Perhaps the most notable change in phospholipid composition was a significant change in DAG. There was approximately twice the amount of DAG in the formula 4–24 diet compared with Jazz-mix, which directly correlated with a doubling of DAG in the larval tissue from animals reared on the Formula 4–24 diet. Conversely, there was no significant difference in DAG during the pupal stage, but nearly 3-times the amount of DAG in adult animals reared on Jazz-mix compared with those reared on Formula 4–24. DAG is a heavily investigated molecule that is implicated in several vital cellular pathways, perhaps most notably DAG synthesized via the hydrolysis of phospholipid phosphatidylinositol 4,5-biphosphate (PIP2) by PLC producing IP3 and DAG. Subsequently DAG actives PKC leading to several downstream effects including lipid storage, fatty acid oxidation, altered muscle activity through interactions with troponin, SERCA, and myosin, or it can act as a transcription factor for several genes.79 DAG levels have been associated with lifespan by altering rapamycin (TOR) levels, one of the downstream targets of PKC. Lin et al, (2014) demonstrated in both Drosophila and C. Elegans, that overexpressing diacylglycerol lipase (DAGL) or reducing the expression of diacylglycerol kinase (DGK) extends lifespan.80 DAG levels are also associated with phototransduction through interaction with rolling blackout, an integral membrane lipase, or other transient receptor potential channels (TRP/TRPL80,81). Such a combination of effects may thus explain why the flies reared on the Jazz-mix diet have a longer lifespan. Given that cholesterol, TAG, MAG and PI levels did not differ across all 3 stages of development, irrespective of diet, emphasizes a critical role for regulating the amounts of these lipids within the animal. This is even more striking given that the starting media upon which these animals were reared contained significantly different amounts of these lipidic components, with MAG and cholesterol being more abundant in Jazz-mix, and DAG and PI being more abundant in formula 4–24.
Overall, our observations confirm that 2 standard diets can significantly alter fundamental processes associated with development, morphology, physiology and behavior in D. melanogaster. The observed effects are more prevalent in larvae, but persist into adulthood and were especially clear with respect to development and fly lifespan. Assessments of the dietary components that may underlie these developmental, morphological, and behavioral differences revealed several putative factors - PC, PI and DAG - that differ significantly in the tissue of these animals. Nonetheless, we report that altering dietary components in food media can result in a multitude of confounding implications and leave it to the wider scientific community to consider how inter-laboratory differences in dietary media may influence results across a broad range of study types.
Abbreviations
- DAG
diacylglycerol
- MAG
monoacylglycerol
- MCH
4-methylcyclohexane
- OCT
3-octanol
- TAG
triacylglycerol
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a Queen Elizabeth II scholarship in science and technology and National Science and Engineering Research Council of Canada (NSERC) PGS funding to KGO, and a NSERC undergraduate student research award to OKL. JRC acknowledges initial support from the Canadian Institutes of Health Research, and the WSU School of Medicine. PSA was supported by a PhD Scholarship from the WSU School of Medicine. NSERC grants to AJM (46292), and GJT (262 087). GJT would also like to acknowledge Brock University Chancellor's Chair funding.
References
- [1].Kopec S. On the influence of intermittent starvation on the longevity of the imaginal stage of Drosophila melanogaster. J Exp Biol 1928; 5:204-11. [Google Scholar]
- [2].Baumberger JP, Glaser RW. The rearing of drosophila ampelophila loew on solid media. Science 1917; 45:21-2; PMID:17832180; https://doi.org/ 10.1126/science.45.1149.21-b [DOI] [PubMed] [Google Scholar]
- [3].Doubal S, Klemera P. The effect of antioxidants and dietary restriction on mortality curves. Age (Omaha) 1999; 22:101-5; PMID:23604407; https://doi.org/ 10.1007/s11357-999-0012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anagnostou C, Dorsch M, Rohlfs M. Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol Exp Appl 2010; 136:1-11; ; https://doi.org/ 10.1111/j.1570-7458.2010.00997.x [DOI] [Google Scholar]
- [5].Hartman TR, Strochlic TI, Ji Y, Zinshteyn D, O'Reilly AM. Diet controls Drosophila follicle stem cell proliferation via Hedgehog sequestration and release. J Cell Biol 2013; 201:741-57; PMID:23690177; https://doi.org/ 10.1083/jcb.201212094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carvalho M, Schwudke D, Sampaio JL, Palm W, Riezman I, Dey G, Gupta GD, Mayor S, Riezman H, Shevchenko A, et al.. Survival strategies of a sterol auxotroph. Development 2010; 137:3675-85; PMID:20940226; https://doi.org/ 10.1242/dev.044560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Powsner L. The effects of temperature on the durations of the developmental stages of drosophila melanogaster. Physiol Zool 1935; 8:474-520; https://doi.org/ 10.1086/physzool.8.4.30151263 [DOI] [Google Scholar]
- [8].Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J Evol Biol 1993; 6:171-93; https://doi.org/ 10.1046/j.1420-9101.1993.6020171.x [DOI] [Google Scholar]
- [9].Fanson BG, Weldon CW, Pérez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 2009; 8:514-23; PMID:19558564; https://doi.org/ 10.1111/j.1474-9726.2009.00497.x [DOI] [PubMed] [Google Scholar]
- [10].Vigne P, Frelin C. Diet dependent longevity and hypoxic tolerance of adult Drosophila melanogaster. Mech Ageing Dev 2007; 128:401-6; PMID:17606290; https://doi.org/ 10.1016/j.mad.2007.05.008 [DOI] [PubMed] [Google Scholar]
- [11].Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci 1996; 263:755-9; PMID:8763795; https://doi.org/ 10.1098/rspb.1996.0113 [DOI] [PubMed] [Google Scholar]
- [12].Chhabra R, Kolli S, Bauer JH. Organically grown food provides health benefits to Drosophila melanogaster. PLoS One 2013; 8:e52988; PMID:23326371; https://doi.org/ 10.1371/journal.pone.0052988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Riddiford LM, Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol 1991; 82:172-83; PMID:1906823; https://doi.org/ 10.1016/0016-6480(91)90181-5 [DOI] [PubMed] [Google Scholar]
- [14].Alpatov W, W. Experimental Studies on the Duration of Life . XIII. the influence of different feeding during the larval and imaginal stages on the duration of life of the imago of drosophila melanogaster. The American Naturalist 1930; 64:37-55; https://doi.org/ 10.1086/280297 [DOI] [Google Scholar]
- [15].Driver CJ, Cosopodiotis G. The effect of dietary fat on longevity of Drosophila melanogaster. Exp Gerontol 1979; 14:95-100; PMID:110610; https://doi.org/ 10.1016/0531-5565(79)90023-8 [DOI] [PubMed] [Google Scholar]
- [16].Testa ND, Ghosh SM, Shingleton AW. Sex-specific weight loss mediates sexual size dimorphism in Drosophila melanogaster. PLoS One 2013; 8:e58936; PMID:23555608; https://doi.org/ 10.1371/journal.pone.0058936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cortese MD, Norry FM, Piccinali R, Hasson E. Direct and correlated responses to artificial selection on developmental time and wing length in Drosophila buzzatii. Evolution 2002; 56:2541-7; PMID:12583594; https://doi.org/ 10.1111/j.0014-3820.2002.tb00179.x [DOI] [PubMed] [Google Scholar]
- [18].Kalmus H. The Resistance to desiccation of drosophila mutants affecting body colour. Proc R Soc B Biol Sci 1941; 130:185-201; https://doi.org/ 10.1098/rspb.1941.0011 [DOI] [Google Scholar]
- [19].Suster ML, Bate M. Embryonic assembly of a central pattern generator without sensory input. Nature 2002; 416:174-8; PMID:11894094; https://doi.org/ 10.1038/416174a [DOI] [PubMed] [Google Scholar]
- [20].Heimbeck G, Bugnon V, Gendre N, Häberlin C, Stocker RF. Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons. J Neurosci 1999; 19:6599-609; PMID:10414987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shaver SA, Varnam CJ, Hilliker AJ, Sokolowski MB. The foraging gene affects adult but not larval olfactory-related behavior in Drosophila melanogaster. Behav Brain Res 1998; 95:23-9; PMID:9754873; https://doi.org/ 10.1016/S0166-4328(97)00206-4 [DOI] [PubMed] [Google Scholar]
- [22].Gerber B, Scherer S, Neuser K, Michels B, Hendel T, Stocker RF, Heisenberg M. Visual learning in individually assayed Drosophila larvae. J Exp Biol 2004; 207:179-88; PMID:14638844; https://doi.org/ 10.1242/jeb.00718 [DOI] [PubMed] [Google Scholar]
- [23].Pereira HS, Sokolowski MB. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proc Natl Acad Sci U S A 1993; 90:5044-6; PMID:8506349; https://doi.org/ 10.1073/pnas.90.11.5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heiman RG, Atkinson RC, Andruss BF, Bolduc C, Kovalick GE, Beckingham K. Spontaneous avoidance behavior in Drosophila null for calmodulin expression. Proc Natl Acad Sci U S A 1996; 93:2420-5; PMID:8637889; https://doi.org/ 10.1073/pnas.93.6.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Suster ML, Martin J-R, Sung C, Robinow S. Targeted expression of tetanus toxin reveals sets of neurons involved in larval locomotion in Drosophila. J Neurobiol 2003; 55:233-46; PMID:12672020; https://doi.org/ 10.1002/neu.10202 [DOI] [PubMed] [Google Scholar]
- [26].Caldwell JC, Miller MM, Wing S, Soll DR, Eberl DF. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc Natl Acad Sci U S A 2003; 100:16053-8; PMID:14673076; https://doi.org/ 10.1073/pnas.2535546100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carhan A, Reeve S, Dee CT, Baines RA, Moffat KG. Mutation in slowmo causes defects in Drosophila larval locomotor behaviour. Invert Neurosci 2004; 5:65-75; PMID:14673704; https://doi.org/ 10.1007/s10158-003-0028-y [DOI] [PubMed] [Google Scholar]
- [28].Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci 2003; 6:267-73; PMID:12563263; https://doi.org/ 10.1038/nn1009 [DOI] [PubMed] [Google Scholar]
- [29].Kwon Y, Shim H-S, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci 2008; 11:871-3; PMID:18660806; https://doi.org/ 10.1038/nn.2170 [DOI] [PubMed] [Google Scholar]
- [30].Lilly M, Carlson J. smellblind: a gene required for Drosophila olfaction. Genetics 1990; 124:293-302. PMID:2106470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gong Z, Gong Z. A molecular diffusion based utility model for Drosophila larval phototaxis. Theor Biol Med Model 2012; 9:3; PMID:22300450; https://doi.org/ 10.1186/1742-4682-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci U S A 1996; 93:6079-84; PMID:8650222; https://doi.org/ 10.1073/pnas.93.12.6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamamoto A, Ohba S. Strategic differences in thermal adaptation between two Drosophila species, D. virilis and D. immigrans. Oecologia 1982; 52:333-9; https://doi.org/ 10.1007/BF00367956 [DOI] [PubMed] [Google Scholar]
- [34].Skandalis DA, Stuart JA, Tattersall GJ. Responses of Drosophila melanogaster to atypical oxygen atmospheres. J Insect Physiol 2011; 57:444-51; PMID:21241703; https://doi.org/ 10.1016/j.jinsphys.2011.01.005 [DOI] [PubMed] [Google Scholar]
- [35].Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 1985; 157:263-77; PMID:3939242; https://doi.org/ 10.1007/BF01350033 [DOI] [PubMed] [Google Scholar]
- [36].Sokolowski MB. Social interactions in “simple” model systems. Neuron 2010; 65:780-94; PMID:20346755; https://doi.org/ 10.1016/j.neuron.2010.03.007 [DOI] [PubMed] [Google Scholar]
- [37].Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: A model system for the study of aggression. Proc Natl Acad Sci U S A 2002; 99:5664-8; https://doi.org/ 10.1073/pnas.082102599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet 2000; 34:205-32; PMID:11092827; https://doi.org/ 10.1146/annurev.genet.34.1.205 [DOI] [PubMed] [Google Scholar]
- [39].Cosens D. Blue adaptation: an experimental tool for the study of visual receptor mechanisms and behaviour of Drosophila. Biophys Struct Mech 1979; 5:211-22; PMID:22730594; https://doi.org/ 10.1007/BF00535449 [DOI] [PubMed] [Google Scholar]
- [40].Ritchie MG, Halsey EJ, Gleason JM. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou & Hall cycles in D. melanogaster song. Anim Behav. 1999; 58:649-57; PMID:10479381; https://doi.org/ 10.1006/anbe.1999.1167 [DOI] [PubMed] [Google Scholar]
- [41].Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci 2007; 8:341-54; PMID:17453015; https://doi.org/ 10.1038/nrn2098 [DOI] [PubMed] [Google Scholar]
- [42].Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol 2009; 19:345-53; PMID:19660932; https://doi.org/ 10.1016/j.conb.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nichols CD, Becnel J, Pandey UB. Methods to assay Drosophila behavior. J Vis Exp 2012; 7:3795; PMID:22433384; https://doi.org/ 10.3791/3795PMID:.7629319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol 1995; 356:275-87; PMID:7629319; https://doi.org/ 10.1002/cne.903560210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ormerod KG, Hadden JK, Deady LD, Mercier AJ, Krans JL. Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J Neurophysiol 2013; 110:1984-96; PMID:23904495; https://doi.org/ 10.1152/jn.00431.2013 [DOI] [PubMed] [Google Scholar]
- [46].Clark J, Milakovic M, Cull A, Klose MK, Mercier AJ. Evidence for postsynaptic modulation of muscle contraction by a Drosophila neuropeptide. Peptides 2008; 29:1140-9; PMID:18394755; https://doi.org/ 10.1016/j.peptides.2008.02.013 [DOI] [PubMed] [Google Scholar]
- [47].Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol 1976; 262:189-214; PMID:11339; https://doi.org/ 10.1113/jphysiol.1976.sp011592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hong S-T, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci 2006; 26:7245-56; PMID:16822982; https://doi.org/ 10.1523/JNEUROSCI.5426-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37:911-7; PMID:13671378; https://doi.org/ 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- [50].Churchward MA, Rogasevskaia T, Höfgen J, Bau J, Coorssen JR. Cholesterol facilitates the native mechanism of Ca2+-triggered membrane fusion. J Cell Sci 2005; 118:4833-48; PMID:16219690; https://doi.org/ 10.1242/jcs.02601 [DOI] [PubMed] [Google Scholar]
- [51].Wright EP, Partridge MA, Padula MP, Gauci VJ, Malladi CS, Coorssen JR. Top-down proteomics: enhancing 2D gel electrophoresis from tissue processing to high-sensitivity protein detection. Proteomics 2014; 14:872-89; PMID:24452924; https://doi.org/ 10.1002/pmic.201300424 [DOI] [PubMed] [Google Scholar]
- [52].Churchward MA, Rogasevskaia T, Brandman DM, Khosravani H, Nava P, Atkinson JK, Coorssen JR. Specific lipids supply critical negative spontaneous curvature–an essential component of native Ca2+-triggered membrane fusion. Biophys J 2008; 94:3976-86; PMID:18227127; https://doi.org/ 10.1529/biophysj.107.123984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 2012; 141:2-18; PMID:21823805; https://doi.org/ 10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- [54].Piper MDW, Skorupa D, Partridge L. Diet, metabolism and lifespan in Drosophila. Exp Gerontol 2005; 40:857-62; PMID:16137851; https://doi.org/ 10.1016/j.exger.2005.06.013 [DOI] [PubMed] [Google Scholar]
- [55].Vijendravarma RK, Narasimha S, Kawecki TJ. Effects of parental larval diet on egg size and offspring traits in Drosophila. Biol Lett 2010; 6:238-41; PMID:19875510; https://doi.org/ 10.1098/rsbl.2009.0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A 2007; 104:8253-6; PMID:17494737; https://doi.org/ 10.1073/pnas.0702726104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 2005; 50:447-77; PMID:15355245; https://doi.org/ 10.1146/annurev.ento.50.071803.130404 [DOI] [PubMed] [Google Scholar]
- [58].Armstrong GAB, Meldrum Robertson R. A role for octopamine in coordinating thermoprotection of an insect nervous system. J Therm Biol 2006; 31:149-58; https://doi.org/ 10.1016/j.jtherbio.2005.11.022 [DOI] [Google Scholar]
- [59].Honda T, Lee C-Y, Yoshida-Kasikawa M, Honjo K, Furukubo-Tokunaga K. Induction of associative olfactory memory by targeted activation of single olfactory neurons in Drosophila larvae. Sci Rep 2014; 4:4798; PMID:24762789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Saraswati S, Fox LE, Soll DR, Wu C-F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol 2004; 58:425-41; PMID:14978721; https://doi.org/ 10.1002/neu.10298 [DOI] [PubMed] [Google Scholar]
- [61].Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol 2003; 284:C718-28; PMID:12444020; https://doi.org/ 10.1152/ajpcell.00359.2002 [DOI] [PubMed] [Google Scholar]
- [62].Wierenga JM, Hollingworth RM. Octopamine uptake and metabolism in the insect nervous system. J Neurochem 1990; 54:479-89; PMID:2105376; https://doi.org/ 10.1111/j.1471-4159.1990.tb01897.x [DOI] [PubMed] [Google Scholar]
- [63].Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett 2002; 329:324-8; PMID:12183041; https://doi.org/ 10.1016/S0304-3940(02)00596-7 [DOI] [PubMed] [Google Scholar]
- [64].Certel SJ, Leung A, Lin CY, Perez P, Chiang AS, Kravitz EA. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS One 2010; 5:e13248; PMID:20967276; https://doi.org/ 10.1371/journal.pone.0013248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Garen A, Kauvar L, Lepesant J-A. Roles of ecdysone in Drosophila development (steroid hormone/temperature-sensitive mutants). PNAS 1977; 74:5099-103; PMID:16592466; https://doi.org/ 10.1073/pnas.74.11.5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Horner MA, Pardee K, Liu S, King-Jones K, Lajoie G, Edwards A, Krause HM, Thummel CS. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev 2009; 23:2711-6; PMID:19952106; https://doi.org/ 10.1101/gad.1833609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bouvaine S, T Behmer S, Lin GG, Faure ML, Grebenok RJ, Douglas AE. The physiology of sterol nutrition in the pea aphid Acyrthosiphon pisum. J Insect Physiol 2012; 58:1383-9; PMID:22878342; https://doi.org/ 10.1016/j.jinsphys.2012.07.014 [DOI] [PubMed] [Google Scholar]
- [68].Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, Deanfield J, Descamps OS, Rietzschel ER, Dias KC, et al.. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J 2012; 33:444-51; PMID:22334625; https://doi.org/ 10.1093/eurheartj/ehr441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 2010; 67:373-80; PMID:20696376; https://doi.org/ 10.1016/j.neuron.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Keene AC, Mazzoni EO, Zhen J, Younger MA, Yamaguchi S, Blau J, Desplan C, Sprecher SG. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J Neurosci 2011; 31:6527-34; PMID:21525293; https://doi.org/ 10.1523/JNEUROSCI.6165-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Graf SA, Sokolowski MB. Rover/sitterDrosophila melanogaster larval foraging polymorphism as a function of larval development, food-patch quality, and starvation. J Insect Behav 1989; 2:301-13; https://doi.org/ 10.1007/BF01068057 [DOI] [Google Scholar]
- [72].Sameoto DD, Miller RS. Selection of pupation site by Drosophila melanogaster and D. simulans. Ecology 1968; 49:177-80; https://doi.org/ 10.2307/1933580 [DOI] [Google Scholar]
- [73].Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 2007; 450:294-8; PMID:17994098; https://doi.org/ 10.1038/nature06223 [DOI] [PubMed] [Google Scholar]
- [74].Pandey MB, Singh BN. Effect of biotic and abiotic factors on pupation height in four species of Drosophila. Indian J Exp Biol 1993; 31:912-7; PMID:8112766 [PubMed] [Google Scholar]
- [75].Spassky B. Effect of temperature and moisture content of the nutrient medium on the viability of chromosomal types in Drosophila pseudoobscura. Am Nat 1951; 85:177-80; https://doi.org/ 10.1086/281667 [DOI] [Google Scholar]
- [76].Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab 2010; 12:533-44; PMID:21035763; https://doi.org/ 10.1016/j.cmet.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Heinrichsen ET, Haddad GG. Role of high-fat diet in stress response of Drosophila. PLoS One 2012; 7:e42587; PMID:22870336; https://doi.org/ 10.1371/journal.pone.0042587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bleijerveld OB, Brouwers JFHM, Vaandrager AB, Helms JB, Houweling M. The CDP-ethanolamine pathway and phosphatidylserine decarboxylation generate different phosphatidylethanolamine molecular species. J Biol Chem 2007; 282:28362-72; PMID:17673461; https://doi.org/ 10.1074/jbc.M703786200 [DOI] [PubMed] [Google Scholar]
- [79].McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 2002; 31:151-75; PMID:11988466; https://doi.org/ 10.1146/annurev.biophys.31.082901.134259 [DOI] [PubMed] [Google Scholar]
- [80].Lin Y-H, Chen YC, Kao TY, Lin YC, Hsu TE, Wu YC, Ja WW, Brummel TJ, Kapahi P, Yuh CH, et al.. Diacylglycerol lipase regulates lifespan and oxidative stress response by inversely modulating TOR signaling in Drosophila and C. elegans. Aging Cell 2014; 13:755-64; https://doi.org/ 10.1111/acel.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leung H-T, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 2008; 58:884-96; PMID:18579079; https://doi.org/ 10.1016/j.neuron.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


