ABSTRACT
In Drosophila melanogaster the progenitors of the germ-line stem cells, the primordial germ cells (PGCs) are formed on the outside surface of the early embryo, while the somatic gonadal precursor cells (SGPs) are specified during mid-embryogenesis. To form the primitive embryonic gonad, the PGCs travel from outside of the embryo, across the mid-gut and then migrate through the mesoderm to the SGPs. The migratory path of PGCs is dictated by a series of attractive and repulsive cues. Studies in our laboratory have shown that one of the key chemoattractants is the Hedgehog (Hh) ligand. Although, Hh is expressed in other cell types, the long-distance transmission of this ligand is specifically potentiated in the SGPs by the hmgcr isoprenoid biosynthetic pathway. The distant transmission of the Hh ligand is gated by restricting expression of hmgcr to the SGPs. This is particularly relevant in light of the recent findings that an ABC transporter, mdr49 also acts in a mesoderm specific manner to release the germ cell attractant. Our studies have demonstrated that mdr49 functions in hh signaling likely via its role in the transport of cholesterol. Given the importance of cholesterol in the processing and long distance transmission of the Hh ligand, this observation has opened up an exciting avenue concerning the possible role of components of the sterol transport machinery in PGC migration.
KEYWORDS: cell migration, embryonic patterning, gonad coalescence, Hedgehog signaling, primordial germ cells
Cell migration
Cell migration plays a key role in a variety of normal and pathogenic biologic processes, including embryogenesis, the immune responses, wound healing, and cancer metastasis.1-5 For example, directed cell migration is essential for the proper functioning of mammalian immune systems, as leukocytes must specifically migrate from circulation to tissues infected with bacteria and other microorganisms.6 Another biologic context in which migration plays a central role is during the development of multicellular organisms. Cell migration is required at many steps ranging from the gastrulation of the embryo to the coalescence and morphogenesis of complex tissues and organs.7-9
The process of gonad coalescence can be divided into temporally discrete steps with distinct mechanistic basis
Primordial Germ Cell (PGC) migration and gonad morphogenesis in a Drosophila embryo have provided an excellent context to elucidate mechanisms underlying directed cell motility.10-14 As the PGCs ultimately give rise to the germ-line stem cells, directed migration and proper gonad coalescence are critical for the successful propagation of genetic information. The 2 cell types that populate the embryonic gonad namely the SGPs and the PGCs arise in different regions of the embryo and distinct mechanisms are responsible for their specification. The SGPs are mesodermal in origin and are specified in parasegments 10–13 whereas the PGCs form by precocious cellularization at the posterior pole of blastoderm stage embryo and are determined by maternal factors. To coalesce with the SGPs into a gonad, the PGCs follow a stereotypical trajectory in a temporally coordinated manner. This is a multistep process that begins at gastrulation when the PGCs are carried into the interior of the embryo by the midgut invagination. The PGCs then pass through the midgut epithelium, and move along the dorsal surface of the midgut until they split into 2 groups. The PGCs in each group migrate laterally to come into contact with the gonadal mesoderm on either side of the embryo (Fig. 1A). PGCs align themselves in a row with the SGPs in parasegments 10–13 and these juxtaposed cells coalesce into the embryonic gonad (Fig. 1B).
Figure 1.
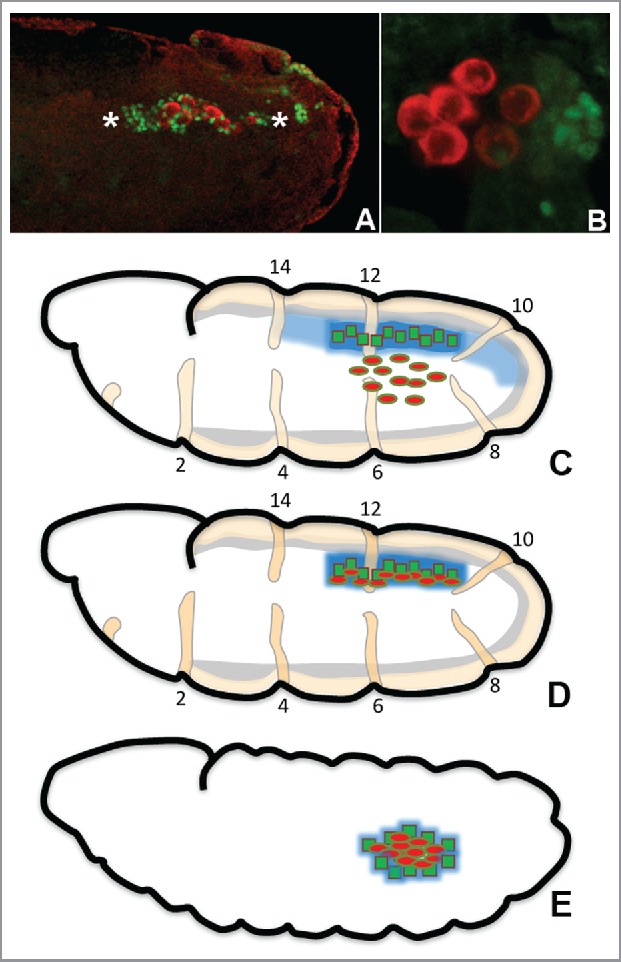
PGCs follow a defined trajectory and temporally discrete steps to reach SGPs which is their ultimate destination. The primitive embryonic gonad coalescence in Drosophila involves directed migration, recognition and sustained association between the 2 cell types, PGCs and SGPs. To coalesce with SGPs, PGCs follow a stereotypical trajectory that begins at gastrulation when the PGCs are carried into the interior of the embryo by the midgut invagination. The PGCs then pass through the midgut epithelium, and move along the surface of the midgut until they split into 2 groups. The PGCs in each group migrate laterally and this brings them into contact with the gonadal mesoderm on either side of the embryo (Panel A). The germ cells align themselves in a row with the SGPs in parasegments 10–13 and these juxtaposed cells coalesce into the embryonic gonad (Panel B), Wild type embryos stained with anti-Eyes absent (DSHB, anti-mouse monoclonal used at 1:10; imaged in Green) and, anti-Vasa (Kind gift from Paul Lasko, anti-rabbit used at 1:1000; imaged in Red) antibodies. Eyes absent antibody labels SGPs while Vasa is a PGC specific marker. In all the panels, embryos are shown with anterior to the left, posterior to the right, dorsal on the top and ventral at the bottom, Panel A: Late stage 13 embryo showing PGCs lined up against SGPs. PGCs (red) lined up against the SGPs (green) are bracketed by 2 asterisk symbols. (For equivalent schematic see panel D), Panel B: Stage 15 embryo showing coalesced gonad consisting of clustered PGCs with intermingled SGPs (For equivalent schematic see panel E), Schematic diagram showing critical steps during germ cell migration, Panel C: PGCs migrating laterally across the mesoderm toward the SGPs at late stage 11 under the attractive influence of the guidance cue (such as Hh), Panel D: PGCs align against the SGPs which are arranged in a linear manner across the para-segments 11–13 by stage 13, Panel E: PGCs and SGPs coalesce into a gonad by stage 15, PGCs: red ellipse; SGPs: green squares; hh (tan) and mdr49 (gray) are expressed in a relatively uniform manner across the mesodermal segments whereas, hmgcr expression (blue) gets restricted to SGPs by stage 12/13 when PGCs are actively migrating toward SGPs. The localized expression underlies the ability of hmgcr to specifically potentiate Hh signal emanating from the SGPs.
Identification of crucial players that ‘entice and repel’
A combination of repulsive and attractive cues guides PGC migration through the midgut and toward the SGPs. Once the PGCs exit the midgut, repulsive clues, generated by Wunen and Wunen2, are thought to direct their bilateral movement on the surface of the midgut.15,16 Subsequently, attractive cues produced by the SGPs guide the PGCs toward the lateral mesoderm and promote their association with the SGPs. One of the first genes implicated in the production of the PGC attractant was hmgcr. In hmgcr mutants, PGCs fail to migrate toward the SGPs and instead either remain associated with the midgut or scatter through the mesoderm. Conversely, ectopic expression in, for example, the nervous system, induces PGCs to migrate toward this tissue.17
hmgcr encodes HMGCoA reductase which is responsible for the synthesis of mevalonic acid. In flies mevalonic acid is a precursor for the biosynthesis of geranylgeranyl-pyrophosphate (GGP) and the genes downstream of hmgcr (fpps and qm) in the synthesis of this isoprenoid are also required for the production of the PGC attractant.18 However, of the genes in this pathway, hmgcr plays the pivotal role in ensuring that the attractant is only “produced” by the SGP. The reason for this is the unique expression pattern of hmgcr. Whereas the downstream isoprenoid biosynthetic genes are broadly expressed in the embryo, hmgcr expression becomes restricted to the SGPs at the time when the PGCs begin migrating toward the SGPs. In spite of its central role in the production of the PGC attractant, the isoprenoid GGP is most probably not used directly in its synthesis. Instead, GGP is used for protein geranylation. Genetic epistasis experiments show that a key target for geranylation in the hmgcr→GGP PGC attractant pathway is the G protein γ subunit 1 (Gγ1) of the heterotrimeric GαGβGγ1 χομπλεξ.19,20 Among other things this complex mediates the intracellular trafficking of membrane vesicles, and the complex must be anchored to the membrane through geranylation of Gγ1 to function.
Another gene implicated in the process of PGC migration encodes the signaling molecule Hedgehog (Hh).20-24 Both ectopic Hh expression and mutations that compromise its production and/or transmission induce mismigration. Since Hh functions as a morphogen in other contexts, an explanation for its effects on PGCs is that it acts indirectly by inappropriately specifying new SGPs. However, arguing against this is the finding that 2 Hh “receptors," patched (ptc) and smoothened (smo) are required in PGCs for proper migration. In the absence of the Hh ligand, the transmembrane receptor Ptc inhibits the 7-pass transmembrane protein Smo from mediating signal transduction. Hh binding to Ptc is thought to relieve the negative influence of Ptc, resulting in the relocalization of Smo to the cell membranes, and this in turn activates the signal transduction cascade downstream of the Hh signal. Consistent with their reciprocal functions in hh signaling, PGCs compromised for ptc or smo activity behave differently. For ptc, the PGCs clump prematurely near the midgut as if they've received sufficient signal while for smo the PGCs behave as if they are ‘signal-blind’ and scatter in the posterior of the embryo.
Localized expression of hmgcr is necessary and sufficient for the potentiation of Hh signaling
Since hh is expressed in a wide range of embryonic tissues and functions in patterning throughout much of development,25-30 a puzzling and clearly critical question is what distinguishes the Hh ligand produced in the SGPs from that in other cell types in the mesoderm and ectoderm. Here one answer seems to be hmgcr and its pivotal role in controlling the level of activity of the hmgcr→GGP→GαGβGγ1 pathway. We found that hmgcr, the downstream genes in the synthesis of GGP (fpps and qm) and, Gγ1 are required for the release of Hh from the basolateral membranes of hh expressing cells in the ectoderm of embryos mutant in one of the hmgcr→ GGP→GαGβGγ1 pathway genes, Hh accumulates in large puncta along the basolateral membranes of the hh expressing cells. Guerrero and coworkers (pers. comm.) have independently shown that this same pathway is critical for the long distance transmission of cholesterol modified Hh from the basolateral membranes in the wing disk.
Several additional findings support the idea that the role of the hmgcr→GGP→GαGβGγ1 pathway in the production of the “PGC attractant” is to potentiate the transmission of hh signals emanating from the SGPs. As was found for gγ1, genetic epistasis experiments place another hh signaling factor, Shifted (Shf), downstream of hmgcr in the “production” of the PGC attractant. Shf is an extracellular protein that interacts with the proteoglycan matrix and is specifically required for the long distance transmission of Hh.31,32 While shf mutations disrupt wing development because they compromise long distance signaling, long distance signaling isn't essential for embryonic patterning and the only phenotype that has been observed in shf mutant embryos is in PGC migration. Like the hmgcr→GGP→GαGβGγ1 pathway, Shf promotes the transmission of cholesterol modified Hh from basolateral membranes of hh expressing cells, but doesn't potentiate the transmission of a mutant form of Hh, Hh-N, that lacks the cholesterol modification and is secreted from apical membranes. Precisely the same specificity is observed when hmgcr is co-expressed in the nervous system with either wild type Hh or Hh-N. The former exacerbates the PGC migration defects induced by hmgcr ectopic expression, while the later doesn't. Finally, visualization of the effects of co-expression of hmgcr and hh in the nervous system using a GFP tagged Hh protein shows that Hmgcr substantially enhances the transmission of Hh-GFP from expressing cells in the nervous system into and through the underlying mesodermal tissue toward the migrating PGCs. Furthermore, Hh-GFP can be seen in direct contact with migrating PGCs, where it is often found concentrated in speckles that are located in close proximity to cellular blebs or protrusions. Interestingly, since the initial discovery that hh guides PGC migration, hh family ligands have been shown to direct cell movement in many other cellular contexts. For example, in mammals, Sonic Hedgehog mediates cell migration and axon guidance during spinal cord development.33-35
Well begun is half done: Efficient release is the key to successful transmission
While the hmgcr isoprenoid biosynthetic pathway potentiates the transmission of the Hh ligand, other mechanisms must also contribute to its production and long distance signaling activity. Intriguingly, in flies and in mammals cholesterol plays a central role in Hh signaling.36-38 First, cholesterol is required for the autoprocessing of the Hh ligand. Second, the resulting covalent cholesterol modification then plays a critical role in the intracellular sorting of the processed Hh peptide, and its subsequent transmission and release from basolateral membranes. Flies are cholesterol auxotrophs and must obtain this sterol through their diet. Consequently hh signaling is sensitive to the levels of dietary cholesterol. If hh functions as a PGC attractant, we would predict that the process of PGC migration would also be sensitive to cholesterol availability. Interestingly this notion is indeed supported by our recently published studies on an ABC transporter, mdr49, which has been implicated in PGC migration.39
Ricardo and Lehmann screened embryos mutant in different ABC transporters for PGC migration defects.40 Of the genes tested, mdr49 was the only transporter that had a significant effect on PGC migration. They found that a moderate loss of function allele called mdr49δ3.16 caused PGC migration defects qualitatively similar to those seen in the hmgcr mutant embryos, and restoration of mdr49 activity by ectopic expression in the mesoderm (where it is normally expressed) was sufficient to rescue the migration defects. Conserved from bacteria to humans, ABC transporters transport hydrophobic, lipophilic compounds. For instance, the ABC transporter Ste6p is required for export of a farnesylated pheromone in budding yeast. Since Mdr49 shares sequence similarity with Ste6p, Ricardo and Lehmann proposed that migrating PGCs might be attracted by an equivalent but unknown isoprenoid-modified peptide that is exported from SGPs by Mdr49.40
The ABC transporters are a very large family and the fly genome encodes many different proteins in this family.41 While Ste6p and Mdr49 share significant sequence similarity (30% identity) there are other fly ABC transporters that are more closely related to Ste6p {CG1824 (40% identity) MRP (33%), CG4562 (32%), CG31789 (32%) and White (32%)} than Mdr49. Conversely, the mammalian transporter Mdr1/P-cg1 is more closely related to Mdr49 than Step 6p (42% identity).42 Among the many small molecules Mdr1/P-cg1 has been reported to translocate across different cellular compartments is cholesterol. Moreover, Tessner and Stenson (2000) have shown that overexpression of Mdr1 upregulates the import of cholesterol in intestinal cells.43 Thus an alternative function for Mdr49 in PGC migration would be to potentiate Hh signaling by providing sufficient levels of cholesterol in SGPs for the processing of the Hh ligand. To explore this possibility, we first asked whether Mdr49 is needed for Hh signaling.
Connecting mdr49 with hh signaling
We have previously shown that different components of germ cell migration pathway also have a role in potentiating Hh signaling in other developmental contexts. We thus sought to assess whether mdr49 can exert similar influence on Hh pathway.
mdr49 suppresses hhMrt
Flies lack the enzymes required to synthesize cholesterol from farnesyl-PP and must obtain this sterol in their diet (18). Since both hh autoprocessing and long distance basolateral hh signaling require the addition of cholesterol to the Hh ligand, we reasoned that the activity of the hh pathway should be sensitive to dietary levels of cholesterol. This prediction is readily tested by taking advantage of a dominant GOF hh allele, hhMoonrat (hhMrt) that disrupts wing development. In WT hh is expressed in the posterior compartment of the wing disk, and functions as a long-distance morphogen to organize wing patterning. hh orchestrates wing development by inducing the expression of downstream targets in the anterior compartment such as decapentaplegic (dpp) and ptc. In hhMrt Hh is inappropriately expressed in the anterior compartment and activates dpp in a manner that causes overgrowth of anterior tissues and partial duplication of distal wing structures.44 Importantly, the phenotypic effects of hhMrt on wing development can be enhanced or suppressed by manipulating other components of the hh pathway like hmgcr or dispatched.
If mdr49 is needed for hh signaling it might be expected to suppress the GOF wing defects of hhMrt. We tested for suppression by using mdr49δ3.16 and a deficiency Df(2R)Exel7123 that uncovers mdr49. Both mutations suppress the hhMrt wing defects. In the case of the deficiency, 80% of the trans-heterozygotes had WT wings compared with less than 10% for the control hhMrt/+.
mdr49 potentiates hh signaling in the wing disk
Dominant suppression of the hhMrt phenotype suggested that loss of mdr49 function should result in reduction in expression of Hh target genes such as ptc and dpp. Indeed expression of mdr49 RNAi using a wing disk specific driver (nubbin Gal4) resulted in reduction in Patched levels. In the converse experiments we also examined if mdr49 overexpression under identical circumstances can promote Hh signaling. We found that overexpression of Mdr49 substantially upregulates the hh target Ptc in the anterior compartment of the wing disk.
mdr49embryos have reduced expression of hh pathway genes likely due to sequestration of Hh ligand
To investigate whether mdr49 functions in hh signaling in the embryo, we examined the expression of Engrailed (En) and Wingless. These 2 genes are components of an hh→wingless (wg)→engrailed (en) autoregulatory loop.45 In this loop, hh expressing cells in each parasegment signal to the neighboring anterior cells to activate Wg expression. Wg in turn signals back to the row of hh expressing cells and upregulates expression of the En transcription factor, which then promotes transcription of hh. When hh signaling is compromised, Wg expression is reduced. Reduced Wg in turn leads to a downregulation of En expression, and the consequent downregulation of hh. We observed that both Wg and En expression is reduced in mdr49δ3.16 compared with the mdr49δ3.16 /+ control embryos suggesting compromised signaling. Based on these findings we hypothesized that as in the case of hmgcr, this is likely due to sub-optimal engagement of Hh ligand in signaling. Consistent with this prediction we found that Hh is sequestered in the hh expressing cells giving rise to a shallower inter-stripe gradient.
Connecting mdr49 to cholesterol and hh signaling
High cholesterol diet suppresses the mdr49 PGC migration defects
Sequestration of Hh in embryos compromised for mdr49 suggest that Mdr49 protein likely potentiates Hh signaling by promoting its release from Hh expressing cells. To connect this to cholesterol transport, we tested whether feeding a high cholesterol diet to mdr49mutant adults could rescue the PGC migration in their progeny. Satisfyingly a high cholesterol diet suppresses the PGC migration defects in mdr49δ3.16 embryos (Fig. 2). It will thus be of interest to determine whether mutations in the different intracellular cholesterol transporters can influence PGC migration. In this regard Niemann Pick disease protein 1a (npc-1a) deserves a special mention. While the precise cause of lethality induced by loss of npc-1a remains to be determined, the lethality can be rescued by a high cholesterol diet.46-48
Figure 2.
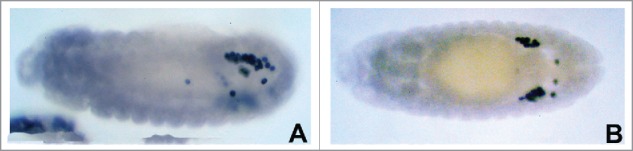
Germ cell migration defects induced by loss of mdr49 are rescued by diet containing excess cholesterol, Either regular medium or medium supplemented with cholesterol was used to raise WT as well as Mdr49 mutant flies. Embryos were collected, fixed with paraformaldehyde and stained with anti-Vasa antibodies to examine the germ cell migration defects. Germ cell migration defects in stage 13–15 embryos were quantified to assess the extent of improvement (39). Panel A: mdr49 embryo (Stage 13) showing germ cell migration defects. More than 10 scattered PGCs are seen in addition to PGCs that have migrated correctly (Vasa positive cells).Panel B: mdr49 embryo derived from flies raised on a cholesterol rich diet. When mdr49 flies are grown on a diet supplemented with excess cholesterol, germ cell migration defects are considerably ameliorated (only 3–4 PGCs are seen away from the gonad). In both the panels, embryos are shown with anterior to the left, posterior to the right.
One speculation for how mdr49 might function in hh signaling (and in PGC migration) is that it mediates the export of cholesterol modified Hh. However since cholesterol is used in Hh autoprocessing, an alternative model (and more likely model given the size of the Hh ligand) is that Mdr49 is needed to ensure that Hh sending cells in the mesoderm have sufficient levels of cholesterol to process the Hh precursor. This idea is supported by the findings of Voght et al. (47), who have suggested that Npc-1a and Npc-1b function in conjunction with an ABC transporter. In this model, the level of autoprocessed Hh or Hh-GFP should be reduced in mdr49 mutants, while this defect should be rescued by a high cholesterol diet. In contrast, the level of Hh-N/Hh-N-GFP should not be sensitive to either mdr49 or dietary cholesterol. Since ABC transporters function in the export of small molecules, it is also possible that cholesterol efflux and/or accumulation in SGPs or elsewhere in the mesoderm will be affected.
Cholesterol, mdr49 and hh: Making connections to a novel long distance signaling mechanism
Patterning of the developing wing disk requires that Hh protein expressed in the posterior compartment travel across many cell diameters to regulate expression of target genes in cells in the anterior compartment. This signal must also be graded across the anterior compartment and the combination of both distance and gradient requires special mechanisms and players to realize. Similar if not more exacting demands are placed on the PGC attractant(s) produced by the SGPs. The attractant must signal the PGCs over very large distances. This signal must also be graded in a manner that conveys directional information to the migrating PGCs. Like Hh signaling in the wing disk, the “classical” mechanism envisioned for a migratory signal would be an extracellular gradient of the attractant with a source and a sink. However, recent studies argue that the morphogenetic gradient is generated by a quite different mechanism–hh expressing cells in the posterior compartment extend Hh containing cytonemes that directly contact receiving cells in the anterior compartment.49 Suggesting that similar mechanisms could be used to communicate directional information to the PGCs, we've found that many of the genes implicated in cytoneme-dependent signaling by Hh also play key roles in the generation and/or transmission of the PGC attractant. In our studies, we have focused on the production/transmission of the PGC attractant. In the recent set of experiments conducted on Mdr49 we explored a newly discovered connection between cholesterol and the production/ transmission of the PGC attractant by the SGPs. Thus, our studies on cholesterol, mdr49 and hh offer the possibility of drawing yet further connections between this novel signaling mechanism and directed cell migration, and as such could have broad implications for cell migration in many other contexts.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
G.D., J.B. and, P.S. were supported by the National Institutes of Health grant [RO1 GM110015]. P.S. is a recipient of a Ministry of Education and Science of the Russian Federation grant. [14. B25.31.0022]. Israel Science Foundation [960/13] and the Legacy Heritage Bio-Medical Program of the Israel Science Foundation [1788/15] supported the research in the Gerlitz laboratory.
References
- [1].Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10:445-57; PMID:19546857; https://doi.org/ 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- [2].Kee Y, Hwang BJ, Sternberg PW, Bronner-Fraser M. Evolutionary conservation of cell migration genes: from nematode neurons to vertebrate neural crest. Genes Dev 2007; 21:391-6; PMID:17322398; https://doi.org/ 10.1101/gad.1509307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704-9; PMID:14657486; https://doi.org/ 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- [4].Franz CM, Jones GE, Ridley AJ. Cell migration in development and disease. Dev Cell 2002; 2:153-8; PMID:11832241; https://doi.org/ 10.1016/S1534-5807(02)00120-X [DOI] [PubMed] [Google Scholar]
- [5].Rorth P. Collective cell migration. Annu Rev Cell Dev Biol 2009; 25:407-29; PMID:19575657; https://doi.org/ 10.1146/annurev.cellbio.042308.113231 [DOI] [PubMed] [Google Scholar]
- [6].Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994; 76:301-14; PMID:7507411; https://doi.org/ 10.1016/0092-8674(94)90337-9 [DOI] [PubMed] [Google Scholar]
- [7].Cai D, Montell DJ. Diverse and dynamic sources and sinks in gradient formation and directed migration. Curr Opin Cell Biol 2014; 30:91-8; PMID:25022255; https://doi.org/ 10.1016/j.ceb.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pocha SM, Montell DJ. Cellular and molecular mechanisms of single and collective cell migrations in Drosophila: themes and variations. Annu Rev Genet 2014; 48:295-318; PMID:25421599; https://doi.org/ 10.1146/annurev-genet-120213-092218 [DOI] [PubMed] [Google Scholar]
- [9].Santos AC, Lehmann R. Germ cell specification and migration in Drosophila and beyond. Curr Biol 2004; 14:R578-89; PMID:15268881; https://doi.org/ 10.1016/j.cub.2004.07.018 [DOI] [PubMed] [Google Scholar]
- [10].Jaglarz MK, Howard KR. The active migration of Drosophila primordial germ cells. Development 1995; 121:3495-503; PMID:8582264 [DOI] [PubMed] [Google Scholar]
- [11].Callaini G, Riparbelli MG, Dallai R. Pole cell migration through the gut wall of the Drosophila embryo: analysis of cell interactions. Dev Biol 1995; 170:365-75; PMID:7649369; https://doi.org/ 10.1006/dbio.1995.1222 [DOI] [PubMed] [Google Scholar]
- [12].Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development 1997; 124:971-82; PMID:9056773 [DOI] [PubMed] [Google Scholar]
- [13].Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development 1995; 121:1815-25; PMID:7600996 [DOI] [PubMed] [Google Scholar]
- [14].Moore LA, Broihier HT, Van Doren M, Lunsford LB, Lehmann R. Identification of genes controlling germ cell migration and embryonic gonad formation in Drosophila. Development 1998; 125:667-78; PMID:9435287. [DOI] [PubMed] [Google Scholar]
- [15].Zhang N, Zhang J, Purcell KJ, Cheng Y, Howard K. The Drosophila protein Wunen repels migrating germ cells. Nature 1997; 385:64-7; PMID:8985246; https://doi.org/ 10.1038/385064a0 [DOI] [PubMed] [Google Scholar]
- [16].Starz-Gaiano M, Cho NK, Forbes A, Lehmann R. Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development 2001; 128:983-91; PMID:11222152 [DOI] [PubMed] [Google Scholar]
- [17].Van Doren M, Broihier HT, Moore LA, Lehmann R. HMG-CoA reductase guides migrating primordial germ cells. Nature 1998; 396:466-9; PMID:9853754; https://doi.org/ 10.1038/24871 [DOI] [PubMed] [Google Scholar]
- [18].Santos AC, Lehmann R. Isoprenoids control germ cell migration downstream of HMGCoA reductase. Dev Cell 2004; 6:283-93; PMID:14960281; https://doi.org/ 10.1016/S1534-5807(04)00023-1 [DOI] [PubMed] [Google Scholar]
- [19].Yi P, Han Z, Li X, Olson EN. The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma1. Science 2006; 313:1301-3; PMID:16857902; https://doi.org/ 10.1126/science.1127704 [DOI] [PubMed] [Google Scholar]
- [20].Deshpande G, Godishala A, Schedl P. Ggamma1, a downstream target for the hmgcr-isoprenoid biosynthetic pathway, is required for releasing the Hedgehog ligand and directing germ cell migration. PLoS Genet 2009; 5:e1000333; PMID:19132091; https://doi.org/ 10.1371/journal.pgen.1000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Deshpande G, Swanhart L, Chiang P, Schedl P. Hedgehog signaling in germ cell migration. Cell 2001; 106:759-69; PMID:11572781; https://doi.org/ 10.1016/S0092-8674(01)00488-3 [DOI] [PubMed] [Google Scholar]
- [22].Deshpande G, Schedl P. HMGCoA reductase potentiates hedgehog signaling in Drosophila melanogaster. Dev Cell 2005; 9:629-38; PMID:16256738; https://doi.org/ 10.1016/j.devcel.2005.09.014 [DOI] [PubMed] [Google Scholar]
- [23].Deshpande G, Sethi N, Schedl P. toutvelu, a regulator of heparan sulfate proteoglycan biosynthesis, controls guidance cues for germ-cell migration. Genetics 2007; 176:905-12; PMID:17409068; https://doi.org/ 10.1534/genetics.107.071415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Deshpande G, Zhou K, Wan JY, Friedrich J, Jourjine N, Smith D, Schedl P. The hedgehog pathway gene shifted functions together with the hmgcr-dependent isoprenoid biosynthetic pathway to orchestrate germ cell migration. PLoS Genet 2013; 9:e1003720; PMID:24068944; https://doi.org/ 10.1371/journal.pgen.1003720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 1994; 76:89-102; PMID:8287482; https://doi.org/ 10.1016/0092-8674(94)90175-9 [DOI] [PubMed] [Google Scholar]
- [26].Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV, et al.. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 1996; 86:21-34; PMID:8689684; https://doi.org/ 10.1016/S0092-8674(00)80074-4 [DOI] [PubMed] [Google Scholar]
- [27].Chen Y, Struhl G. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development 1998; 125:4943-8; PMID:9811578 [DOI] [PubMed] [Google Scholar]
- [28].Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 1994; 368:208-14; PMID:8145818; https://doi.org/ 10.1038/368208a0 [DOI] [PubMed] [Google Scholar]
- [29].Chuang PT, Kornberg TB. On the range of hedgehog signaling. Curr Opin Genet Dev 2000; 10:515-22; PMID:10980429; https://doi.org/ 10.1016/S0959-437X(00)00121-0 [DOI] [PubMed] [Google Scholar]
- [30].Ingham PW. Hedgehog signalling. Curr Biol 2008; 18:R238-41; PMID:18364223; https://doi.org/ 10.1016/j.cub.2008.01.050 [DOI] [PubMed] [Google Scholar]
- [31].Glise B, Miller CA, Crozatier M, Halbisen MA, Wise S, Olson DJ, Vincent A, Blair SS. Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev Cell 2005; 8:255-66; PMID:15691766; https://doi.org/ 10.1016/j.devcel.2005.01.003 [DOI] [PubMed] [Google Scholar]
- [32].Gorfinkiel N, Sierra J, Callejo A, Ibanez C, Guerrero I. The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Dev Cell 2005; 8:241-53; PMID:15691765; https://doi.org/ 10.1016/j.devcel.2004.12.018 [DOI] [PubMed] [Google Scholar]
- [33].Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science 1996; 274, 1123-33; PMID:8895455; https://doi.org/ 10.1126/science.274.5290.1123 [DOI] [PubMed] [Google Scholar]
- [34].Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 2003; 113:11-23; PMID:12679031; https://doi.org/ 10.1016/S0092-8674(03)00199-5 [DOI] [PubMed] [Google Scholar]
- [35].Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 2009; 62:349-62; PMID:19447091; https://doi.org/ 10.1016/j.neuron.2009.03.022 [DOI] [PubMed] [Google Scholar]
- [36].Gallet A, Rodriguez R, Ruel L, Therond PP. Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev Cell 2003; 4:191-204; PMID:12586063; https://doi.org/ 10.1016/S1534-5807(03)00031-5 [DOI] [PubMed] [Google Scholar]
- [37].Dawber RJ, Hebbes S, Herpers B, Docquier F, van den Heuvel M. Differential range and activity of various forms of the Hedgehog protein. BMC Dev Biol 2005; 5:21; PMID:16197551; https://doi.org/ 10.1186/1471-213X-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guerrero I, Chiang C. A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol 2007; 17:1-5; PMID:17126548; https://doi.org/ 10.1016/j.tcb.2006.11.002 [DOI] [PubMed] [Google Scholar]
- [39].Deshpande G, Manry D, Jourjine N, Mogila V, Mozes H, Bialistoky T, Gerlitz O, Schedl P. Role of the ABC transporter Mdr49 in Hedgehog signaling and germ cell migration. Development 2016; 143:2111-20; PMID:27122170; https://doi.org/ 10.1242/dev.133587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ricardo S, Lehmann R. An ABC transporter controls export of a Drosophila germ cell attractant. Science 2009; 323:943-6; PMID:19213920; https://doi.org/ 10.1126/science.1166239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 1992; 8:67-113; PMID:1282354; https://doi.org/ 10.1146/annurev.cb.08.110192.000435 [DOI] [PubMed] [Google Scholar]
- [42].Metherall JE, Li H, Waugh K. Role of multidrug resistance P-glycoproteins in cholesterol biosynthesis. J Biol Chem 1996; 271:2634-40; PMID:8576233; https://doi.org/ 10.1074/jbc.271.5.2634 [DOI] [PubMed] [Google Scholar]
- [43].Tessner TG, Stenson WF. Overexpression of MDR1 in an intestinal cell line results in increased cholesterol uptake from micelles. Biochem Biophys Res Commun 2000; 267:565-71; PMID:10631102; https://doi.org/ 10.1006/bbrc.1999.1996 [DOI] [PubMed] [Google Scholar]
- [44].Felsenfeld AL, Kennison JA. Positional signaling by hedgehog in Drosophila imaginal disc development. Development 1995; 121:1-10; PMID:7867491 [DOI] [PubMed] [Google Scholar]
- [45].Sanson B, Alexandre C, Fascetti N, Vincent JP. Engrailed and hedgehog make the range of Wingless asymmetric in Drosophila embryos. Cell 1999; 98:207-16; PMID:10428032; https://doi.org/ 10.1016/S0092-8674(00)81015-6 [DOI] [PubMed] [Google Scholar]
- [46].Huang X, Suyama K, Buchanan J, Zhu AJ, Scott MP. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development 2005; 132:5115-24; PMID:16221727; https://doi.org/ 10.1242/dev.02079 [DOI] [PubMed] [Google Scholar]
- [47].Voght SP, Fluegel ML, Andrews LA, Pallanck LJ. Drosophila NPC1b promotes an early step in sterol absorption from the midgut epithelium. Cell Metab 2007; 5:195-205; PMID:17339027; https://doi.org/ 10.1016/j.cmet.2007.01.011 [DOI] [PubMed] [Google Scholar]
- [48].Fluegel ML, Parker TJ, Pallanck LJ. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics 2006; 172:185-96; PMID:16079224; https://doi.org/ 10.1534/genetics.105.046565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development 2014; 141:729-36; PMID:24496611; https://doi.org/ 10.1242/dev.086223 [DOI] [PMC free article] [PubMed] [Google Scholar]


