Abstract
DNA extracted from eight human cervical carcinomas, one lymph node metastasis and related control tissue was examined for the presence of herpes simplex virus (HSV) DNA sequences. Southern blot transfers of tumour and control DNA were hybridised with radioactively labelled cloned probes representing 70% of the HSV-2 genome. Specific hybridisation to HSV DNA sequences was observed in one of eight carcinoma tissues analysed. Hybridisation of HSV-2 DNA probes to BamHI and XhoI restriction enzyme fragments of tumour cell DNA which co-migrated with authentic HSV-2 viral fragments identified co-linear HSV-2 DNA sequences comprising 3% of the HSV-2 genome, between map coordinates 0.582 and 0.612. The remaining eight tumour and all control tissues analysed, showed no specific hybridisation to any of the probes used at levels of sensitivity which would detect 0.5 copies/cell of HSV-2 DNA restriction fragments of 2 kb or greater.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E., Kaufman R. H., Melnick J. L., Levy A. H., Rawls W. E. Seroepidemiologic studies of herpesvirus type 2 and carcinoma of the cervix. IV. Dysplasia and carcinoma in situ. Am J Epidemiol. 1973 Aug;98(2):77–87. doi: 10.1093/oxfordjournals.aje.a121541. [DOI] [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Rigby P. W. Cloning and characterization of the integrated viral DNA from three lines of SV40-transformed mouse cells. Cell. 1981 Aug;25(2):547–559. doi: 10.1016/0092-8674(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Docherty J. J., Subak-Sharpe J. H., Preston C. M. Identification of a virus-specific polypeptide associated with a transforming fragment (BglII-N) of herpes simplex virus type 2 DNA. J Virol. 1981 Oct;40(1):126–132. doi: 10.1128/jvi.40.1.126-132.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
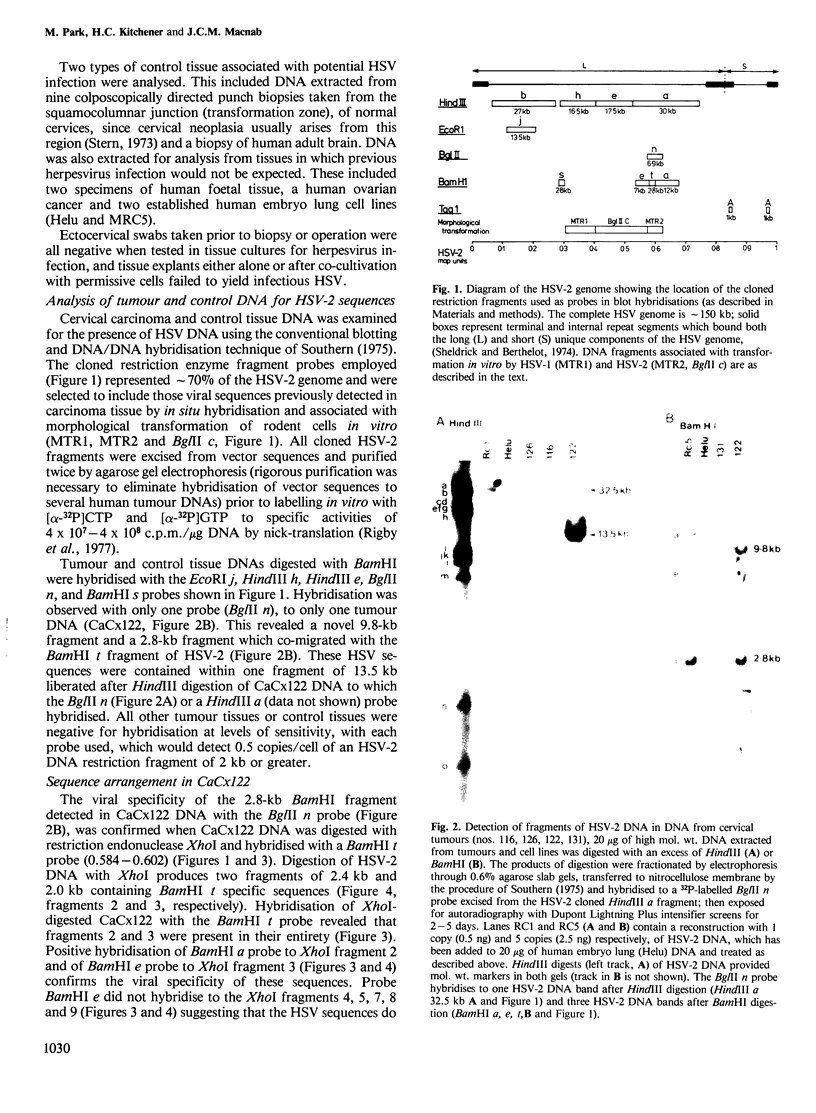
- Dreesman G. R., Burek J., Adam E., Kaufman R. H., Melnick J. L., Powell K. L., Purifoy D. J. Expression of herpesvirus-induced antigens in human cervical cancer. Nature. 1980 Feb 7;283(5747):591–593. doi: 10.1038/283591a0. [DOI] [PubMed] [Google Scholar]
- Eglin R. P., Sharp F., MacLean A. B., Macnab J. C., Clements J. B., Wilkie N. M. Detection of RNA complementary to herpes simplex virus DNA in human cervical squamous cell neoplasms. Cancer Res. 1981 Sep;41(9 Pt 1):3597–3603. [PubMed] [Google Scholar]
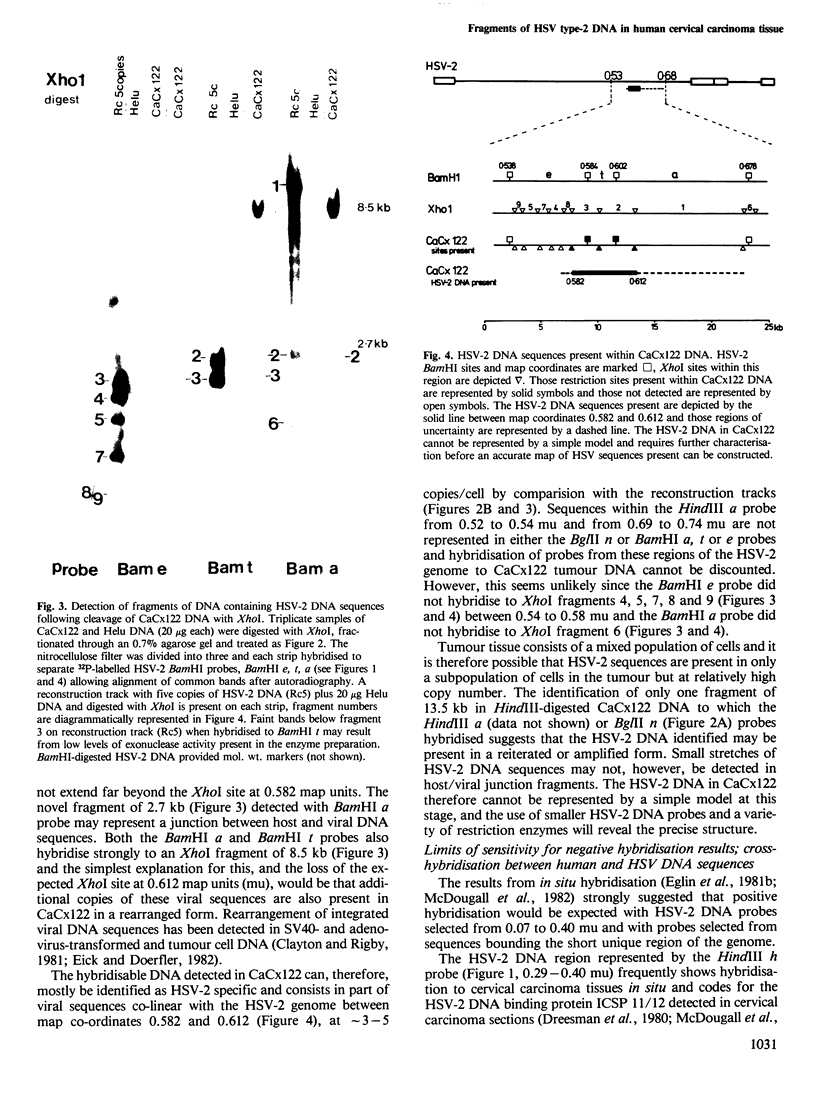
- Eick D., Doerfler W. Integrated adenovirus type 12 DNA in the transformed hamster cell line T637: sequence arrangements at the termini of viral DNA and mode of amplification. J Virol. 1982 Apr;42(1):317–321. doi: 10.1128/jvi.42.1.317-321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Locker H., Vlazny D. A. Studies of defective herpes simplex viruses. Ann N Y Acad Sci. 1980;354:347–370. doi: 10.1111/j.1749-6632.1980.tb27977.x. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B., Cassai E., Nahmias A. A DNA fragment of Herpes simplex 2 and its transcription in human cervical cancer tissue. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3784–3789. doi: 10.1073/pnas.69.12.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Goldstein L. C., Lewis J. B. Identification of proteins encoded by a fragment of herpes simplex virus type 2 DNA that has transforming activity. J Virol. 1982 May;42(2):530–537. doi: 10.1128/jvi.42.2.530-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. Transformation of rodent cells by a cloned DNA fragment of herpes simplex virus type 2. J Virol. 1981 May;38(2):749–760. doi: 10.1128/jvi.38.2.749-760.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Docherty J. J., Clarke A., Rawls W. E. Reaction patterns of herpes simplex virus type 1 and type 2 proteins with sera of patients with uterine cervical carcinoma and matched controls. Cancer Res. 1980 Dec;40(12):4640–4647. [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., deVilliers E. M., zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982 Feb 15;29(2):143–146. doi: 10.1002/ijc.2910290205. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Sanders P. R., Loewenstein P. M., Freel J. H., Eisinger M., Switlyk S. A. Isolation of a human papillomavirus from a patient with epidermodysplasia verruciformis: presence of related viral DNA genomes in human urogenital tumors. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4437–4441. doi: 10.1073/pnas.79.14.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Aaronson S. A., Derge J. G., Chakrabarty M., Showalter S. D., Dunn C. Y. Activation of an endogenous mouse type C virus by ultraviolet-irradiated herpes simplex virus types 1 and 2. Proc Natl Acad Sci U S A. 1976 Feb;73(2):646–650. doi: 10.1073/pnas.73.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Tumorigenic transformation induced by a specific fragment of DNA from herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2279–2283. doi: 10.1073/pnas.77.4.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZAL H. L., LONG J. P. Squamous cell papillomas of the uterine cervix; a report of 20 cases. Cancer. 1958 Sep-Oct;11(5):1049–1059. doi: 10.1002/1097-0142(195809/10)11:5<1049::aid-cncr2820110527>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kessler I. I. Human cervical cancer as a venereal disease. Cancer Res. 1976 Feb;36(2 Pt 2):783–791. [PubMed] [Google Scholar]
- Maitland N. J., Kinross J. H., Busuttil A., Ludgate S. M., Smart G. E., Jones K. W. The detection of DNA tumour virus-specific RNA sequences in abnormal human cervical biopsies by in situ hybridization. J Gen Virol. 1981 Jul;55(Pt 1):123–137. doi: 10.1099/0022-1317-55-1-123. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Crum C. P., Fenoglio C. M., Goldstein L. C., Galloway D. A. Herpesvirus-specific RNA and protein in carcinoma of the uterine cervix. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3853–3857. doi: 10.1073/pnas.79.12.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall J. K., Galloway D. A., Fenoglio C. M. Cervical carcinoma: detection of herpes simplex virus RNA in cells undergoing neoplastic change. Int J Cancer. 1980 Jan 15;25(1):1–8. doi: 10.1002/ijc.2910250102. [DOI] [PubMed] [Google Scholar]
- Menczer J., Yaron-Schiffer O., Leventon-Kriss S., Modan M., Modan B. Herpesvirus type 2 in adenocarcinoma of the uterine cervix: a possible association. Cancer. 1981 Oct 1;48(7):1497–1499. doi: 10.1002/1097-0142(19811001)48:7<1497::aid-cncr2820480702>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie M. J., McGeoch D. J. DNA sequence analysis of an immediate-early gene region of the herpes simplex virus type 1 genome (map coordinates 0.950 to 0.978). J Gen Virol. 1982 Sep;62(Pt 1):1–15. doi: 10.1099/0022-1317-62-1-1. [DOI] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E. Cytology and histopathology of cervical herpes simplex infection. Cancer. 1966 Jul;19(7):1026–1031. doi: 10.1002/1097-0142(196607)19:7<1026::aid-cncr2820190718>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Novak U., Dilworth S. M., Griffin B. E. Coding capacity of a 35 percent fragment of the polyoma virus genome is sufficient to initiate and maintain cellular transformation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3278–3282. doi: 10.1073/pnas.77.6.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S. Diseases and mechanisms of persistent DNA virus infection: latency and cellular transformation. J Infect Dis. 1975 Aug;132(2):209–223. doi: 10.1093/infdis/132.2.209. [DOI] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Puga A., Cantin E. M., Notkins A. L. Homology between murine and human cellular DNA sequences and the terminal repetition of the S component of herpes simplex virus type 1 DNA. Cell. 1982 Nov;31(1):81–87. doi: 10.1016/0092-8674(82)90407-x. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Melnick J. L. The association of herpesvirus type 2 and carcinoma of the uterine cervix. Am J Epidemiol. 1969 May;89(5):547–554. doi: 10.1093/oxfordjournals.aje.a120967. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., LaFemina R., Hayward S. D., Hayward G. S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Royston I., Aurelian L. The association of genital herpesvirus with cervical atypia and carcinoma in situ. Am J Epidemiol. 1970 Jun;91(6):531–538. doi: 10.1093/oxfordjournals.aje.a121164. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Skinner G. R. Transformation of primary hamster embryo fibroblasts by type 2 simplex virus: evidence for a "hit and run" mechanism. Br J Exp Pathol. 1976 Aug;57(4):361–376. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stern E. Cytohistopathology of cervical cancer. Cancer Res. 1973 Jun;33(6):1368–1378. [PubMed] [Google Scholar]
- Stow N. D. Localization of an origin of DNA replication within the TRS/IRS repeated region of the herpes simplex virus type 1 genome. EMBO J. 1982;1(7):863–867. doi: 10.1002/j.1460-2075.1982.tb01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Umene K., Enquist L. W. Reiterated sequences within the intron of an immediate-early gene of herpes simplex virus type 1. Nucleic Acids Res. 1981 Aug 25;9(16):4189–4199. doi: 10.1093/nar/9.16.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet. 1982 Dec 18;2(8312):1370–1372. doi: 10.1016/s0140-6736(82)91273-9. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Wolf H., Dörries K., Egger H. Attempts to detect virus-specific DNA in human tumors. II. Nucleic acid hybridizations with complementary RNA of human herpes group viruses. Int J Cancer. 1974 May 15;13(5):657–664. doi: 10.1002/ijc.2910130510. [DOI] [PubMed] [Google Scholar]