Abstract
Bangladesh has a population with a low gastric cancer risk but high prevalence of Helicobacter pylori infection. Several studies have examined virulence genes in H. pylori from Bangladesh. We analyzed cagA and vacA subtypes and their association with severe histology phenotypes, and analyzed population types among Bangladeshi strains. We included patients who underwent endoscopy in Dhaka. Sequences of virulence genes and seven housekeeping genes were obtained by next generation sequencing and confirmed by Sanger sequencing. We isolated 56 H. pylori strains from 133 patients, of which 73.2% carried cagA, and all were considered Western-type. Patients infected with cagA-positive strains had more severe histological scores than patients infected with cagA-negative strains. Among vacA s1 and m1 genotypes, the s1a (97.8%, 43/44) and m1c (28/30, 93.3%) genotypes were predominant. All strains containing s1 and m1 (30/56, 53.6%) also had i1, d1, and c1. In contrast, all strains containing the less-virulent genotypes s2 and m2 (12/56, 21.4%) also possessed i2, d2, and c2. Multivariate analysis indicated that subjects infected with vacA m1-genotype strains only had a significantly higher risk of antrum atrophy than patients infected with m2-genotype strains. Of the two main H. pylori populations in this study, hpAsia2 strains were associated with higher activity and inflammation in the antrum compared to hpEurope strains; however, only vacA s1m1i1d1c1 strains, independent of population type, were significantly associated with inflammation in the antrum, unlike the s2m2i2d2c2 genotype. In conclusion, Bangladeshi strains were divided into two main populations of different genotypes. The low incidence of gastric cancer in Bangladesh might be attributable to the high proportion of less-virulent genotypes, which may be a better predictor of gastric cancer risk than the ancestral origin of the H. pylori strains. Finally, the vacA m region may be a better virulence marker than other regions.
Introduction
Helicobacter pylori, a gram-negative bacterium responsible for several gastroduodenal diseases, produces a number of virulence factors that are essential for colonization of the stomach and survival in the hostile gastric environment [1]. A well-known virulence factor is cagA, which encodes CagA, a 120–145-kDa immunodominant protein [2]. CagA activates a number of signal transduction pathways that are involved in binding and perturbing the function of epithelial junctions, resulting in aberrations in tight junction function, cell polarity, and cellular differentiation [3]. H. pylori strains can be categorized as cagA-positive or -negative. CagA expression in cagA-positive strains has been associated with inflammation and an increased risk for more severe clinical outcomes when compared to cagA-negative strains in H. pylori-infected patients [1]. cagA-positive strains that have EPIYA motifs, which are tyrosine-phosphorylated by Src and Abl family kinases, impair a variety of intracellular signaling systems after they infect gastric epithelial cells [4]. An increased risk of gastric cancer is observed in individuals infected with strains possessing cagA with an EPIYA-D segment (an East Asian-type cagA-positive strain) than with strains possessing an EPIYA-C segment (a Western-type cagA-positive strain) [1].
The CagA multimerization (CM) sequence is located within the EPIYA-C segment (FPLKRHDKVDDLSKVG) and, downstream of the EPIYA-D segment (FPLRRSAAVNDLSKVG). The type and number of CM motifs may affect the potential for multimerization of individual CagA proteins in host cells, which may, in turn, affect the ability of CagA to disturb host cell function via Src homology region 2-containing protein tyrosine phosphatase 2 (SHP2) deregulation [5]. An amino acid sequence alignment of the pre-EPIYA region of cagA, located approximately 300-bp upstream of the first EPIYA motif, revealed that a 39-bp deletion was present in most strains isolated from East Asia. This deletion was absent in most strains from Western countries, which were subsequently denoted as the no-deletion type of H. pylori [6, 7].
Virulent strains of H. pylori also produce vacuolating cytotoxin A (VacA), which enters host cells via endocytosis and induces multiple cellular activities, including membrane channel formation, cytochrome c release from mitochondria leading to apoptosis, and binding to cell membrane receptors that initiates a proinflammatory response [8]. The gene encoding vacA displays allelic diversity, including diversity in the signal (s) regions s1 and s2 and in the middle (m) regions m1 and m2. Based on in vitro experiments, s1m1 strains are the most cytotoxic, as they consistently induce cell vacuolation. s1m2 strains do not consistently induce cell vacuolation, and s2m2 strains show no cytotoxic activity [1]. The s1 and m1 types can be further subdivided into s1a, s1b, and s1c, and m1a, m1b, and m1c, respectively [1].
The intermediate (i) region is a third disease-related region of vacA. It is located between the s region and the m region and is divided in two subtypes: i1 and i2. It has been suggested that the i region may be a better predictor of disease severity than either the s or the m region [9, 10]. The deletion (d) region, located between the i and m regions, remains poorly studied. The 69–81-bp deletion (d2) type was reported to be less virulent than the no-deletion (d1) type [11]. One study reported that the frequency of the vacA d1 genotype was 43.4% and that its frequency was significantly higher in H. pylori isolates from patients with peptic ulcer disease (71.4%) than in isolates from patients with gastritis (27.4%) [12]. Recently, a novel polymorphic site in the 3′ end of vacA, denoted as c1 and c2 (with c1 being more virulent than c2), was reported to be a strong predictor of gastric cancer in male patients [13].
Multi-locus sequence typing (MLST) using seven housekeeping genes identified seven population types: hpEurope, hpEastAsia, hpAfrica1, hpAfrica2, hpAsia2, hpNEAfrica, and hpSahul; these strains reflect geography and human migration out of Africa [14–16]. Interestingly, in addition to virulence factors, population types were reported to be closely associated with the incidence of gastric cancer and partly explained the “Asian enigma,” the phrase that refers to an area with a low incidence of gastric cancer despite a high prevalence of H. pylori infection [17]. A previous study suggested that a European-origin strain (hpEurope) was associated with premalignant histological lesions, while infection with African strains (hpAfrica1) was not [18, 19]; however, there have been no previous studies focusing on the relationship between the phylogeographic origin of H. pylori and gastric cancer risk in Asia, particularly for hpAsia2 [17].
Bangladesh is a South Asian country with a population of more than 160 million, making it the world's eighth most populous country. The prevalence of H. pylori infection is reportedly high (60.2%), which is similar to that of other developing countries (53.8% among those aged 12–19 years) [20]. However, the age-standardized incidence rate (ASR) of gastric cancer in Bangladesh is low for Asian countries (5.4/100,000; available from the International Agency for Research on Cancer; GLOBOCAN2012, http://globocan.iarc.fr/). Several studies have characterized Bangladeshi strains based on their cagA and vacA status [21–23], but there have been few studies that have analyzed the association of cagA or vacA subtypes with histological scores of disease severity. Therefore, in this study, we analyzed cagA and vacA subtypes in Bangladeshi strains and their association with severe histological scores. Moreover, we analyzed Bangladeshi strains using seven housekeeping genes and identified two predominant populations. The results of this study might explain the low gastric cancer prevalence in Bangladesh.
Materials and methods
Patients
This study enrolled patients who underwent endoscopy at Dhaka Medical College in November 2014. Exclusion criteria were the following: a history of partial gastric resection; previous eradication therapy for H. pylori; or treatment with bismuth-containing compounds, H2-receptor blockers, or proton pump inhibitors (PPI) in the previous four weeks. Experienced endoscopists collected gastric biopsy specimens during each endoscopy session, including two samples (for culture and histology) from the lesser curvature of the antrum approximately 3 cm from the pyloric ring and one sample from the greater curvature of the corpus (for histology). Peptic ulcer diseases, including gastric and duodenal ulcers, were diagnosed by endoscopic observation, while chronic gastritis was diagnosed by histologic examination. Biopsy specimens for bacterial culture were immediately placed at −20°C and stored at −80°C within a day of collection for later use. Written informed consent was obtained from all participants, and the protocol was approved by the Ethics Committee of the Bangladesh Medical Research Council (BMRC), Dhaka, Bangladesh, and the Oita University Faculty of Medicine, Japan.
Histology and immunohistochemistry
One antrum and one corporal specimen from each patient was histologically examined. Biopsy materials were fixed in 10% buffered formalin and embedded in paraffin. Serial sections were stained with hematoxylin and eosin, as well as with May and Giemsa stains. The degree of inflammation, neutrophil activity, atrophy, intestinal metaplasia, and bacterial density were classified into four grades, in accordance with the updated Sydney system: 0, ‘normal’; 1, ‘mild’; 2, ‘moderate’; and 3, ‘marked’ [24]. Samples with grades of 1 or higher of atrophy were considered atrophy-positive. In addition, the gastritis stage was assessed based on the topographic location (antrum or corpus) of atrophy according to the Operative Link on Gastritis Assessment (OLGA) system [25].
Immunohistochemistry was performed as previously described [26]. Briefly, after antigen retrieval and inactivation of endogenous peroxidase activity, tissue sections were incubated overnight at 4°C with anti-H. pylori antibody (Dako, Glostrup, Denmark), anti-CagA antibody (b-300, Santa Cruz Biotechnology, Dallas, TX, USA), or anti-East Asian-type CagA-specific antibody (α-EAS Ab), which was immunoreactive only with the East Asian-type CagA strains and not with Western-type CagA strains [26], diluted 1:2000 (Dako). After washing, the sections were incubated with biotinylated goat anti-rabbit or anti-rat IgG (Nichirei Co., Tokyo, Japan), which was followed by an incubation with a solution of avidin-conjugated horseradish peroxidase (Vectastain Elite ABC Kit; Vector Laboratories Inc., Burlingame, CA, USA). Peroxidase activity was detected using an H2O2/diaminobenzidine substrate solution.
H. pylori isolation and genotyping
For H. pylori culture, antral biopsy specimens were homogenized and inoculated onto antibiotics selection plates and were subsequently subcultured on Mueller Hinton II Agar medium (Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with 10% horse blood without antibiotics (Nippon Biotest Laboratories Inc., Tokyo, Japan). The plates were incubated for up to 10 days at 37°C under microaerophilic conditions (10% O2, 5% CO2, and 85% N2). H. pylori isolates were identified based on colony morphology; Gram staining results; and oxidase, catalase, and urease reactions. Isolated strains were stored at −80°C in Brucella Broth (Difco, Franklin Lakes, NJ, USA) containing 10% dimethyl sulfoxide and 10% horse serum (Nippon Biotest Laboratories Inc., Tokyo, Japan).
Genomic DNA was extracted from confluent plate cultures expanded from a single colony of H. pylori using a commercially available kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s directions. DNA samples were subjected to next generation sequencing (NGS) (Illumina, Inc., San Diego, CA). MiSeq output was used to create contig sequences using CLC Genomics Workbench 7.0.4 (CLC Bio-Qiagen, Aarhus, Denmark). Genomics Workbench was also used to analyze seven housekeeping genes (atpA, efp, mutY, ppa, trpC, ureI, and yphC), full-length cagA, and full-length vacA, with sequences confirmed by polymerase chain reaction (PCR) amplification and direct sequencing, as described previously [27]. DNA sequencing was performed using a Big Dye Terminator v3.1 Cycle Sequencing Kit on an AB 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Analysis of the H. pylori population structure
Seven multi-locus sequence types of genes in the genomic DNA of Bangladeshi strains and 430 representative MLST sequences representing various populations were downloaded from the PubMLST database (http://pubmlst.org/). A neighbor-joining tree (Kimura’s two-parameter model) was constructed using the dataset. Next, using similar strains, we analyzed the bacterial population structure using STRUCTURE (v.2.3.2) software [28]. Markov Chain Monte Carlo (MCMC) simulations were run in STRUCTURE using a non-admixture model with a burn-in of 20,000, followed by 30,000 iterations for each run.
Data analysis
Discrete variables were tested using a chi-square test; continuous variables were tested using Mann-Whitney U tests. A multivariate logistic regression model was used to calculate the odds ratios (OR) of various clinical outcomes, with age, sex, and H. pylori genotypes as predictors. All determinants with P values less than 0.10 were included in the full logistic regression model, and the model was reduced by excluding variables with P values greater than 0.10. OR and 95% confidence intervals (CI) were used to estimate risk. A P value less than or equal to 0.05 was considered statistically significant. SPSS statistical software package version 18.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Prevalence of H. pylori
From 133 consecutive patients (61 males and 72 females; age range, 18 to 65 years; mean age, 35.2 ± 11.8 years), a total of 56 H. pylori strains were isolated: 38 from patients living in Dhaka city and 18 from patients living in the village outside of Dhaka city. All of the patients were ethnically Bengali. Isolates were obtained from 26 male (age range, 18 to 56 years; mean age, 34.2 ± 11.6 years) and 30 female patients (age range, 19 to 65 years; mean age 36.1 ± 12.1 years). Of these patients, 53 had chronic gastritis, and three had peptic ulcer diseases. No association between age, sex, and diagnosis was found (P >0.05).
Virulence genes
In total, 41 of 56 strains possessed cagA (73.2%). cagA in two strains was not detected by either NGS or PCR; however, these strains contained partial cag pathogenicity island (PAI) genes, as confirmed by PCR of the cag PAI empty site. Therefore, we considered these strains to be “cagA undetermined”. Thirty-nine of 41 strains positive for cagA were confirmed to be immunoreactive with anti-CagA antibody by immunohistochemistry.
Based on NGS results, which were confirmed by PCR-based sequence analyses, the cagA genotypes included 27 ABC, 8 ABCC, 2 ABCCC, and 1 ABBC types (Table 1). The remaining three strains were AB type. All of the strains were considered Western-type cagA. The AB type was regarded as Western-type cagA based on the similarities of its B segment sequences with those of Western-type cagA sequences [29]. In agreement with the sequencing results, all cagA-positive strains showed no immunoreactivity to the α-EAS Ab, which is specific for East Asian-type cagA.
Table 1. Helicobacter pylori virulence factors and clinical outcome.
| Description of genotype | Total | Gastritis | Peptic ulcer disease | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Total studied | 56 | 53 | 3 | |||
| Mean age (yr) | 35.2 ± 11.8 | 35.4 ± 11.9 | 31.7 ± 10.4 | |||
| Sex | ||||||
| Female | 30 | 53.6% | 28 | 93.3% | 2 | 6.7% |
| Male | 26 | 46.4% | 25 | 96.2% | 1 | 3.8% |
| cagA | ||||||
| cagA-positive | 41 | 73.2% | 39 | 95.1% | 2 | 4.9% |
| AB | 3 | 7.3% | 3 | 100.0% | 0 | 0.0% |
| ABC | 27 | 65.9% | 26 | 96.3% | 1 | 3.7% |
| ABBC | 1 | 2.4% | 1 | 100.0% | 0 | 0.0% |
| ABCC | 8 | 19.5% | 7 | 87.5% | 1 | 12.5% |
| ABCCC | 2 | 4.9% | 2 | 100.0% | 0 | 0.0% |
| cagA-negative | 13 | 23.2% | 12 | 92.3% | 1 | 7.7% |
| cagA undetermined | 2 | 3.6% | 2 | 100.0% | 0 | 0.0% |
| Pre-EPIYA type (no deletion) | 41 | 100.0% | 39 | 95.1% | 2 | 4.9% |
| vacA | ||||||
| s1 | 44 | 78.6% | 41 | 93.2% | 3 | 6.8% |
| s2 | 12 | 21.4% | 12 | 100.0% | 0 | 0.0% |
| m1 | 30 | 53.6% | 30 | 100.0% | 0 | 0.0% |
| m2 | 26 | 46.4% | 23 | 88.5% | 3 | 11.5% |
| i1 | 38 | 67.9% | 36 | 94.7% | 2 | 5.3% |
| i2 | 18 | 32.1% | 17 | 94.4% | 1 | 5.6% |
| d1 | 37 | 66.1% | 35 | 94.6% | 2 | 5.4% |
| d2 | 19 | 33.9% | 18 | 94.7% | 1 | 5.3% |
| c1 | 30 | 53.6% | 30 | 100.0% | 0 | 0.0% |
| c2 | 26 | 46.4% | 23 | 88.5% | 3 | 11.5% |
| vacA s1m1i1d1c1 | 30 | 53.6% | 30 | 100.0% | 0 | 0.0% |
| s1m2i1d1c2 | 7 | 12.5% | 5 | 71.4% | 2 | 28.6% |
| s1m2i1d2c2 | 1 | 1.8% | 1 | 100.0% | 0 | 0.0% |
| s1m2i2d2c2 | 6 | 10.7% | 5 | 83.3% | 1 | 16.7% |
| s2m2i2d2c2 | 12 | 21.4% | 12 | 100.0% | 0 | 0.0% |
| cagA-positive/vacA s1m1i1d1c1 | 28 | 68.3% | 28 | 100.0% | 0 | 0.0% |
| s1m2i1d1c2 | 7 | 17.1% | 5 | 71.4% | 2 | 28.6% |
| s1m2i1d2c2 | 1 | 2.4% | 1 | 100.0% | 0 | 0.0% |
| s1m2i2d2c2 | 3 | 7.3% | 3 | 100.0% | 0 | 0.0% |
| s2m2i2d2c2 | 2 | 4.9% | 2 | 100.0% | 0 | 0.0% |
| cagA undetermined/vacA s1m1i1d1c1 | 2 | 100.0% | 2 | 100.0% | 0 | 0.0% |
| cagA-negative/vacA s1m2i2d2c2 | 3 | 23.1% | 2 | 66.7% | 1 | 33.3% |
| s2m2i2d2c2 | 10 | 76.9% | 10 | 100.0% | 0 | 0.0% |
EPIYA motifs were also evaluated (Table 2). In total, 134 EPIYA motifs were identified in the 41 cagA-positive strains. In agreement with our previous studies [27, 29], we found two types of motifs: EPIYA (130/134, 97.0%) and EPIYT (4/134, 3.0%). The EPIYA-B contained two types of motifs that included five amino acids (EPIYA and EPIYT), and EPIYT was found only in this motif. After excluding three strains of the AB type, we analyzed 38 CM motifs in Western-type cagA strains in the EPIYA-B segment and in the first distal repeat of the EPIYA-C segment (Table 3). The majority of the CM motifs in Western-type cagA strains were somewhat different from the typical CM motifs (FPLKRHDKVDDLSKVG) observed in strains circulating in Western countries; for examples the first motifs were divergent (20/38, 52.6%), including FPLKKHDKVDDLSKVG or YPLKRHDKVDDLSKVG, but the second motif was not (30/38, 78.9% typical sequences). Most of the first motif sequences were identical to those in the second motif (20/38, 52.6%); for example, both motifs were FPLKRHDKVDDLSKVG. Sequence analyses of the 300-bp region upstream of the first EPIYA motif, called the pre-EPIYA, revealed that no strains contained a deletion that is typically observed in strains from Western countries.
Table 2. Frequencies of EPIYA motifs in Bangladeshi strains.
| All motifs | No. | A motif | No. | B motif | No. | C motif | No. |
|---|---|---|---|---|---|---|---|
| EPIYA | 130 | EPIYA | 41 | EPIYA | 38 | EPIYA | 51 |
| EPIYT | 4 | EPIYT | 4 | ||||
| Total | 134 | 41 | 42 | 51 |
Table 3. CagA multimerization (CM) motif in Western-type cagA H. pylori strains from Bangladesh.
| Peptide sequences | |||
|---|---|---|---|
| 1st motif | No. | 2nd motif | No. |
| FPLKRHDKVDDLSKVG | 18 | FPLKRHDKVDDLSKVG | 30 |
| FPLKK*HDKVDDLSKVG | 17 | FPLKK*HDKVDDLSKVG | 4 |
| Y*PLKRHDKVDDLSKVG | 3 | Others | 4 |
| Total | 38 | Total | 38 |
*Bold letters are used to highlight the different between typical and atypical CM motifs.
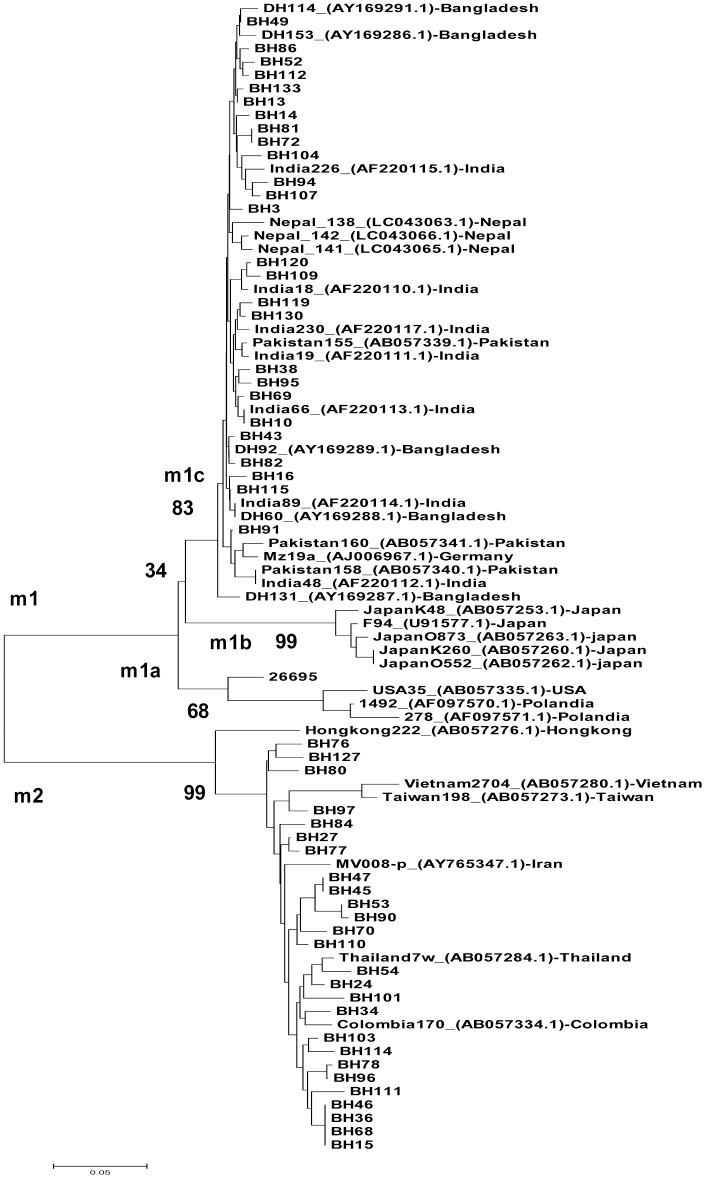
The predominant vacA s type was s1 (44/56, 78.6%), with 97.8% (43/44) of these being genotype s1a (Table 1), which is typical of South Asia strains. The remaining strain (BH15) was not classified in any subtype by the vacA signal region. The vacA s2 genotype accounted for 21.4% (12/56) of the isolates. The prevalence of the vacA m1 genotype was 53.6% (30/56), while the vacA m2 genotype accounted for 46.4% (26/56) of all strains. Results of a sequence analysis of the 0.7-kb m region of vacA were consistent with PCR results and indicated a clear distinction between the m1 and m2 sequences (Fig 1). The genotype m1c, a typical South Asian genotype, was predominant (28/30, 93.3%), with no strains of genotype m1a or m1b detected. The remaining two strains (BH73 and BH63) were not classified as any subtype, based on the vacA m region. Detailed data on the vacA i, c, and d regions are shown in Table 1.
Fig 1. Phylogenetic tree of Helicobacter pylori nucleotide sequences, including the vacA m region.
Genetic distances were estimated using the six-parameter method, and phylogenetic trees were constructed using the neighbor-joining method. Reference strains are shown with strain names and GenBank accession numbers. Bootstrap values are shown along each main branch of the tree. The lengths of the horizontal bars indicate the number of nucleotide substitutions per site.
When examining combinations of the vacA genotypes, it was found that all strains that contained s1 and m1 also possessed i1, d1, and c1 (30/56, 53.6%). In contrast, all strains that contained the less-virulent genotypes s2 and m2 possessed i2, d2, and c2 (12/56, 21.4%), and all strains that possessed s1m2i2 also contained d2 and c2 (6/56, 10.7%) (Table 1). An examination of strains that had both cagA and vacA showed that all cagA-negative strains possessed m2i2d2c2, while m1i1d1c1 was predominant (28/41, 68.3%) in cagA-positive strains.
Histology
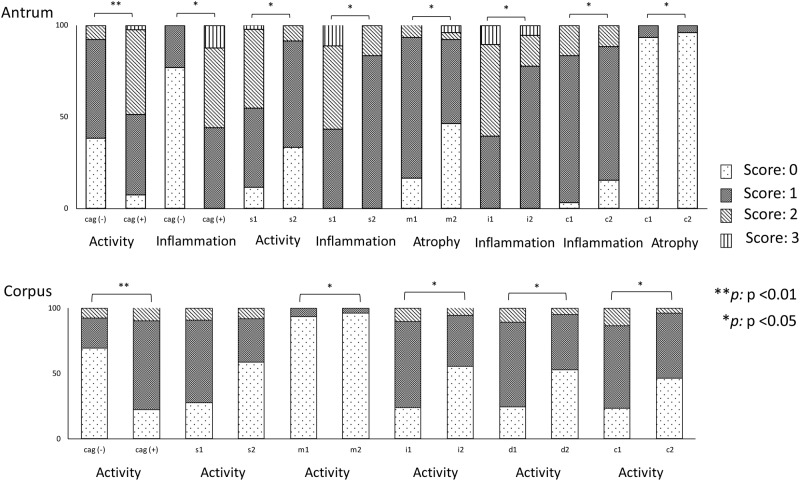
Of the 56 patients infected with H. pylori, only 3 (5.4%) and 1 (1.8%) patients suffered atrophic gastritis and intestinal metaplasia, respectively. Histological analysis (Fig 2) showed that activity in the antrum and corpus was significantly greater in subjects infected with cagA-positive strains than in those infected with cagA-negative strains (mean [median]; 1.23 [1] vs. 0.69 [1], P = 0.001 and 0.75 [1] vs. 0.38 [0], P = 0.007, respectively). In addition, inflammation in the antrum was also significantly greater in subjects infected with cagA-positive strains than in those infected with cagA-negative strains (1.57 [1] vs. 1.23 [1], P = 0.03). Compared to subjects infected with cagA-negative strains, those infected with cagA-positive strains had a significantly higher risk of antrum and corpus activity after adjusting for age and sex (OR = 7.56, 95% CI = 1.46 to 39.2 and OR = 9.15, 95% CI = 2.14 to 39.1, respectively). There was no significant difference in histological scores between individuals infected with single-repeat and multiple-repeat Western-type cagA (i.e., ABC vs. ABCC or ABCCC). Additionally, there were no significant differences in histological scores between subjects infected with strains containing EPIYA and EPIYT motifs, or between those with identical and non-identical CM motifs (P > 0.05).
Fig 2. Association between histological findings and genotypes in H. pylori strains from Bangladesh.
The X-axis describes the degree of histological severity, which was classified into four grades based on the updated Sydney system (scores from 0 to 3), and the Y-axis indicates the genotypes of cagA and vacA. cagA-positive strains induced more severe histological damage in the antrum and corpus. Similar results were also found for vacA genotypes, including s1 vs. s2 only in the antrum, and m1 vs. m2, i1 vs. i2, c1 vs. c2, and d1 vs. d2 in both the antrum and corpus.
Subjects infected with vacA s1 strains showed activity and inflammation in the antrum that was significantly greater than in those infected with vacA s2 strains (mean [median]; 1.36 [1] vs. 0.75 [1], P = 0.01 and 1.68 [2] vs. 1.17 [1], P = 0.013, respectively). Inflammation and atrophy scores for the antrum were significantly higher in subjects infected with strains containing vacA m1 than in those infected with vacA m2 strains (mean [median]; 1.70 [2] vs. 1.42 [1], P = 0.05 and 0.90 [1] vs. 0.65 [1], P = 0.047, respectively). In addition, activity in the corpus and OLGA scores were significantly increased in subjects infected with strains containing vacA m1, relative to those infected with vacA m2 strains (mean [median]; 0.90 [1] vs. 0.58 [1], P = 0.049 and 0.90 [1] vs. 0.65 [1], P = 0.047, respectively). Similarly, subjects who were infected with virulent i and d types (i1 and d1) had inflammation in the antrum and activity in the corpus that was significantly greater than that of subjects infected with vacA i2 and d2 strains (P = 0.02 and P = 0.042, respectively). Inflammation and atrophy scores for the antrum, activity in the corpus, and OLGA scores were significantly increased in subjects infected with strains containing vacA c1, relative to those infected with vacA c2 (for both, P = 0.047).
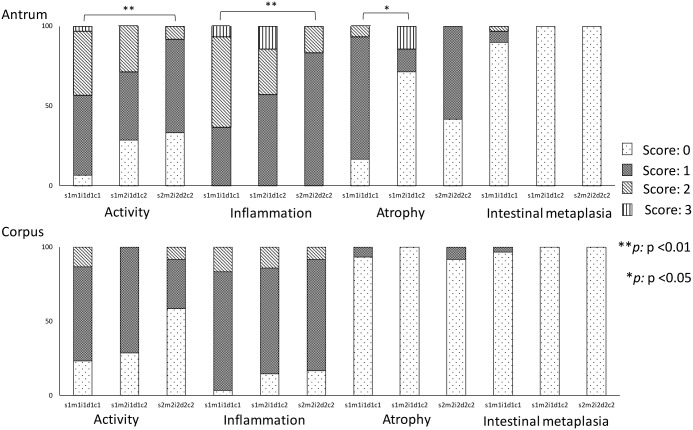
Histological analysis of specimens from subjects infected with combination vacA genotypes showed that subjects infected with vacA s1m1i1d1c1 strains had more antral atrophy than was seen in subjects infected with strains of genotype s1m2i1d1c2 (0.90 [1] vs. 0.57 [0], P = 0.034, Fig 3). Subjects infected with vacA s1m1i1d1c1 strains also showed antral activity and inflammation that was increased, relative to activity and inflammation in subjects infected with s2m2i2d2c2 (1.40 [1] vs. 0.75 [1], P = 0.008 and 1.70 [2] vs. 1.17 [1], P = 0.007, respectively). Multivariate analysis showed that subjects infected with vacA m1 strains had a significantly higher risk of antral atrophy after adjusting for age, sex, and cagA status (OR = 11.90, 95% CI = 1.685 to 83.98), relative to those infected with the m2 genotype.
Fig 3. Histological analysis of specimens from subjects infected with combination vacA genotypes.
Histological severity scores based on the updated Sydney system are indicated on the X-axis and the genotype combinations of vacA are described on the Y-axis. In line with the analysis of single sub-genotypes of vacA, s1m1i1d1c1 strains induced significantly greater atrophy, activity, and inflammation in the antrum compared to s1m2i1d1c2, s2m2i2d2c2, or s2m2i2d2c2 strains.
Population structure
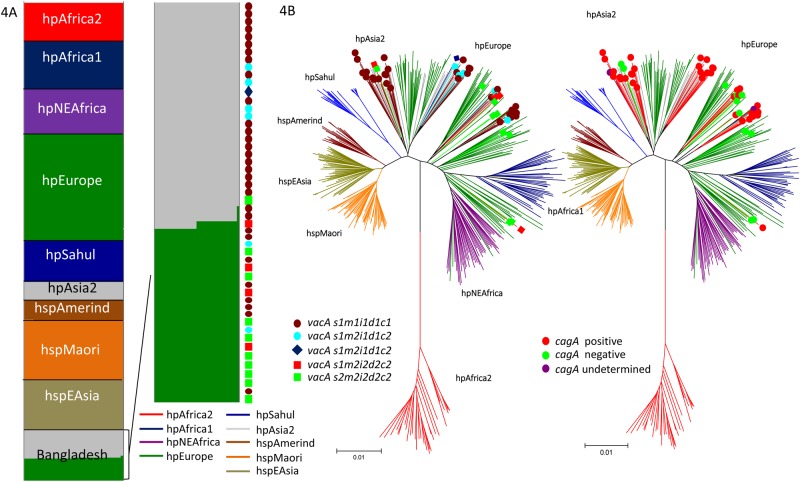
A total of 51 strains were analyzed using STRUCTURE. Five strains were excluded because one of the seven housekeeping genes was undetectable in the NGS data and because of the poor quality of sequencing results. We investigated the population structure of the Bangladeshi strains using the highest posterior probability of five runs (K = 15). A non-admixture STRUCTURE analysis estimated the geographic origins of each of the ancestral populations. Interestingly, although we obtained samples from subjects of one ethnicity (Bengali), we found that the Bangladeshi strains were divided into two main populations in similar proportions: the hpAsia2 (grey, 54.9%, 28/51) and hpEurope (green, 43.1%, 22/51) populations (Fig 4A). STRUCTURE results were supported by a neighbor-joining tree constructed in MEGA 3.1, using a Kimura-2 parameter model that revealed clear geographic associations for the two populations (Fig 4B). No association between population type and either sex or place of residence (P = 0.07 and P = 0.45) was observed. Histological analysis revealed unexpected results: the subjects infected with hpAsia2 strains showed greater activity and inflammation in the antrum compared to subjects infected with hpEurope strains (1.39 [1.5] vs. 0.95 [1], P = 0.025 and 1.75 [2] vs. 1.36 [1], P = 0.047).
Fig 4. Population analysis of Bangladeshi strains.
The number of tentative populations (K) was set from 7 to 15, and 5 to 15 runs were executed for each of the K population components, which are represented in K colors. Each vertical line in the bar chart represents a single strain, and the line colors indicate the population to which each strain might belong. The lengths of the colored lines are proportional to the probability that the strain belongs to that particular population. The population segregated into two populations, hpAsia2 and hpEurope, as confirmed by non-admixture STRUCTURE analysis (4A). We used a different line color to distinguish each population type in both STRUCTURE and in the neighbor-joining tree constructed in 4B (e.g., hpEurope is shown in green, and hpAsia2 is shown in grey). The position of strains in relation to their genotypes are shown in colored boxes or circles.
Results of a detailed analysis combining population structure and genotype are shown in Table 4. The hpEurope population contained more cagA-negative strains (76.9%, 10/22) compared to the hpAsia2 population (15.4%, 2/28, P = 0.002). In addition, hpAsia2 comprised a higher proportion of the more virulent vacA genotype (s1m1i1d1c1) and a lower proportion of the less-virulent genotype (s2m2i2d2c2) compared to hpEurope (75.0%, 21/28 vs. 36.4%, 8/22; 3.6%, 1/28 vs. 40.9%, 9/22, P = 0.001, respectively). Multivariate analysis showed that, compared to subjects infected with cagA-negative strains, subjects infected with cagA-positive strains, independent of their population types, had a higher risk of antral activity after adjusting for age, sex, and population type, although this difference was not statistically significant (OR = 5.063, 95% CI = 0.819 to 31.304, P = 0.08). Moreover, compared to subjects infected with the s2m2i2d2c2 genotype, those infected with the vacA s1m1i1d1c1-only genotype, independent of their population types, had a significantly higher risk of inflammation in the antrum after adjusting for age, sex, and population type (OR = 7.451, 95% CI = 1.027 to 54.077, P = 0.047).
Table 4. H. pylori population and genotypes.
| Genotype | Total | H. pylori population (%) | ||
|---|---|---|---|---|
| hpAsia2** | hpEurope | hpAsia2-hpEurope | ||
| cagA-negative | 13* | 2 (15.4) | 10 (76.9)*** | 0 |
| cagA-positive | 41** | 25 (61.0) | 11 (26.8) | 1 (2.4) |
| Group 1: vacA s1m1i1d1c1 | 29 | 21 (75.0) | 8 (36.4) *** | 0 |
| Group 2: vacA s1m2i1d1c2 | 6 | 4 (14.2) | 2 (9.1) | 0 |
| Group 3: vacA s1m2i1d1c2 | 1 | 1 (3.6) | 0 (0.0) | 0 |
| Group 4: vacA s1m2i2d2c2 | 5 | 1 (3.6) | 3 (13.6) | 1 (100.0) |
| Group 5: vacA s2m2i2d2c2 | 10 | 1 (3.6) | 9 (40.9) | 0 |
| Total | 51 | 28 | 22 | 1 |
* One strain was excluded because one of the seven housekeeping genes was undetected in the NGS data and because of the poor quality of sequencing results.
**hpAsia2 strains induced greater antral histological severity than hpEurope strains.
*** the hpEurope population contained more cagA-negative and less-virulent vacA genotypes than the hpAsia2 population.
Discussion
We confirmed that H. pylori strains from a Bengali population in Bangladesh were divided into two main populations. We could not confirm which population is older because of our study design; however, we assumed that the hpAsia2 population was distributed evenly in the Indian subcontinent before being reduced through competition with novel strains (hpEurope), as has been seen in previous studies that will be described below. It has been suggested that Ancestral Europe 1 (AE1) originated in Central Asia, where it evolved into hpAsia2, which is commonly found in South Asia (6). A hybridization between AE1 and AE2, which appears to have evolved in northeast Africa, became the more recently diverged hpEurope (4). This supports the surprising results that the H. pylori of the Iceman—a mummy found in the European Alps—is hpAsia2, with a low proportion of its genome reflecting AE2 ancestry [30], confirming that the introduction of hpAsia2 into the European continent occurred at least 5,000 years ago. However, other studies found that all Indian strains that were sequenced from Native Indian people, who are mainly of Aryan and Dravidian ancestry, have significant homology to the hpEurope population, irrespective of religion (Muslim or Hindu) suggested the possibility that H. pylori arrived with Indo-Aryan migration (approximately 4,000–10,000 years ago) [31]. In contrast, Ladakhi strains were categorized as mainly hpAsia2, with some hpEurope, irrespective of religion (Muslim or Buddhist) [31], when sequences were compared with sequences of similar strains from a previous publication [32]. The presence of an hpAsia2 population in Bangladeshi strains supports the hypothesis that hpAsia2 was an independent population that was widespread throughout the Indian subcontinent after the Dravidians retreated to avoid Indo-European dominance [33]. This is supported by the difference in the distribution of cagA and vacA genotypes in the two populations, although their vacA m sequences were similar to one another, as well as to strains with the vacA m1c allele from India (Fig 1). In fact, our previous report revealed that Nepalese strains also contained an hpAsia2 population, instead of a population specific to Nepal [7]. The hpEurope population spread to Southeast Asia, including Cambodia, Thailand, and Malaysia, likely within the last 3,000 years [34].
Through multivariate analysis, our study also confirmed an unexpected finding that subjects infected with hpAsia2 strains had greater activity and inflammation in the antrum than subjects infected with hpEurope strains. Although no previous study has examined histological severity by H. pylori population, the hpEurope population is believed to be associated with a higher risk of gastric cancer than is hpAsia2, based on the incidence of gastric cancer in people from each continent (9.4/100,000 vs. 6.7/100,000 for Europe and south-central Asia, respectively; http://globocan.iarc.fr/). This is likely because of the higher proportion of the less-virulent cagA and vacA genotypes among hpEurope strains that were associated with less severe histological damage. The Bangladeshi strains analyzed in this study had a high proportion of less-virulent cagA-positive and vacA-positive strains, similar to that observed in strains circulating in neighboring countries (Table 5) [7, 35–40]. Therefore, this is likely only a coincidence and not causal. Previously, we suggested that virulence factors, especially cagA, were a better predictor of gastric cancer risk than the ancestral origin of H. pylori [41]. However, the question remains: why were the less-virulent types of cagA and vacA not associated with the hpAsia2 population that are typical in South Asia? Another possibility is that antral gastritis predominantly induces a hyperacidity that predisposes an H. pylori-infected person to gastric metaplasia of the duodenal mucosa, allowing H. pylori colonization of the duodenum and propagation of duodenal ulceration [8]. South Asia is a region with a high prevalence of duodenal ulcers. Therefore, hpAsia2 strain colonization may be a marker for duodenal ulcer risk in Bangladesh. However, within the hpAsia2 population, only one strain was isolated from a patient with duodenal and gastric ulcers.
Table 5. Summary of prevalence and genotypes of H. pylori in South Asia.
| Country | Gastric cancer incidence | Prevalence of H. pylori | cagA- positive | cagA type prevalence | vacA s1m1 |
|---|---|---|---|---|---|
| Bangladesh | 5.8 | 47.0% | 73.2% | Western-type cagA (100.0%) | 53.6% |
| Nepal [7] | 12.1 | 38.4% | 100.0% | Western-type cagA (94.1%)* | 60.8% |
| India [35–37, 42] | 6.1 | 58.0% | 64.0–79.1% | Western-type cagA (100.0%) | 37.2–59.2% |
| Pakistan [38] | 3.0 | 61.3% | 80.4% | Western-type cagA (90.0%) | 58.7% |
| Bhutan [39, 40] | 17.2 | 73.4% | 98.6% | East Asian-type cagA (91.7%) | 33%** |
*Includes an East Asian-type and Western-type cagA recombinant (23.6%)
**Included in the 38.3% of strains with the s1 m1-m2 chimeric genotype
Our study confirmed that, among Western strains, vacA s1m1 and s2m2 strains were exclusively i1 and i2, respectively, and that the s1m2 strains vary in their i types [10]. We also found that the more virulent genotypes (i.e., i1, d1, and c1) were associated with significantly more severe histological scores than were i2, d2, and c2. However, in contrast with results of a previous study in Iran showing that i1- and c1-type strains were strongly associated with gastric adenocarcinoma, irrespective of their vacA s or m type or cag status [13, 43], we confirmed that in our Bangladesh study, only the vacA m region was independently associated with a higher risk of atrophy in the antrum after adjusting for age, sex, and cagA status. In addition, all strains containing s1 and m1 possessed i1, d1, and c1, while strains containing s2 and m2 possessed i2, d2, and c2. All strains possessing s1m2i2 also contained d2 and c2. Therefore, in countries such as Bangladesh that have a low prevalence of gastric cancer, the i, d, and c genotypes may simply reflect the s and m regions, and the vacA m region may be used as a virulence marker instead of using the overall vacA genotype. Moreover, combining the cagA and vacA genotypes instead of the s region showed that all cagA-negative strains possessed m2i2d2c2 and that the m1i1d1c1 genotype was predominant in cagA-positive strains, suggesting the possibility that vacA genotypes only reflect the presence of these other virulence factors, which primarily represent differences related to human migration [44].
Interestingly, although the first CM motif in half of the strains was different from that of typical Western-type cagA-positive strains, the CM motifs consisted of only three types (Table 3). The EPIYA motifs in these strains only consisted of two types (EPIYA and EPIYT), which is a low number in comparison with that found in our previous publication in Okinawa, which identified six types of EPIYA motifs (EPIYA, EPIYT, ESIYA, ESIYT, ELIYA, QPIYA, and EPVYA) [27]. In addition, an analysis of the 300-bp region upstream of the first EPIYA motif showed that no strains contained the deletion that is typically observed in strains from Western countries. Although the same Western-type cagA was predominant, our previous report showed that Nepalese strains contained three types of pre-EPIYA sequences (no-deletion type, 6-bp type, 18-bp type) [7]. Moreover, all B-segments of Bangladeshi cagA strains contained no chimeric EPIYA segments, and all were categorized as type BC, which is typical of Western-type cagA-positive strains (Table 5) [1]. These “homogenous” genotypes include a few recombinants among the Bangladeshi strains. In general, recombination is extremely frequent in H. pylori [45], as a result of adaptation during chronic colonization [46].
All Bangladeshi strains of H. pylori were categorized as Western-type cagA, which is the less-virulent cagA genotype [1]. Sequence analysis indicated that the predominant vacA genotype of Bangladeshi strains, s1m1, was associated with higher scores related to activity, inflammation, and atrophy when compared to the scores associated with s2m2 H. pylori strains. However, most of the vacA s regions were of the s1a subtype, which is less frequently found in patients with gastric cancer than in those with peptic ulcers and chronic gastritis [47] or is present only in dyspeptic patients [48]. In addition, the predominant m region subtype was m1c, which is specific to South Asian countries with a low ASR of gastric cancer. Moreover, the higher proportion of vacA s2 and m2 genotypes, even in cagA-positive strains, reflects the low prevalence of gastric cancer-inducing strains, which is in agreement with an in vitro study that showed that this genotype fails to induce cell vacuolation [1]. These results might explain, at least in part, the “Asian enigma” in Bangladesh, where the H. pylori infection rate in the Bangladeshi population is high, but the risk of gastric cancer is low.
There are several limitations in this study. First, the number of strains included was small. Further studies with a larger number of samples, balanced for each diagnosis, will be necessary to better understand the association between virulence factors and clinical outcomes in Bangladesh. Secondly, we obtained samples from a single hospital in Dhaka, which is the capital and largest metropolis in Bangladesh. The physical and cultural landscape varies by region in Bangladesh. Therefore, our results cannot be generalized across all of Bangladesh.
In conclusion, we revealed that Bangladeshi strains segregated into two main populations with different genotypes. The low incidence of gastric cancer in Bangladesh might be attributable to the high proportion of less -virulent genotypes, which may be a better predictor of gastric cancer risk than is the ancestral origin of H. pylori strains. Finally, we determined that the vacA m region, instead of the overall vacA genotype, can be used as a virulence marker.
Data Availability
Sequencing data for cagA, vacA and seven housekeeping genes are available under DDBJ accession numbers LC187614–LC187654, LC187347–LC187613, and LC186099–LC186455, respectively.
Funding Statement
This work was supported in part by grants from the National Institutes of Health (DK62813) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (25293104, 26640114 and 16H05191) (YY). This work was also supported by the Okinawa Prefectural Government. It was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits (YY) and the Strategic Funds for the Promotion of Science and Technology from the Japan Science and Technology Agency (JST) (YY). HA was a dissertation PhD student supported by the JSPS RONPAKU program in 2013. MM was a PhD student and PS is a PhD student supported by the Japanese government (MEXT) scholarship program in 2012 and 2013, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629–41. doi: 10.1038/nrgastro.2010.154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(12):5791–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15(3):163–76. doi: 10.1111/j.1523-5378.2010.00759.x . [DOI] [PubMed] [Google Scholar]
- 4.Backert S, Moese S, Selbach M, Brinkmann V, Meyer TF. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Molecular microbiology. 2001;42(3):631–44. . [DOI] [PubMed] [Google Scholar]
- 5.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nature reviews Cancer. 2004;4(9):688–94. doi: 10.1038/nrc1433 . [DOI] [PubMed] [Google Scholar]
- 6.Uchida T, Nguyen LT, Takayama A, Okimoto T, Kodama M, Murakami K, et al. Analysis of virulence factors of Helicobacter pylori isolated from a Vietnamese population. BMC microbiology. 2009;9:175 doi: 10.1186/1471-2180-9-175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miftahussurur M, Sharma RP, Shrestha PK, Suzuki R, Uchida T, Yamaoka Y. Molecular Epidemiology of Helicobacter pylori Infection in Nepal: Specific Ancestor Root. PloS one. 2015;10(7):e0134216 doi: 10.1371/journal.pone.0134216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical microbiology reviews. 2006;19(3):449–90. doi: 10.1128/CMR.00054-05 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135(1):91–9. doi: 10.1053/j.gastro.2008.03.041 . [DOI] [PubMed] [Google Scholar]
- 10.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133(3):926–36. doi: 10.1053/j.gastro.2007.06.056 . [DOI] [PubMed] [Google Scholar]
- 11.Ogiwara H, Sugimoto M, Ohno T, Vilaichone RK, Mahachai V, Graham DY, et al. Role of deletion located between the intermediate and middle regions of the Helicobacter pylori vacA gene in cases of gastroduodenal diseases. Journal of clinical microbiology. 2009;47(11):3493–500. doi: 10.1128/JCM.00887-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basiri Z, Safaralizadeh R, Bonyadi MJ, Somi MH, Mahdavi M, Latifi-Navid S. Helicobacter pylori vacA d1 genotype predicts risk of gastric adenocarcinoma and peptic ulcers in northwestern Iran. Asian Pacific journal of cancer prevention: APJCP. 2014;15(4):1575–9. . [DOI] [PubMed] [Google Scholar]
- 13.Bakhti SZ, Latifi-Navid S, Mohammadi S, Zahri S, Bakhti FS, Feizi F, et al. Relevance of Helicobacter pylori vacA 3'-end Region Polymorphism to Gastric Cancer. Helicobacter. 2015. doi: 10.1111/hel.12284 . [DOI] [PubMed] [Google Scholar]
- 14.Moodley Y, Linz B, Yamaoka Y, Windsor HM, Breurec S, Wu JY, et al. The peopling of the Pacific from a bacterial perspective. Science. 2009;323(5913):527–30. doi: 10.1126/science.1166083 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falush D, Wirth T, Linz B, Pritchard J, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299(5612):1582–5. doi: 10.1126/science.1080857 . [DOI] [PubMed] [Google Scholar]
- 16.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445(7130):915–8. doi: 10.1038/nature05562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12(2):203–13. doi: 10.1016/j.meegid.2011.12.002 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60(9):1189–95. doi: 10.1136/gut.2010.234468 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodaman N, Pazos A, Schneider BG, Piazuelo MB, Mera R, Sobota RS, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc Natl Acad Sci U S A. 2014;111(4):1455–60. doi: 10.1073/pnas.1318093111 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuhisa T, Aftab H. Observation of gastric mucosa in Bangladesh, the country with the lowest incidence of gastric cancer, and Japan, the country with the highest incidence. Helicobacter. 2012;17(5):396–401. doi: 10.1111/j.1523-5378.2012.00967.x ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman M, Mukhopadhyay AK, Nahar S, Datta S, Ahmad MM, Sarker S, et al. DNA-level characterization of Helicobacter pylori strains from patients with overt disease and with benign infections in Bangladesh. Journal of clinical microbiology. 2003;41(5):2008–14. doi: 10.1128/JCM.41.5.2008-2014.2003 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta S, Chattopadhyay S, Chowdhury A, Santra A, Saha DR, Ramamurthy T, et al. Diagnosis and genotyping of Helicobacter pylori by polymerase chain reaction of bacterial DNA from gastric juice. Journal of gastroenterology and hepatology. 2005;20(8):1253–9. doi: 10.1111/j.1440-1746.2005.03811.x . [DOI] [PubMed] [Google Scholar]
- 23.Kibria KM, Hossain ME, Sultana J, Sarker SA, Bardhan PK, Rahman M, et al. The Prevalence of Mixed Helicobacter pylori Infections in Symptomatic and Asymptomatic Subjects in Dhaka, Bangladesh. Helicobacter. 2015;20(5):397–404. doi: 10.1111/hel.12213 . [DOI] [PubMed] [Google Scholar]
- 24.Dixon M, Genta R, Yardley J, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–81. . [DOI] [PubMed] [Google Scholar]
- 25.Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56(5):631–6. doi: 10.1136/gut.2006.106666 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida T, Kanada R, Tsukamoto Y, Hijiya N, Matsuura K, Yano S, et al. Immunohistochemical diagnosis of the cagA-gene genotype of Helicobacter pylori with anti-East Asian CagA-specific antibody. Cancer Sci. 2007;98(4):521–8. Epub 2007/02/08. doi: 10.1111/j.1349-7006.2007.00415.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsunari O, Shiota S, Suzuki R, Watada M, Kinjo N, Murakami K, et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. Journal of clinical microbiology. 2012;50(3):876–83. doi: 10.1128/JCM.05562-11 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangmankongworakoon N, Mahachai V, Thong-Ngam D, Vilaichone RK, Tumwasorn S, Kullavanijaya P. Pattern of drug resistant Helicobacter pylori in dyspeptic patients in Thailand. J Med Assoc Thai. 2003;86 Suppl 2:S439–44. . [PubMed] [Google Scholar]
- 29.Xia Y, Yamaoka Y, Zhu Q, Matha I, Gao X. A comprehensive sequence and disease correlation analyses for the C-terminal region of CagA protein of Helicobacter pylori. PloS one. 2009;4(11):e7736 doi: 10.1371/journal.pone.0007736 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maixner F, Krause-Kyora B, Turaev D, Herbig A, Hoopmann MR, Hallows JL, et al. The 5300-year-old Helicobacter pylori genome of the Iceman. Science. 2016;351(6269):162–5. doi: 10.1126/science.aad2545 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devi SM, Ahmed I, Francalacci P, Hussain MA, Akhter Y, Alvi A, et al. Ancestral European roots of Helicobacter pylori in India. BMC genomics. 2007;8:184 doi: 10.1186/1471-2164-8-184 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirth T, Wang X, Linz B, Novick RP, Lum JK, Blaser M, et al. Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):4746–51. doi: 10.1073/pnas.0306629101 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu A, Mukherjee N, Roy S, Sengupta S, Banerjee S, Chakraborty M, et al. Ethnic India: a genomic view, with special reference to peopling and structure. Genome research. 2003;13(10):2277–90. doi: 10.1101/gr.1413403 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breurec S, Guillard B, Hem S, Brisse S, Dieye FB, Huerre M, et al. Evolutionary history of Helicobacter pylori sequences reflect past human migrations in Southeast Asia. PloS one. 2011;6(7):e22058 doi: 10.1371/journal.pone.0022058 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Kumar A, Dixit VK. Genetic diversity in strains of Helicobacter pylori from India and their relatedness to strains from other parts of the world. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11(1):242–7. doi: 10.1016/j.meegid.2010.09.001 . [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay AK, Kersulyte D, Jeong JY, Datta S, Ito Y, Chowdhury A, et al. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. Journal of bacteriology. 2000;182(11):3219–27. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sodhi JS, Javid G, Zargar SA, Tufail S, Shah A, Khan BA, et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. Journal of gastroenterology and hepatology. 2013;28(5):808–13. doi: 10.1111/jgh.12178 . [DOI] [PubMed] [Google Scholar]
- 38.Rasheed F, Campbell BJ, Alfizah H, Varro A, Zahra R, Yamaoka Y, et al. Analysis of clinical isolates of Helicobacter pylori in Pakistan reveals high degrees of pathogenicity and high frequencies of antibiotic resistance. Helicobacter. 2014;19(5):387–99. doi: 10.1111/hel.12142 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilaichone RK, Mahachai V, Shiota S, Uchida T, Ratanachu-ek T, Tshering L, et al. Extremely high prevalence of Helicobacter pylori infection in Bhutan. World journal of gastroenterology. 2013;19(18):2806–10. doi: 10.3748/wjg.v19.i18.2806 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsunari O, Miftahussurur M, Shiota S, Suzuki R, Vilaichone RK, Uchida T, et al. Rare Helicobacter pylori Virulence Genotypes in Bhutan. Scientific reports. 2016;6:22584 doi: 10.1038/srep22584 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiota S, Matsunari O, Watada M, Yamaoka Y. Virulence factors or ancestral origin of Helicobacter pylori: which is a better predictor of gastric cancer risk? Gut. 2012;61(3):469–70. doi: 10.1136/gutjnl-2011-300317 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiya K, Nagata N, Uchida T, Kobayakawa M, Asayama N, Akiyama J, et al. Different gastric mucosa and CagA status of patients in India and Japan infected with Helicobacter pylori. Digestive diseases and sciences. 2014;59(3):631–7. doi: 10.1007/s10620-013-2961-x . [DOI] [PubMed] [Google Scholar]
- 43.Yordanov D, Boyanova L, Markovska R, Gergova G, Mitov I. Significance of Helicobacter pylori vacA intermediate region genotyping-a Bulgarian study. Diagnostic microbiology and infectious disease. 2012;74(3):253–7. doi: 10.1016/j.diagmicrobio.2012.07.008 . [DOI] [PubMed] [Google Scholar]
- 44.Yamaoka Y, Graham DY. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014;10(8):1487–500. doi: 10.2217/fon.14.29 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suerbaum S, Achtman M. Evolution of Helicobacter pylori: the role of recombination. Trends in microbiology. 1999;7(5):182–4. . [DOI] [PubMed] [Google Scholar]
- 46.Kraft C, Suerbaum S. Mutation and recombination in Helicobacter pylori: mechanisms and role in generating strain diversity. International journal of medical microbiology: IJMM. 2005;295(5):299–305. doi: 10.1016/j.ijmm.2005.06.002 . [DOI] [PubMed] [Google Scholar]
- 47.Lin HJ, Perng CL, Lo WC, Wu CW, Tseng GY, Li AF, et al. Helicobacter pylori cagA, iceA and vacA genotypes in patients with gastric cancer in Taiwan. World journal of gastroenterology: WJG. 2004;10(17):2493–7. doi: 10.3748/wjg.v10.i17.2493 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Vidal Y, Ponce-de-Leon S, Castillo-Rojas G, Barreto-Zuniga R, Torre-Delgadillo A. High diversity of vacA and cagA Helicobacter pylori genotypes in patients with and without gastric cancer. PloS one. 2008;3(12):e3849 doi: 10.1371/journal.pone.0003849 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data for cagA, vacA and seven housekeeping genes are available under DDBJ accession numbers LC187614–LC187654, LC187347–LC187613, and LC186099–LC186455, respectively.