Abstract
Objective
The aim of this study was to report the basic cerebrospinal fluid (CSF) profile in patients with primary progressive multiple sclerosis (PPMS).
Methods
The results of CSF analysis from 254 patients with PPMS were collected at four university hospitals in Germany. Routine CSF parameters and different indices of intrathecal immunoglobulin synthesis were evaluated. We assessed possible correlations between the various CSF parameters and the expanded disability status scale (EDSS) both at the time of lumbar puncture and during the course of the disease.
Results
The median cell count and albumin concentration in the CSF did not deviate from normal values. The CSF-serum albumin-quotient (QALB) was elevated in 29.6% of the patients, while intrathecal immunoglobulin G (IgG) oligoclonal bands (OCBs) were detected in 91.1% of the patients. CSF-lactate levels as well as local IgM- and IgA-synthesis were correlated with the yearly disease progression rate, as assessed by EDSS.
Conclusion
We present the results of the hitherto largest and most detailed CSF biomarker profile in a cohort of 254 patients with PPMS. As reported previously, OCBs are the most sensitive marker for intrathecal IgG synthesis. CSF-lactate concentrations are positively correlated with the progression rate, which might suggest that mitochondrial dysfunction plays a relevant role in PPMS. The negative correlation between intrathecally produced IgM and IgA and disease progression may indicate their hitherto unexplored protective role.
Introduction
Primary progressive multiple sclerosis (PPMS) is currently considered an entity in multiple sclerosis (MS) disease spectrum, representing about 15% of MS patients[1]. However, PPMS patients differ in many clinical, pathological, and imaging aspects, which explains the necessity of various diagnostic guidelines[2]. Indeed, the diagnostic guidelines for PPMS have been revised over the last few years[3–6]. In contrast to relapsing-remitting multiple sclerosis (RRMS), the cerebrospinal fluid (CSF) examination remains a part of the McDonald diagnostic criteria[6]. At present, many studies addressing the CSF profiles of various MS subtypes have been published. However, these studies had obvious limitations, including a relatively small number of PPMS patients (only one study reported data from more than 100 PPMS patients) as well as limited CSF parameter datasets[7–9]. Therefore, we initiated a multicenter study to systematically collect and analyze detailed CSF profiles containing all parameters commonly assessed in clinical practice in a well-characterized PPMS cohort consisting of more than 250 patients.
Methods
Data collection
CSF data were collected from four university hospitals in Germany (Ulm, Frankfurt, Rostock, and Freiburg). We included PPMS in- and outpatients treated between 2010 and 2015. Each PPMS diagnosis was established according to the 2010 revisions of the McDonald criteria[6] after careful exclusion of relevant differential diagnoses. Lumbar puncture (LP) was performed for diagnostic purposes only with the written consent of all patients. CSF and serum samples were taken on the same day and stored according to consensus protocol for the standardization of CSF collection and biobanking[10]. Records of all available patients matching these criteria were retrospectively reviewed regarding age at onset, initial neurological complaints, age at first diagnosis, time between clinical onset and diagnosis, expanded disability status scale (EDSS) at the time of LP (EDSSLP), EDSS at the last documented follow-up (EDSSFU), and treatments. Age at clinical onset and initial complaints were obtained from the available medical records and assessed according to the first documented neurological symptoms attributable to the disease. We divided the initial complaints into four main categories: motor, sensory, cerebellar, and other. The “other” category comprised brain stem syndromes, visual disturbances, cognitive symptoms, complex partial seizures, and bladder dysfunction. The clinical severity of MS was assessed using the EDSS score[11] and determined by a certified EDSS rater. We calculated the yearly progression rate by dividing the EDSSFU over the period between clinical onset and date of the EDSSFU.
CSF analysis included basic parameters, such as the total cell count; the CSF-serum quotient for albumin (QALB); quotients of immunoglobulin G, M, and A (IgG, IgM, and IgA); CSF-lactate concentration; oligoclonal bands (OCB) pattern[9]; and measles, rubella and zoster (MRZ) reaction[12]. In cases of repeated LP for the same patient, only the results of the LP used to establish the diagnosis were analyzed. QALB was used as an indicator for the blood-CSF barrier (BCB) function[13], and it was assessed according to the age-related reference range (4+ age/15)[14]. The IgX index was calculated using the following formula: (IgX CSF / IgX Serum: Albumin CSF / Albumin Serum)[15]. IgG-index values > 0.7 [16], IgM-index values > 0.061 [17], and IgA-index values > 0.34 [18] were considered elevated. The concentration of intrathecal IgG, IgM, and IgA -synthesis (IgGloc, IgMloc, and IgAloc) was calculated according to the following formula: IgXloc = (QIgX—Qlim). IgXserum. Following Reiber, the formula for Qlim was Qlim = a/b √QALB2 + b2 –c, where a/b, b2, and c values differ for each immunoglobulin type[19]. Negative values were reported as zero. IgG OCBs were classified into the following five patterns[20]: no bands detected in CSF or serum (pattern 1), bands detected in CSF only (pattern 2), bands detected in CSF plus additional identical bands in CSF and serum (pattern 3), identical bands detected in CSF and serum (pattern 4), and monoclonal bands detected in CSF and serum (pattern 5).
We considered the MRZ reaction to be positive when the reported antibody index (AI) against at least two of the included indices was > 1.4 [21].
The patients were further classified into three categories. PPMST included patients who received medical treatment over the disease course; PPMSTN included treatment-naïve patients, and LPD included those who received diagnostic LP before any medical treatment began.
Statistics
All statistical tests were performed using IBM SPSS Statistics, version 21 (Armonk, USA). We used the Shapiro–Wilk test to examine the distribution of the data and the Mann–Whitney U test or the Kruskal–Wallis test to compare the medians of different variables. Fisher’s exact test was used for qualitative variants, and Spearman’s rho test was used to measure correlation. A p-value < 0.05 was considered statistically significant. No Bonferroni correction was done due to the exploratory nature of this study.
Protocol approval
The study was reviewed by the appropriate ethics committee of the University of Ulm (approval number 20/10) and was performed in accordance with the ethical standards the 1964 Declaration of Helsinki. Written informed consent for the LP was obtained from all patients participating in this study.
Results
Clinical characteristics of the patients
The clinical features of the 254 enrolled PPMS patients are summarized in Table 1and Fig 1. Overall, 154 patients received one or more of the following therapies over the course of the disease: 3-month pulse steroid therapy (n = 71), mitoxantrone (n = 29), cyclosporine (n = 1), rituximab (n = 6), intravenous immunoglobulins (n = 1), dimethyl fumarate (n = 1), interferon beta-1a (n = 2), glatiramer acetate (n = 1), azathioprine (n = 4), and more than one therapy (n = 38). However, we did not include details such as the duration of the therapies because this was not the focus of the study. It should be mentioned that none of these therapies is approved for PPMS.
Table 1. Clinical features of primary progressive multiple sclerosis (PPMS) patients.
| Clinical features of primary progressive multiple sclerosis (PPMS) patients (median, interquartile range) | |
|---|---|
| Number | 254 |
| Gender (male: female) | 1: 1.1 |
| Age at first symptom | 44 (37–51) |
| Age at diagnosis | 47 (41–55) |
| Time between first symptom and diagnosis in years | 3 (1–5) |
| Expanded disability status scale (EDSS) at the date of lumbar puncture (LP) | 4.0 (3.0–5.5) |
| Follow up period in months | 27 (1–74) |
| EDSS at follow-up | 6.0 (4.0–6.5) |
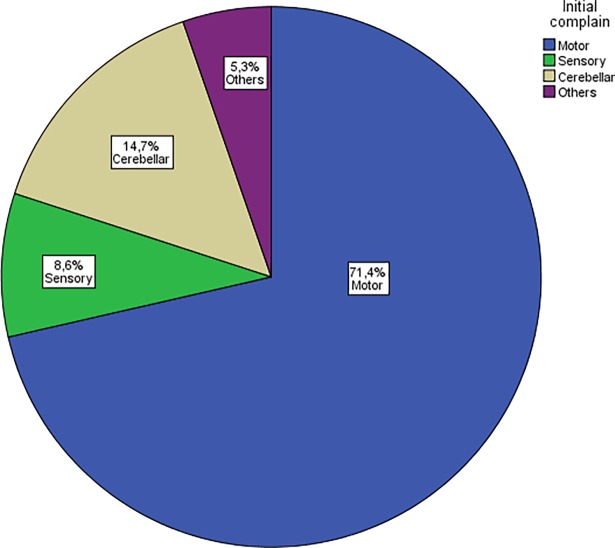
Fig 1. Distribution of the initial manifestations.
Motor weakness was the most common initial symptom in about 71.4% (175/245) of patients included in our multicenter cohort, followed by cerebellar manifestations in 14.7% (36/245) and sensory disturbances in 8.6% (21/245). Other initial manifestations such as brain stem syndromes, visual disturbances, cognitive symptoms, complex focal seizures or urinary disturbances were present in 6.3% (13/245).
Patients with a motor symptom at clinical onset (PPMSMotor) scored higher on the EDSSLP compared to patients with cerebellar manifestations (PPMSCerebellar) or patients with sensory symptoms (PPMSSensory) (4.5 vs 3.5 or 3.5, p < 0.001, n = 201). The yearly progression rate did not differ between these three subgroups with different initial symptoms (p = 0.3). Similarly, analysis of PPMSTN revealed a higher EDSSLP in the PPMSMotor group compared to the PPMSCerebellar and PPMSSensory groups (4.5 vs 3.5 vs 4.0, p = 0.03). The yearly progression rate did not differ between the groups (p = 0.2).
CSF profile in PPMS
In some patients, clinical or CSF data were not obtainable from the records or could not be retrospectively evaluated. A summary of these results is provided in Table 2.
Table 2. Summary of the results of lumbar puncture (LP) in patients with primary progressive multiple sclerosis (PPMS).
| CSF parameter | Median (maximum) | interquartile range | Missing data | |||
|---|---|---|---|---|---|---|
| Total PPMS cohort | LPD | Total PPMS cohort | LPD | Total PPMS cohort (total number = 254) |
LPD (total number = 209) | |
| Cell count (/μl) | 2 (101) | 3 (101) | 1–5 | 1–5 | 20 | 10 |
| Total protein (mg/l) | 485 (1775) | 485 (1775) | 181–323 | 366–602 | 21 | 10 |
| Albumin (mg/l) | 252 (1300) | 247 (1300) | 374–593 | 176–332 | 78 | 63 |
| Alb-Q (x103)% | 5.8 (37.8) | 5.8 (37.8) | 4.3-7-8 | 4.2–7.9 | 27 | 17 |
| Lactat (mmol/l) | 1.7 (5.6) | 1.7 (2.9) | 1.5–1.9 | 1.5–1.9 | 153 | 127 |
| IgG index | 0.7 (6.0) | 0.8 (6.0) | 0.6–1.10 | 0.6–1.1 | 75 | 65 |
| IgGloc | 2.70 (271.7) | 3.3 (271.7) | 0–24.4 | 0–24.1 | 75 | 65 |
| IgA index | 0.3 (2.3) | 0.3 (2.1) | 0.2–0.3 | 0.3–0.3 | 78 | 68 |
| IgAloc | 0 (72.7) | 0 (72.7) | 0 | 0 | 78 | 68 |
| IgM index | 0.08 (0.97) | 0.08 (0.97) | 0.05–0.13 | 0.05–0.2 | 78 | 68 |
| IgMloc | 0 (4.5) | 0 (2.5) | 0 | 0 | 78 | 68 |
Loc = local synthesis in mg/dl, LPD: diagnostic lumbar puncture.
Cell count, total protein, albumin, and lactate
The cell count was elevated in 28.6% (n = 67) of patients. CSF-lactate levels were not elevated with a median of 1.7 mmol/L. The age-matched QALB was elevated in 29.6% (n = 67/226) of the patients.
Similar results were found for the LPD subgroup, in which 28.6% (n = 57) of patients had an elevated cell count. The CSF-lactate median was 1.7 mmol/L (0.5–2.9, n = 63), and the QALB was elevated in 32.7% of the patients (n = 48/147).
Intrathecal immunoglobulin synthesis
Pattern 1 was found in 8.2% of the patients (n = 18/220). With 78.6%, pattern 2 was observed most commonly in our patients (n = 173/220) whereas pattern 3 was found in 12.3% of the patients (n = 27/220). Two cases showed pattern 4 OCBs.
The MRZ reaction was positive in 77 of 148 patients (52%). Patients having a biphasic reaction (only 2 AI are positive) constituted 25% (37/148) of patients with positive MRZ-Reaction. A triphasic positive reaction (M+,R+ and V+) was found in 27.0% (n = 40/148), whereas a monophasic positive reaction (M+ or R+ or V+) was detected in 31.1% (n = 46/148). The Prevalence of positive AI against single virus and of the various combination is mentioned in Table 3.
Table 3. Prevalence of single positive AI against measles (M), Rubella (R) and varicella-zoster virus (Z) and different combinations in our PPMS cohort.
| Positive Antibody index | Measles (M) | Rubella (R) | Varicella Zoster (Z) | M+R+Z- | M+R-Z+ | M-R+Z+ | M+R+Z+ | Positive MRZ-reaction (≥ two AI > 1.4) |
|---|---|---|---|---|---|---|---|---|
| Total PPMS cohort | 24/148 (16.2%) |
7/148 (4.7%) |
15/148 (10.1%) |
13/148 (8.8%) |
13/148 (8.8%) |
11/148 (7.4%) |
40/148 (27.0%) |
77/148 (52.0%) |
| OCB+ | 24/139 (17.3%) |
7/139 (5.0%) |
14/139 (10.1%) |
13/139 (9.4%) |
12/139 (8.6%) |
11/139 (7.9%) |
35/139 (25.2%) |
71/139 (51.1%) |
| OCB- | 0/9 |
0/9 |
1/9 (11.1%) |
0/9 |
1/9 (11.1%) |
0/9 |
5/9 (55.6%) |
6/9 (66.7%) |
| LPD | 18/127 (14.2%) |
7/127 (5.5%) |
13/127 (10.2%) |
11/127 (8.7%) |
12/127 (9.4%) |
11/127 (8.7%) |
34/127 (26.8%) |
68/127 (53.5%) |
OCB+: patients with positive oligoclonal bands, OCB-: patients with negative oligoclonal bands, LPD: diagnostic lumbar puncture.
The IgG-index values were elevated in 49.2% (n = 88/179), and IgGloc tested positive in 61.7%. The IgM index was elevated in 58.5% (n = 103/176), and the IgMloc was positive in 21.0% (n = 37/176). While the IgA index was elevated in 24.4% (n = 43/176), IgAloc was detectable in 17.6% of the patients (n = 31/176).
Analysis of the LPD subgroup revealed similar results. The IgG-index values were elevated in 51.4% (n = 74/144), while IgGloc tested positive in 63.1% of this subgroup.
Correlations between CSF findings and clinical parameters
QALB
Comparing the analysis of the whole group with that of the LPD subgroup revealed no differences between the median EDSSLP and median yearly progression rate, which were 4.0 and 0.58 in both groups, respectively, in patients with normal and elevated QALB. In the PPMSTN subgroup, the median yearly progression rates were 0.73 and 0.54 in patients with normal and elevated QALB, respectively (p = 0.6, n = 34).
CSF lactate
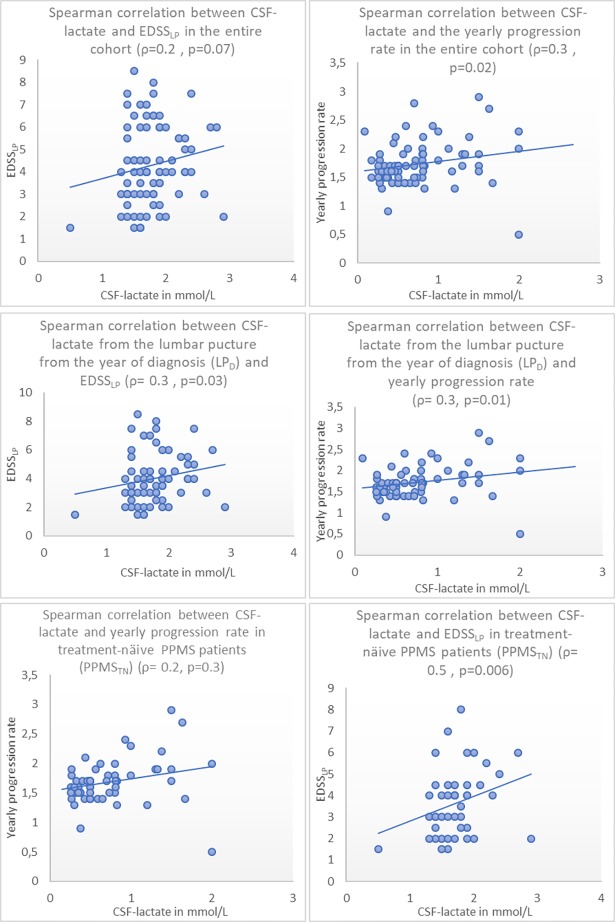
CSF-lactate levels were consistently correlated with the progression rate in the entire cohort, the LPD and PPMSTN subgroups. After excluding one case that had an extremely high lactate level (5.6 mmol/L), we found a correlation between CSF-lactate levels and EDSSLP scores in the LPD subgroup but not in the entire cohort or the PPMSTN subgroup (see Table 4and Fig 2). CSF-lactate levels did not correlate with the patients’ age at the time of LP.
Table 4. Spearman correlation (ρ) between CSF-lactate and EDSS at the lumbar puncture (EDSSLP) and the yearly progression rate in the entire cohort, treatment-naïve PPMS patients (PPMSTN) and at the LP at the year of diagnosis (LPD).
| Entire cohort | PPMSTN | LPD | |
|---|---|---|---|
| Correlation between CSF-lactate in (mmol/L) and EDSSLP | ρ = 0.2 (p = 0.07) (n = 80) | ρ = 0.2 (p = 0.3) (n = 33) | ρ = 0.3 (p = 0.03) (n = 66) |
| Correlation between CSF-lactate in (mmol/L) and yearly progression rate | ρ = 0.3 (p = 0.02) (n = 76) | ρ = 0.5 (p = 0.006) (n = 32) | ρ = 0.3 (p = 0.01) (n = 62) |
Fig 2. Correlation between CSF-lactate and EDSSLP and yearly progression rate in the entire cohort, EDSSLP and LPD.
Spearman correlation between the CSF-lactate and expanded disability score scale at the LP (EDSSLP) and yearly progression rate showed consistently statistically significant positive correlation in the entire cohort, treatment-näive patients PPMSTN and in the results of the diagnostic LP (LPD). On the other side, a significant correlation between CSF-lactate and EDSSLP was found only in the LPD.
Intrathecal synthesis of immunoglobulins
The median EDSSLP and the yearly progression rate did not differ based on the OCB pattern, MRZ reaction or among patients with elevated or normal IgG, IgM, and IgA index values.
We found no correlation between IgG–production and clinical parameters in the entire cohort and those in the LPD subgroup. In the PPMSTN subgroup, however, quantitative markers of intrathecal IgG synthesis (IgG index and IgGloc) were correlated with the yearly progression rate (ρ = 0.4; p = 0.01 and 0.02, respectively; n = 35). In patients with intrathecal IgM/A synthesis, we found a moderate negative correlation between both IgMloc, IgAloc, and the yearly progression rate (ρ = -0.4, p = 0.03, n = 28, and ρ = -0.5, p = 0.01, n = 25, respectively).
Discussion
Our study is, to the best of our knowledge, the largest CSF cohort reported in the literature thus far, with 254 PPMS patients. Motor impairment was the most frequent initial symptom, followed by cerebellar disturbances and sensory manifestations. These findings, along with the mean age and sex distribution, are typical for PPMS and consistent with other published cohorts[1, 2, 22]. The higher EDSSLP rating for patients in the PPMSmotor group is probably due to the relatively high influence of motor symptoms on the EDSS overall[23]. The large proportion of PPMS patients under treatment that has not been approved illustrates the greatest known difficulty in treating patients with a severe progressive disease, as no approved therapies were available at the time of writing this article.
The median cell count did not deviate from the normal values, which is consistent with previous reports on other CSF cohorts with MS[24]. The BCB dysfunction was found in only one-third of the patients, which is in accordance with the hypothesis that inflammation is compartmentalized behind an intact blood-brain barrier (BBB)[25]. Other factors causing increased Alb-Q, such as reduced flow rate, were not assessed in our study.
Until recently, the clinical significance of CSF lactate in MS was not known[26]. Thus, in more than the half of our patients, CSF-lactate levels had not been routinely measured. Nevertheless, we have reported a normal CSF-lactate median in patients with PPMS[27]. Simone et al.[28] and Regenold et al.[29] reported increased levels of lactate in MS patients, while Aasly et al.[30] reported lower levels compared to healthy controls. However, all three studies did not include PPMS patients.
We have reported a weak to moderate positive correlation between CSF-lactate levels and the yearly progression rate in the entire cohort and all subgroups. A similar association was reported recently in a study with 118 RRMS patients[26]. A previous study also reported a positive correlation between serum lactate and the disease severity in all clinical subtypes of MS[31]. In other studies, CSF lactate correlated with the cell count, inflammatory and gadolinium-enhancing MRI lesions in MS patients[28, 32]. This might be explained through the mitochondrial dysfunction in progressive MS subtypes. Mitochondrial dysfunction with subsequent cellular hypoxia is especially relevant for the neurodegeneration of susceptible, chronically demyelinated axons that are commonly found in progressive MS subtypes[33]. However, this is rather speculative and prospective studies with a larger number of patients are essential to validate the prognostic value of testing CSF-lactate levels in PPMS.
The higher incidence of intrathecal IgG production indicated by OCBs compared to the elevated IgG-index and IgGloc confirms the well-known higher sensitivity of isoelectric focusing (IEF)[34]. Our results were consistent with McLean et al.’s study, which reported OCB in 86–88% of samples from a cohort that included 31 progressive MS patients[9], but higher than those reported from the PROMiSe trial cohort (80%)[35]. OCBs pattern 2 was found the most frequently in our PPMS patients (79% of patients), matching the results of McLean et al.’s study[9]. In contrast, Villar et al. observed a predominance (64%) of OCBs pattern 3, but the sample size was much smaller than that of our study (n = 39)[36]. The role of systemic inflammation in the disease progression of PPMS is described elsewhere[37], [38]. In our study, we did not find any differences between yearly progression rates in patients with OCBs patterns 2 and 3. Nevertheless, studies with a larger sample size are needed to confirm our results.
In our study, the frequency of elevated IgG-index values was lower than in the cohort described by Izquierdo et al.[39]. However, Izquierdo et al.’s study included only 23 PPMS patients, who were diagnosed according to the diagnostic criteria specified by Poser. We did not find any positive correlations between EDSSLP or disease progression rates and intrathecal IgG synthesis, except in the PPMSTN group. However, because of the small number of patients in this group, these results should be evaluated with caution.
The MRZ reaction has been proposed as a highly specific (rule-in rather than rule-out) marker of MS[40, 41], which discriminates well between MS and neuromyelitis optica [42, 43] and possibly also between MS and MOG encephalomyelitis [44]. A positive MRZ reaction was found in in slightly more than half of the PPMS patients, a slightly lower percentage than what was observed in clinically isolated syndrome (CIS) and RRMS 60–70%[12, 41, 45] and is similar to a recent report from one of the study centres [46]. The difference might reflect lower prevalence of polyspecific immunoglobulin synthesis in PPMS or may be explained by various assays applied in different studies.
Data reports on the prevalence of intrathecal IgM synthesis in PPMS are scarce. We only found one such study, which reported elevated IgM index in 32.3% of its 29 patients with chronic progressive MS[47]. Increased IgM-index values might include false-positive results due to the linear formula used. Likewise, no studies investigating the intrathecal IgA synthesis in PPMS have been published thus far.
For the first time, a negative correlation between absolute levels of intrathecally produced IgM and IgA in CSF and disease progression has been reported in a large PPMS cohort. The correlation we found might indicate a possible protective role (e.g., anti-inflammatory or remyelinating) for IgM and IgA in PPMS. This role, postulated from in-vitro results, may be explained by the stimulatory effects of IgM on the oligodendrocytes as well as axonal protection[48, 49].
By using the IgX index and the Reiber formula to calculate the absolute amounts of intrathecal synthesis of immunoglobulins in mg/l, it becomes clear that the discrepancies among the prevalence of intrathecal IgG, IgM, and IgA synthesis are caused by the linear (IgX index) versus non-linear (IgXloc) relationships between the QALB and QIgX[19].
The main limitations of this study include the retrospective cohort design and the incomplete clinical and CSF data for some patients. However, the large number of patients in our cohort and the multicentric aspect of the design are major advantages of study, which may be the last one to include the treatment of naïve PPMS patients as different effective disease-modifying drugs are expected to enter the market in the near future [50, 51].
In summary, our study included the most detailed CSF results of the largest PPMS cohort hitherto reported, with 254 PPMS patients. The main findings were the following: a) the high diagnostic sensitivity of intrathecally produced OCBs, which mainly consisted of pattern 2; b) the positive correlation of CSF-lactate levels with clinical severity and yearly progression rates; and c) the possible protective role of intrathecally synthesized IgM and IgA, which may be of therapeutic relevance.
Acknowledgments
We would like to thank Alyssa Perry for reviewing and correcting the manuscript.
Data Availability
All relevant data are within the paper. For further details authors may be contacted at ahmed.abdelhak@uni-ulm.de.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Thompson AJ, Polman CH, Miller DH, McDonald WI, Brochet B, Filippi MMX, et al. Primary progressive multiple sclerosis. Brain: a journal of neurology. 1997;120 (Pt 6):1085–96. . [DOI] [PubMed] [Google Scholar]
- 2.Miller DH, Leary SM. Primary-progressive multiple sclerosis. The Lancet Neurology. 2007;6(10):903–12. doi: 10.1016/S1474-4422(07)70243-0 . [DOI] [PubMed] [Google Scholar]
- 3.Thompson AJ, Montalban X, Barkhof F, Brochet B, Filippi M, Miller DH, et al. Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Annals of neurology. 2000;47(6):831–5. . [PubMed] [Google Scholar]
- 4.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of neurology. 2001;50(1):121–7. . [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of neurology. 2005;58(6):840–6. doi: 10.1002/ana.20703 . [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69(2):292–302. doi: 10.1002/ana.22366 ; PubMed Central PMCID: PMCPMC3084507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lourenco P, Shirani A, Saeedi J, Oger J, Schreiber WE, Tremlett H. Oligoclonal bands and cerebrospinal fluid markers in multiple sclerosis: associations with disease course and progression. Multiple sclerosis. 2013;19(5):577–84. doi: 10.1177/1352458512459684 . [DOI] [PubMed] [Google Scholar]
- 8.Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Annals of neurology. 2009;66(4):460–71. doi: 10.1002/ana.21867 . [DOI] [PubMed] [Google Scholar]
- 9.McLean BN, Luxton RW, Thompson EJ. A study of immunoglobulin G in the cerebrospinal fluid of 1007 patients with suspected neurological disease using isoelectric focusing and the Log IgG-Index. A comparison and diagnostic applications. Brain: a journal of neurology. 1990;113 (Pt 5):1269–89. . [DOI] [PubMed] [Google Scholar]
- 10.Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914–22. doi: 10.1212/WNL.0b013e3181c47cc2 ; PubMed Central PMCID: PMCPMC2839806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. . [DOI] [PubMed] [Google Scholar]
- 12.Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Multiple sclerosis. 1998;4(3):111–7. doi: 10.1177/135245859800400304 . [DOI] [PubMed] [Google Scholar]
- 13.Reiber H. Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. Journal of the neurological sciences. 1994;122(2):189–203. . [DOI] [PubMed] [Google Scholar]
- 14.Reiber H, Otto M, Trendelenburg C, Wormek A. Reporting cerebrospinal fluid data: knowledge base and interpretation software. Clin Chem Lab Med. 2001;39(4):324–32. doi: 10.1515/CCLM.2001.051 . [DOI] [PubMed] [Google Scholar]
- 15.Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37(5):397–401. doi: 10.1080/00365517709091498 . [DOI] [PubMed] [Google Scholar]
- 16.Zettl UK LR, Mix E. Klinische Liquordiagnostik. De Gruyter Verlag; 2003. [Google Scholar]
- 17.Forsberg P, Henriksson A, Link H, Ohman S. Reference values for CSF-IgM, CSF-IgM/S-IgM ratio and IgM index, and its application to patients with multiple sclerosis and aseptic meningoencephalitis. Scand J Clin Lab Invest. 1984;44(1):7–12. . [DOI] [PubMed] [Google Scholar]
- 18.Lolli F, Halawa I, Link H. Intrathecal synthesis of IgG, IgA, IgM and IgD in untreated multiple sclerosis and controls. Acta Neurol Scand. 1989;80(3):238–47. . [DOI] [PubMed] [Google Scholar]
- 19.Reiber H, Uhr M. Liquordiagnostik. 2011:143–78. 10.1007/978-3-642-16920-5_6.
- 20.Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Archives of neurology. 2005;62(6):865–70. doi: 10.1001/archneur.62.6.865 . [DOI] [PubMed] [Google Scholar]
- 21.Hottenrott T, Dersch R, Berger B, Rauer S, Eckenweiler M, Huzly D, et al. The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS. 2015;12:27 doi: 10.1186/s12987-015-0024-8 ; PubMed Central PMCID: PMCPMC4677451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottrell DA, Kremenchutzky M, Rice GP, Koopman WJ, Hader W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain: a journal of neurology. 1999;122 (Pt 4):625–39. . [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58 doi: 10.1186/1471-2377-14-58 ; PubMed Central PMCID: PMCPMC3986942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tourtellotte WW, Ma BI. Multiple sclerosis: the blood-brain-barrier and the measurement of de novo central nervous system IgG synthesis. Neurology. 1978;28(9 Pt 2):76–83. . [DOI] [PubMed] [Google Scholar]
- 25.Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis. Journal of the neurological sciences. 2008;274(1–2):42–4. doi: 10.1016/j.jns.2008.06.032 . [DOI] [PubMed] [Google Scholar]
- 26.Albanese M, Zagaglia S, Landi D, Boffa L, Nicoletti CG, Marciani MG, et al. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J Neuroinflammation. 2016;13(1):36 doi: 10.1186/s12974-016-0502-1 ; PubMed Central PMCID: PMCPMC4750170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leen WG, Willemsen MA, Wevers RA, Verbeek MM. Cerebrospinal fluid glucose and lactate: age-specific reference values and implications for clinical practice. PloS one. 2012;7(8):e42745 doi: 10.1371/journal.pone.0042745 ; PubMed Central PMCID: PMCPMC3412827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simone IL, Federico F, Trojano M, Tortorella C, Liguori M, Giannini P, et al. High resolution proton MR spectroscopy of cerebrospinal fluid in MS patients. Comparison with biochemical changes in demyelinating plaques. Journal of the neurological sciences. 1996;144(1–2):182–90. . [DOI] [PubMed] [Google Scholar]
- 29.Regenold WT, Phatak P, Makley MJ, Stone RD, Kling MA. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. Journal of the neurological sciences. 2008;275(1–2):106–12. doi: 10.1016/j.jns.2008.07.032 ; PubMed Central PMCID: PMCPMC2584157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aasly J, Garseth M, Sonnewald U, Zwart JA, White LR, Unsgard G. Cerebrospinal fluid lactate and glutamine are reduced in multiple sclerosis. Acta Neurol Scand. 1997;95(1):9–12. . [DOI] [PubMed] [Google Scholar]
- 31.Amorini AM, Nociti V, Petzold A, Gasperini C, Quartuccio E, Lazzarino G, et al. Serum lactate as a novel potential biomarker in multiple sclerosis. Biochimica et biophysica acta. 2014;1842(7):1137–43. 10.1016/j.bbadis.2014.04.005. doi: 10.1016/j.bbadis.2014.04.005 . [DOI] [PubMed] [Google Scholar]
- 32.Lutz NW, Viola A, Malikova I, Confort-Gouny S, Audoin B, Ranjeva JP, et al. Inflammatory multiple-sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PloS one. 2007;2(7):e595 doi: 10.1371/journal.pone.0000595 ; PubMed Central PMCID: PMCPMC1899231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. The Lancet Neurology. 2009;8(3):280–91. doi: 10.1016/S1474-4422(09)70043-2 . [DOI] [PubMed] [Google Scholar]
- 34.Lunding J, Midgard R, Vedeler CA. Oligoclonal bands in cerebrospinal fluid: a comparative study of isoelectric focusing, agarose gel electrophoresis and IgG index. Acta Neurol Scand. 2000;102(5):322–5. . [DOI] [PubMed] [Google Scholar]
- 35.Wolinsky JS, Narayana PA, O'Connor P, Coyle PK, Ford C, Johnson K, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Annals of neurology. 2007;61(1):14–24. doi: 10.1002/ana.21079 . [DOI] [PubMed] [Google Scholar]
- 36.Villar LM, Masjuan J, Sadaba MC, Gonzalez-Porque P, Plaza J, Bootello A, et al. Early differential diagnosis of multiple sclerosis using a new oligoclonal band test. Archives of neurology. 2005;62(4):574–7. doi: 10.1001/archneur.62.4.574 . [DOI] [PubMed] [Google Scholar]
- 37.Ukkonen M, Wu K, Reipert B, Dastidar P, Elovaara I. Cell surface adhesion molecules and cytokine profiles in primary progressive multiple sclerosis. Multiple sclerosis. 2007;13(6):701–7. doi: 10.1177/1352458506075378 . [DOI] [PubMed] [Google Scholar]
- 38.Romme Christensen J, Bornsen L, Ratzer R, Piehl F, Khademi M, Olsson T, et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PloS one. 2013;8(3):e57820 doi: 10.1371/journal.pone.0057820 ; PubMed Central PMCID: PMCPMC3585852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izquierdo G, Angulo S, Garcia-Moreno JM, Gamero MA, Navarro G, Gata JM, et al. Intrathecal IgG synthesis: marker of progression in multiple sclerosis patients. Acta Neurol Scand. 2002;105(3):158–63. . [DOI] [PubMed] [Google Scholar]
- 40.Jarius S, Eichhorn P, Jacobi C, Wildemann B, Wick M, Voltz R. The intrathecal, polyspecific antiviral immune response: Specific for MS or a general marker of CNS autoimmunity? Journal of the neurological sciences. 2009;280(1–2):98–100. doi: 10.1016/j.jns.2008.08.002 . [DOI] [PubMed] [Google Scholar]
- 41.Jarius S, Eichhorn P, Franciotta D, Petereit HF, Akman-Demir G, Wick M, et al. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. Journal of neurology. 2017;264(3):453–66. doi: 10.1007/s00415-016-8360-4 . [DOI] [PubMed] [Google Scholar]
- 42.Jarius S, Franciotta D, Bergamaschi R, Rauer S, Wandinger KP, Petereit HF, et al. Polyspecific, antiviral immune response distinguishes multiple sclerosis and neuromyelitis optica. Journal of neurology, neurosurgery, and psychiatry. 2008;79(10):1134–6. doi: 10.1136/jnnp.2007.133330 . [DOI] [PubMed] [Google Scholar]
- 43.Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation. 2012;9:14 doi: 10.1186/1742-2094-9-14 ; PubMed Central PMCID: PMCPMC3283476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280 doi: 10.1186/s12974-016-0718-0 ; PubMed Central PMCID: PMCPMC5086042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brettschneider J, Tumani H, Kiechle U, Muche R, Richards G, Lehmensiek V, et al. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PloS one. 2009;4(11):e7638 doi: 10.1371/journal.pone.0007638 ; PubMed Central PMCID: PMCPMC2766627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hottenrott T, Dersch R, Berger B, Rauer S, Huzly D, Stich O. The MRZ reaction in primary progressive multiple sclerosis. Fluids Barriers CNS. 2017;14(1):2 doi: 10.1186/s12987-016-0049-7 ; PubMed Central PMCID: PMCPMC5294835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reboul J, Lambin P, Gervais A, Gaillard O, Delattre J, Bricaire F, et al. Frequency and relevance of IgM intrathecal synthesis in multiple sclerosis. Eur J Neurol. 1995;2(5):429–34. doi: 10.1111/j.1468-1331.1995.tb00152.x . [DOI] [PubMed] [Google Scholar]
- 48.Wootla B, Denic A, Warrington AE, Rodriguez M. A monoclonal natural human IgM protects axons in the absence of remyelination. J Neuroinflammation. 2016;13(1):94 doi: 10.1186/s12974-016-0561-3 ; PubMed Central PMCID: PMCPMC4850699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warrington AE, Rodriguez M. Method of identifying natural antibodies for remyelination. J Clin Immunol. 2010;30 Suppl 1:S50–5. doi: 10.1007/s10875-010-9406-5 ; PubMed Central PMCID: PMCPMC4108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helwick C. Ocrelizumab Benefit in Primary Progressive MS Consistent in Subgroups: Medscape; 2016 [cited 2016 2016]. Available from: http://www.medscape.com/viewarticle/859222.
- 51.Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: A randomised, double-blind, placebo-controlled study. Multiple sclerosis. 2016. doi: 10.1177/1352458516667568 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. For further details authors may be contacted at ahmed.abdelhak@uni-ulm.de.