ABSTRACT
Synthesis of sugars from simple carbon sources is critical for survival of animals under limited nutrient availability. Thus, sugar-synthesizing enzymes should be present across the entire metazoan spectrum. Here, we explore the evolution of glucose and trehalose synthesis using a phylogenetic analysis of enzymes specific for the two pathways. Our analysis reveals that the production of trehalose is the more ancestral biochemical process, found in single cell organisms and primitive metazoans, but also in insects. The gluconeogenic-specific enzyme glucose-6-phosphatase (G6Pase) first appears in Cnidaria, but is also present in Echinodermata, Mollusca and Vertebrata. Intriguingly, some species of nematodes and arthropods possess the genes for both pathways. Moreover, expression data from Drosophila suggests that G6Pase and, hence, gluconeogenesis, initially had a neuronal function. We speculate that in insects—and possibly in some vertebrates—gluconeogenesis may be used as a means of neuronal signaling.
KEYWORDS: brain, Drosophila, evolution, gluconeogenesis, glucose, trehalose, glucose-6-phosphatase, NPF, neuronal signaling, trehalose-6-phosphatase
Sugar synthesis pathways
Claude Bernard, the great French physiologist, first reported hepatic glucose production in dogs more than 150 years ago. Ever since, the unique place of gluconeogenesis in blood glucose homeostasis has been well documented in many other vertebrates. Bernard's observation reflects animals' need for tightly controlled glucose levels in the blood, induced by the action of two counteracting hormones, glucagon and insulin, secreted by α- and β− cells of the liver, respectively. When glucose levels are low (i.e., under scarce dietary carbohydrate availability), glucagon is secreted. Glucagon first initiates glycogenolysis, the breakdown of stored glycogen into glucose. When glycogen is depleted, the activity of additional glucagon-dependent enzymes increases to build glucose from more basic carbon sources (lactic acid, amino acids). Insulin is secreted when glucose levels are high, leading ultimately to increase production of glycogen (glycogenesis) and fats (lipogenesis).
Gluconeogenesis involves up to eleven enzymatic reactions (Fig. 1).1 Eight enzymes in this cascade function bi-directionally, also catalyzing the reverse reactions in glycolysis, whereas three enzymes—phosphoenolpyruvate carboxykinase (Pepck), fructose-1, 6-bisphosphatase (FBPase), and glucose-6-phosphatase (G6Pase)—are specific to gluconeogenesis. In insects, where trehalose (a glucose disaccharide) is the main sugar constituent of the circulatory system, synthesis follows the same enzymatic pathway up to the very last step, the production of glucose-6-phosphate (see below).2
Figure 1.
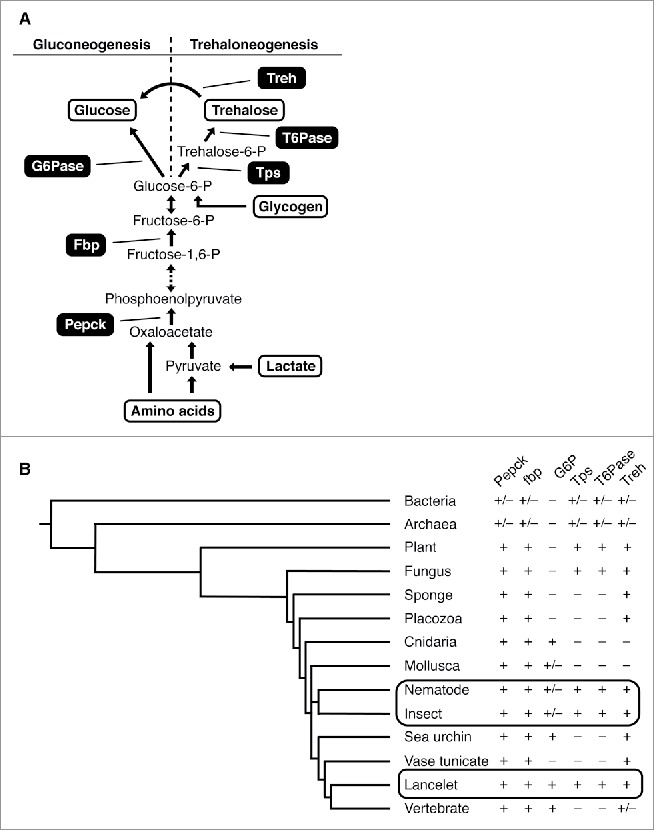
Evolution of gluconeogenesis and trehaloneogenesis (A) Sugar biosynthetic pathways: only the steps of the pathway involving enzymes specific for gluconeogenesis and trehaloneogenesis are shown. Gluconeogenic substrates are shown in red. (B) Presence (+) or absence of key enzymes for gluconeogenesis and trehaloneogenesis across different phyla/animal groups. Boxed area highlights phyla/subphyla in which some species contain both the trehalo- and gluco-neogenic enzymes. Ch indicates subphyla belonging to the phyla of Chordata.
In contrast to mammals, fish, and insects, little is known about the main sugar constituents in the circulatory system of other animal lineages and their biosynthetic pathway(s). By performing a phylogenetic analysis for gluconeogenic and trehaloneogenic enzymes, we show that the basic trehalose synthesizing enzyme machinery is present not only in the most ancient organisms, bacteria, but also in the phyla of Nematoda and Arthropoda. In contrast, G6Pase, the key enzyme for glucose production, first appears in the phylum Cnidaria, the first animals with dedicated sensory neurons (albeit not yet well-developed nervous systems). A gene for G6Pase (G6P) is also present in Echinodermata and most Chordata, including the Vertebrata. Intriguingly, we observed that in a few species, including the fruit fly Drosophila, genes for both pathways are present, but appear to serve different roles: trehaloneogenesis is employed in fat cells for systemic production of trehalose, the main hemolymph sugar. Intriguingly, expression profiling suggests that the production of glucose occurs mainly in the brain, and we propose that this process is used for neural signaling, rather than systemic glucose production. Together, our analysis provides new insights into the evolutionary history of these important biochemical processes.
Gluconeogenic and trehaloneogenic enzymes
Gluconeogenesis has been studied mostly in mammals, some fish, and a few other vertebrates, where it is the critical anabolic regulatory pathway for glucose homeostasis under restrictive dietary carbohydrate availability.3 Gluconeogenesis is initiated after glucacon secretion, for example, in response to dietary carbohydrate restriction, and is largely restricted to the liver and, to a lower extent, to kidneys and intestine.4,5 In addition to mammals and fish, insects represent the only other major animal class where sugar homeostasis has been studied in some detail. The main circulatory sugar of insects is trehalose, albeit in some species, the hemolymph also contains lower amounts of glucose and fructose.6 Similar to mammals, insects adjust trehalose levels in the fat body (the insect liver) through hormonal secretion of insulin-like peptides (ILPs) and adipokinetic hormone (AKH, a glucagon ortholog) from neuroendocrine cells in the brain.7 Trehaloneogenesis follows the same enzymatic cascade as gluconeogenesis, up until the last common substrate, glucose 6-phosphate (Fig. 1A). Instead of dephosphorylation, glucose 6-phosphate is converted via two tightly linked enzymatic steps involving trehalose-6-phosphate synthase (Tps) and trehalose-6-phosphatase (T6Pase) into trehalose. Trehalose is then distributed via the hemolymph to cells in need of energy, where it is converted into glucose by trehalase (Treh). Thus, animals with trehalose as their main energy carrier in their hemolymph are expected to have genes for these respective enzymes (Tps, T6Pase and Treh).
Interestingly, trehalose is a major cellular constituent in organisms other than insects, including bacteria, yeast and some metazoan phyla.8 While trehalose synthesis has been well studied in a few bacterial model systems, no systematic phylogenetic analysis of its biosynthetic pathway has been performed. We therefore investigated the evolutionary orgin of enzymes necessary for both trehaloneogenesis and gluconeogenesis (Fig. 1A).
Identification of genes encoding sugar synthesizing enzymes
When amino acid sequences of Pepck and Fbp enzymes which are common to both sugar synthetic pathways from S. cerevisiae and D. melanogaster were queried against DNA and protein databases of 125 different species of metazoa, fungi, archaea, bacteria, and plants (Fig. 1B; Supplemental Table 1), we found orthologous enzymes in at least some species from all phyla, in addition to those known from previous studies (Archaea and Bacteria). This indicates that sugar biosynthesis is an enzymatic pathway important for species across most if not all animal phyla.
BLAST searches using human and Drosophila G6Pase protein sequences revealed that the phylogenetic spectrum of organisms possessing an orthologous gene was confined to higher animals (all Chordata with the exception of the single representative of Tunicata), many, but not all, arthropods and mollusks, and, intriguingly, hydra and jellyfish, representatives of the phylum Cnidaria. In contrast, BLAST searches using protein sequences of Tps and T6Pase from S. cerevisiae and D. melanogaster identified a roughly, but not entirely, complementary phyla representation, compared to G6Pase harboring species. Genes encoding Tps and T6Pase were found in the most ancient domains (Bacteria, Archaea) and kingdoms (Planta and Fungi), and they were also present in some non-chordate phyla (Nematoda, and Arthropoda). However, they were absent in the more primitive Porifera and Placozoa, as well as the more advanced Mollusca, Echinodermata and Chordata, with the exception of the single representative of lancelets (subphylum Cephalochordata). Thus, the appearance of enzymes necessary for trehaloneogenesis (Tps, T6Pase, and Treh) preceded that of enzymes necessary for gluconeogenesis (G6Pase). The fact that the entire set of trehaloneogenic genes appeared in single cell organisms, long before metazoan with complex circulatory systems evolved, indicates that trehalose served processes other than maintaining sugar/energy homeostasis.
Non-classical role of trehaloneogenesis
In both bacteria and yeast, trehalose is a constituent of the cytoplasm, and, as such, it not only serves as a nutrient, but also as an important substrate to counter stress. In E. coli and S. cerevisiae, low temperature or humidity induces production of trehalose, which protects proteins and other cellular components from damage incurred by freezing and dehydration, respectively.9,10,11 Thus, the initial role of trehalose biosynthesis was likely to increase trehalose concentration for counteracting external stresses.
Trehalose can ameliorate these and other stresses [such as the presence of reactive oxygen species (ROS) and osmotic shock] also in arthropods, as well as some plants. Interestingly, in some of these organisms, it was shown that certain stress signals lead to upregulation of enzymes necessary for trehaloneogenesis.8 In addition, trehalose and trehaloneogenic intermediates (such as trehalose 6-phosphate) have been postulated to function as signaling molecules during development in both plants and worms.12 For example, in A. thaliana, mutations in TPS1 lead to embryonic lethality.13 In contrast, Tps1 mutant C. elegans have no discernable phenotype, whereas T6Pase mutant worms die in the early larval stage.14 These observations suggest that trehalose 6-phosphate may function as a signaling molecule, whereby lack of it in Arabidopsis or inappropriate accumulation in the worm larvae interferes with proper development. Thus, as evolution proceeded to generate more complex life forms, the trehaloneogenic pathway acquired additional roles in modulating/controlling stress-response pathways in animals, as well as in regulating early development.
Trehaloneogenesis: The main pathway for sugar homeostasis in insect
In more complex animals, such as vertebrates and insects, the main and primary function of gluconeogenesis and trehaloneogenesis is to maintain sugar homeostasis when dietary carbohydrates are limited. Surprisingly, some insects, including fruit flies and mosquitos, possess the genes for both pathways (Fig. 1B, Table S1). To further investigate the potential functional relevance for this duplicity, we took advantage of expression data available for Drosophila melanogaster (http://flybase.org) and the mosquito A. gambiae (http://mozatlas.gen.cam.ac.uk/mozatlas/). In the fly, the trehaloneogenic-specific enzymes encoded by tps1 (Drosophila tps1 gene encodes a fusion protein of Tps and T6Pase) and enzymes shared by both pathways (Pepck and Fbp) are abundantly expressed in the fat body, while expression of G6P is found exclusively in the brain (Fig. 2A), a profile that is also supported by expression data from mosquitoes (data not shown). Moreover, both Pepck and Fbp are not only expressed in the fat body, but also found in the brain, at levels similar to those of G6P. While these data are consistent with the dogma that trehaloneogenesis, and not gluconeogenesis, is the mechanism for maintaining sugar homeostasis in these insects,7 they also suggest that glucose can be produced in the insect brain through the gluconeogenic pathway.
Figure 2.
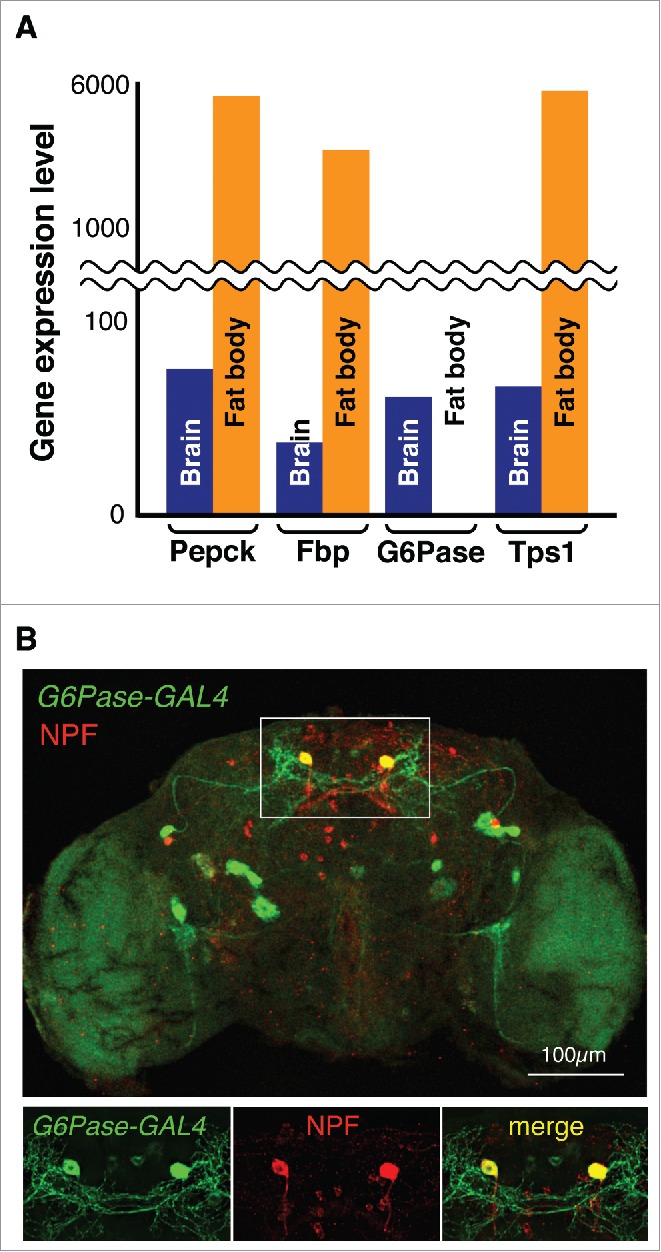
Expression of gluconeogenic and trehaloneogenic genes in D. melanogaster (A) Expression profile obtained from Flybase (http://flybase.org). Note that Drosophila Tps1 gene encodes a fusion protein of Tps and T6Pase.5 (B) G6P-GAL4 expression in the Drosophila brain. UAS-mCD8GFP was used to visualize GAL4 expression (green), while NPF (Red) was detected using an anti-NPF antibdoy.
Novel non-systemic roles for gluconeogenesis
The oldest phylum in which a functional G6P gene is present is Cnidaria, the first animals with diverse types of organized cell populations, including sensory neurons (Fig. 1B). This notion, and the observation that G6P is expressed in an apparent brain-specific fashion in flies and mosquitoes raises the intriguing possibility that gluconeogenesis may have evolved initially as a neuron-specific process. To evaluate the expression profile of gluconeogenic genes in the fly at the cellular level, we examined their expression using the bipartite GAL4/UAS expression system. Indeed, examination of reporter activity in G6P-GAL4/UAS-GFP flies revealed that G6P is expressed in only about 20 neurons in the brain. Moreover, a gene reporter for another gluconeogenic enzyme (fbp-GAL4) showed an overlapping, albeit not identical, pattern of expression (data not shown), suggesting that the cells co-expressing the two genes are gluconeogenesis-competent. Most remarkably, four G6P-GAL4 and Fbp-GAL4 expressing neurons also express neuropeptide F (NPF; Fig. 2B), the insect ortholog of mammalian neuropeptide Y (NPY). Given this small number of cells, it seems unlikely that their purpose is the production of glucose for systemic dissemination; rather, we speculate that gluconeogenesis is used as a cell-intrinsic, internal signaling mechanism, possibly to regulate NPF signaling, in these neurons.
NPY has received broad attention due to its ability to modulate the activity of many organ systems, including the control of food intake regulated in part by the hypothalamus.15 Numerous studies in rodents led to the identification of hypothalamic NPY expressing neurons (as well as cells expressing other neurohormones) whose activity is modulated by changes in external glucose concentrations.16 Glucose mediated signaling by at least some of these neurons is thought to regulate pancreatic/hepatic insulin/glucagon secretion, as well as modulate the activity of thalamus neurons involved in reward seeking behavior.17 Moreover, key gluconeogenic genes were expressed in the brain of many mammals,18-21 but their relationship to NPF-/glucose-responsive neurons remains to be examined. In conclusion, our phylogenetic analysis, in combination with numerous experimental studies on the role of trehalose in bacteria, yeast, C. elegans, and plants, strongly argues for multifaceted roles of gluco- and trehalo-neogenesis. The Drosophila model system is well suited for investigating the postulated novel role of gluconeogenesis in the brain and might stimulate exploration of related studies in mammals.
Methods
Bioinfomatics: Presence of a gene was determined by BLASTP or TBLASTN using sequences from H. sapiens, D. melanogaster, and S. cerevisiae as queries. Genes with the total score ≥80 were defined as orthologues. Representative species for each category, out of more than 100 analyzed, were: Escherichia coli, Anabaena cylindrical, Rhodopirellula sp., Synechococcus sp and Terriglobus saanensis (Bacteria), Agaricus bisporus, Botrytis cinerea, Millerozyma farinose, Saccharomyces cerevisiae and Torulaspora delbrueckii (Fungi), Arabidopsis thaliana, Chlamydomonas reinhardtii, Oryza sativa, Physcomitrella patens and Zea mays (Plants), Amphimedon queenslandica (Sponges), Trichoplax adhaerens (Placozoa), Hydra vulgaris and Nematostella vectensis (Cnidaria), Aplysia californica and Octopus bimaculoides (Mollusca), Ancylostoma ceylanicum, Caenorhabditis elegans, Heterodera glycines, Loa loa and Trichinella spiralis (Nematodes), Aedes aegypti, Apis mellifera, Bombyx mori, Drosophila melanogaster and Tribolium castaneum (Insects), Strongylocentrotus purpuratus (Sea urchin), Ciona intestinalis (Vase tunicate), Branchiostoma floridae (Lancelet), Danio rerio, Gallus gallus, Homo sapiens and Xenopus tropicalis (Vertebrate).
Immunostaining: Immunostaining experiments were performed as previously described.14 Anti-NPF and anti-GFP were obtained from RayBiotech, Inc. and Novus Biologicals USA, respectively. Anti-rabbit Alexa Fluor 488 and anti-chicken Alexa Fluor 555 from Molecular Probes were used as secondary antibodies.
Molecular Biology/Transgenesis: A fragment containing 676 nt sequence upstream of the G6P ATG initiation codon was PCR amplified form genomic DNA and cloned into the pCaSpeR4 transformation vector to generate the G6P-GAL4 construct. Embryo injections were carried out by BestGene (San Diego). UAS-mCD8GFP and G6P-GAL4 were combined in the same flies to visualize G6P-GAL4 expression.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowlegements
We thank Chika Miyamoto for help with the blast searches and Drs. R. Sitcheran and J. Karpac for comments on the manuscript.
Funding
This work was supported by a grant from the NIH (5R01DC013967) to H.A.
References
- [1].Sharabi K, Tavares CD, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med 2015; 46:21-33; PMID:26549348; https://doi.org/ 10.1016/j.mam.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thompson S. The Insect ‘Blood’ SUgar. Adv In Insect Phys 2003; 31:205-85. [Google Scholar]
- [3].Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B 2012; 182:1015-45; PMID:22476584; https://doi.org/ 10.1007/s00360-012-0658-7 [DOI] [PubMed] [Google Scholar]
- [4].Corssmit EP, Romijn JA, Sauerwein HP. Review article: Regulation of glucose production with special attention to nonclassical regulatory mechanisms: a review. Metabolism 2001; 50:742-55; PMID:11436176; https://doi.org/ 10.1053/meta.2001.24195 [DOI] [PubMed] [Google Scholar]
- [5].Mithieux G. The new functions of the gut in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care 2005; 8:445-9; PMID:15930972; https://doi.org/ 10.1097/01.mco.0000172587.17385.aa [DOI] [PubMed] [Google Scholar]
- [6].Fell RD. The qualitative and quantitative analysis of insect hemolymph sugars by high performance thin-layer chromatography. Comp Biochem Physiol Part A Physiol 1990; 95:539-44; https://doi.org/ 10.1016/0300-9629(90)90735-B [DOI] [Google Scholar]
- [7].Owusu-Ansah E, Perrimon N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: implications for aging and metabolic diseases. Dis Model Mech 2014; 7:343-50; PMID:24609035; https://doi.org/ 10.1242/dmm.012989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology 2003; 13:17R-27R; PMID:12626396; https://doi.org/ 10.1093/glycob/cwg047 [DOI] [PubMed] [Google Scholar]
- [9].Petitjean M, Teste MA, Francois JM, Parrou JL. Yeast Tolerance to various stresses relies on the trehalose-6P synthase (Tps1) protein, not on trehalose. J Biol Chem 2015; 290:16177-90; PMID:25934390; https://doi.org/ 10.1074/jbc.M115.653899 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [10].Phadtare S. Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 2004; 6:125-136; PMID:15119823 [PubMed] [Google Scholar]
- [11].Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 1990; 58:209-17; PMID:2256682; https://doi.org/ 10.1007/BF00548935 [DOI] [PubMed] [Google Scholar]
- [12].Erkut C, Kurzchalia TV. The C. elegans dauer larva as a paradigm to study metabolic suppression and desiccation tolerance. Planta 2015; 242:389-96; PMID:25868548; https://doi.org/ 10.1007/s00425-015-2300-x [DOI] [PubMed] [Google Scholar]
- [13].Eastmond PJ, van Dijken AJ, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JD, Smeekens SC, Graham IA. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 2002; 29:225-35; PMID:11851922; https://doi.org/ 10.1046/j.1365-313x.2002.01220.x [DOI] [PubMed] [Google Scholar]
- [14].Kormish JD, McGhee JD. The C. elegans lethal gut-obstructed gob-1 gene is trehalose-6-phosphate phosphatase. Dev Biol 2005; 287:35-47; PMID:16197937; https://doi.org/ 10.1016/j.ydbio.2005.08.027 [DOI] [PubMed] [Google Scholar]
- [15].Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: the universal soldier. Cell Mol Life Sci 2003; 60:350-77; PMID:12678499; https://doi.org/ 10.1007/s000180300029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci 2005; 360:2227-35; PMID:16321792; https://doi.org/ 10.1098/rstb.2005.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karnani M, Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol 2011; 300:R47-55; PMID:21048078; https://doi.org/ 10.1152/ajpregu.00527.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cloix JF, Beaulieu E, Hevor T. Various fructose-1,6-bisphosphatase mRNAs in mouse brain, liver, kidney and heart. Neuroreport 1997; 8:617-22; PMID:9106734; https://doi.org/ 10.1097/00001756-199702100-00008 [DOI] [PubMed] [Google Scholar]
- [19].Ghosh A, Cheung YY, Mansfield BC, Chou JY. Brain contains a functional glucose-6-phosphatase complex capable of endogenous glucose production. J Biol Chem 2005; 280:11114-9; PMID:15661744; https://doi.org/ 10.1074/jbc.M410894200 [DOI] [PubMed] [Google Scholar]
- [20].Velasquez ZD, Perez M, Moran MA, Yanez AJ, Avila J, Slebe JC, Gomez-Ramos P. Ultrastructural localization of fructose-1,6-bisphosphatase in mouse brain. Microsc Res Tech 2011; 74:329-36; PMID:20687127 [DOI] [PubMed] [Google Scholar]
- [21].Zimmer DB, Magnuson MA. Immunohistochemical localization of phosphoenolpyruvate carboxykinase in adult and developing mouse tissues. J Histochem Cytochem 1990; 38:171-8; PMID:1688895; https://doi.org/ 10.1177/38.2.1688895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


