Abstract
Background
Childhood asthma morbidity has been associated with short-term air pollution exposure. To date, most investigations have used time-series models, and it is not well understood how exposure misclassification arising from unmeasured spatial variation may impact epidemiological effect estimates. Here, we develop case-crossover models integrating temporal and spatial individual-level exposure information, toward reducing exposure misclassification in estimating associations between air pollution and child asthma exacerbations in New York City (NYC).
Methods
Air pollution data included: a) highly spatially-resolved intra-urban concentration surfaces for ozone and co-pollutants (nitrogen dioxide and fine particulate matter) from the New York City Community Air Survey (NYCCAS), and b) daily regulatory monitoring data. Case data included citywide hospital records for years 2005–2011 warm-season (June – August) asthma hospitalizations (n = 2,353) and Emergency Department (ED) visits (n = 11,719) among children aged 5 to 17 years. Case residential locations were geocoded using a multi-step process to maximize positional accuracy and precision in near-residence exposure estimates. We used conditional logistic regression to model associations between ozone and child asthma exacerbations for lag days 0 to 6, adjusting for co-pollutant and temperature exposures. To evaluate the effect of increased exposure specificity through spatial air pollution information, we sequentially incorporated spatial variation into daily exposure estimates for ozone, temperature, and co-pollutants.
Results
Percent excess risk per 10 ppb ozone exposure in spatio-temporal models were significant on lag days 1 through 5, ranging from 6.5 (95% CI: 0.2 – 13.1) to 13.0 (6.0 – 20.6) for inpatient hospitalizations, and from 2.9 (95% CI: 0.1 – 5.7) to 9.4 (6.3 – 12.7) for ED visits, with strongest associations consistently observed on lag day 2. Spatio-temporal excess risk estimates were consistently but not statistically significantly higher than temporal-only estimates on lag days 0 to 3.
Conclusion
Incorporating case-level spatial exposure variation produced small, non-significant increases in excess risk estimates. Our modeling approach enables a refined understanding of potential measurement error in temporal-only versus spatio-temporal air pollution exposure assessments. As ozone generally varies over much larger spatial scales than that observed within NYC, further work is necessary to evaluate potential reductions in exposure misclassification for populations spanning wider geographic areas, and for other pollutants.
Keywords: case-crossover, childhood asthma, intraurban variation, ozone, spatio-temporal
BACKGROUND
There is substantial evidence linking short-term variation in ambient air pollution with acute asthmatic response in children (Akinbami et al. 2010; Babin et al. 2007; Dong et al. 2011; Halonen et al. 2010; Samoli et al. 2011; Sheffield et al. 2015; Silverman and Ito 2010; Strickland et al. 2010). Air pollutant concentrations can vary substantially within urban areas (Jerrett et al. 2005; Clougherty et al. 2013), and this exposure variation has been associated with asthma exacerbation risk (Lemke et al. 2014; Laurent et al. 2007). Further, because intraurban variation in ozone is driven by availability of nitrogen oxides and photochemical transformation, patterns of ozone exposures can differ substantially from those of other pollutants [e.g., nitrogen dioxide (NO2), fine particulate matter (PM2.5) (NYC DOHMH 2009)], complicating the interpretability of associations between ozone concentrations and asthma outcomes. Despite these important challenges – and the tremendous public health burden of urban childhood asthma (CDC 2014; Akinbami et al. 2009) – few studies to date have been able to account for fine-scale spatio-temporal variation in ozone, temperature and co-pollutant exposures in analyses of childhood asthma exacerbation.
Case-crossover designs have been widely applied in epidemiology studies of acute air pollution exposures (Carracedo-Martínez et al. 2010), with certain advantages compared to time-series analyses. Specifically, because each case serves as their own control, time-invariant case characteristics (e.g., race, sex) are controlled for by design (Maclure 1991). Further, selecting referent (non-case) days immediately before and/or after the case event day effectively accounts for seasonal patterns (Bateson and Schwartz 1999, 2001). To date, however, a key advantage of case-crossover design for air pollution epidemiology – the ability to incorporate case-level spatial exposure variation – has been under-explored (Carracedo-Martínez et al. 2010). This analytic advantage may be particularly important for reducing exposure misclassification arising from fine-scale spatial variation for short-term air pollution epidemiology.
While there is growing interest in methods for integrating temporal and spatial variation in air pollution exposure assessment (Özkaynak et al. 2014), there are relatively few examples using the case-crossover design. In one example, Delfino et al. (2014) observed significant effect modification of the association between daily air pollution levels (i.e., temporal variation) and asthma morbidity across increasing levels of modeled near-residence traffic-related pollution concentrations (i.e., spatial variation) across Orange County, California. Alternatively, approaches utilizing spatio-temporal exposure metrics (e.g., Ross et al. 2013; Johnson et al. 2013; Maynard et al. 2007) are well-suited to case-crossover design, but, to date, have also been underutilized.
Here we leverage highly spatially-refined air pollution data from the New York City Community Air Survey (NYCCAS) (Clougherty et al 2013; Matte et al. 2013) to incorporate individual-level spatial exposure variation in a case-crossover analysis of daily ozone exposure and childhood asthma exacerbation events in New York City (NYC). We compare percent excess risk estimates derived from aspatial vs. spatio-temporal exposure covariates to evaluate differences in magnitude and precision.
METHODS
Asthma case data
Emergency Department (ED) visit (outpatient) and hospitalization (inpatient) event data for asthma (ICD-9 code: 493) among children aged 5 to 17 years old in NYC from 2005 – 2011 (June 1 – August 31) were obtained from the New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS). Cases were limited to warm season months (June – August) to match the period over which the NYCCAS spatial ozone assessment occurred, and to capture annual-peak ozone levels resulting from higher rates of photochemical transformation (DOHMH 2009a). We excluded case events for children younger than 5 years, due to questionable reliability of asthma diagnosis among younger children (Potter 2010).
Air pollution data and exposure assignment
We estimated short-term near-residence exposures to ozone and co-pollutants by integrating temporally- and spatially-refined data sources. Spatial data consisted of fine-scale summertime O3 and co-pollutant (annual average NO2 and PM2.5) concentrations, derived from two years of NYCCAS monitoring data (2009 – 2010). NYCCAS is an on-going air pollution surveillance initiative of the New York City Department of Health and Mental Hygiene (NYC DOHMH), methods for which are detailed elsewhere (Matte et al. 2013; Clougherty et al. 2013). Briefly, two-week integrated street-level (10 – 12 feet above the ground) samples were collected at 150 monitoring locations across NYC. Land Use Regression (LUR) was used to predict fine-scale seasonal-average concentration estimates corresponding to 300m grid centroids, enabling fine-scale exposure estimates.
Temporal data included hourly monitoring data from five US Environmental Protection Agency Air Quality System (EPA AQS) regulatory monitoring stations in NYC, for the years 2005 – 2011 (May – September). Methods for constructing city-wide daily time-series are detailed elsewhere (Sheffield et al. 2015). Briefly, missing values were interpolated and daily 24-hour average O3 concentration values were computed for each regulatory monitor. We considered the spatial distribution of, and data density within, regulatory monitors to prevent biasing the citywide time trend. To enable adjustment for potential confounding by co-pollutant exposures, fine particulate matter (PM2.5) and nitrogen dioxide (NO2) daily time series were constructed using the average of scaled daily values to account for between-site differences in variance and mean of regulatory monitoring data, as in Schwartz (2000).
We combined these spatial and temporal air pollution data to estimate spatio-temporal near-residence exposures. We first applied a multi-step validation and geocoding process, methods for which are detailed in Supplemental Materials. Briefly, our approach used three address locators – the geographic reference data that translate addresses to latitude and longitude point locations – to sequentially geocode addresses, allowing for maximal accuracy and match rates. This approach also enabled sensitivity analysis for geocoding-induced exposure misclassification, wherein we re-fit all models including only the subset of subjects whose addresses were geocoded with the highest positional accuracy.
We estimated near-residence seasonal-average pollution exposures from NYCCAS spatial data as the mean concentration within a 300 m radial buffer around the geocoded point. To generate daily spatio-temporal exposure estimates, we multiplied daily EPA AQS concentrations by the ratio of near-residence (i.e., 300m buffer mean) concentration to the citywide average of NYCCAS concentrations across populated areas (i.e., census tracts with residential population > 20).
Meteorological covariates
Spatial temperature data consisted of a NYCCAS-derived fine-scale spatial temperature surface. NYCCAS temperature monitoring data included 15-minute measurements from continuous HOBO sensors (Pocasset, MA) deployed at each site throughout all sampling periods, averaged by hour and site. For calculating the spatial predictive surface, overnight (3 – 5 AM) averages (adjusted for trends at the five NYCCAS reference sites) were used as the dependent variable because they presented the most consistent spatial patterns across seasons. As such, overnight temperatures may be more indicative of consistent spatial differences in ambient temperature, compared to average or maximum temperature (due to localized intermittent shading in street canyons, etc.).
Temporal temperature data from the four meteorological stations in the NYC area (JFK International Airport, LaGuardia Airport, Central Park, Newark International Airport) was retrieved from the National Oceanic and Atmospheric Administration (NOAA) National Climatic Data Center (NOAA 2014a). Daily minimum temperature, average temperature (Tavg), daily maximum temperature (Tmax), and dew point temperature (TDP) were averaged across the four stations, and these values were highly correlated across stations: Tmin vs. Tavg rho = 0.93; Tmin vs. Tmax rho = 0.84. Relative humidity (RH) was calculated from Tavg and TDP using the standard NOAA equation (NOAA 2014b). To best match the spatial temperature surface, we created a daily time-series including 3 – 5 AM hourly average temperature from the four NOAA sites (hereafter Tmin). As with spatio-temporal air pollution exposure estimates, we adjusted the daily temperature time-series using the spatial ratio of near-residence (i.e., 300m buffer mean) Tmin to the citywide average Tmin for each case.
Statistical analysis
We used conditional logistic regression with symmetric bi-directional referent sampling in the case-crossover design. Specifically, referent days were defined as the same day of week and year, +/− two weeks from the case event day (as in Bateson and Schwartz 1999), such that each case had four referent days. The functional form of model covariates was determined using Likelihood Ratio Test to compare fit across models of increasing complexity [i.e., linear form, natural spline (ns) with defined degrees of freedom (df), or penalized spline (ps) with unlimited df], one variable at a time. Temperature covariates were modeled using natural splines with 3 degrees of freedom, and co-pollutants were modeled using penalized splines.
Building upon previous analysis of this hospitalization and emergency visit data that did not include spatial exposure information (Sheffield et al. 2015), we estimated percent excess risk per 10 ppb change in estimated ozone exposure for lag days 0 to 6 to capture potential delayed effects of ozone beyond the lag period commonly investigated (e.g., Halonen et al. 2010; Norris et al. 1999; Burnett et al. 1999; Jalaludin et al. 2008). Adjustment covariates included: same-day Tmin (ns, df = 3), lagged 6-day average Tmin (ns, df = 3), and 4-day average (lag days 0 – 3) of estimated PM2.5 exposures (ps, df = 3). NO2 was included in the sensitivity analysis detailed below.
To evaluate how incorporating individual-level (spatial) exposure information changed exposure-response relationships, we sequentially fit four models (A – D) moving from temporal-only (A) to spatio-temporal exposure covariates (D), as follows:
-
-
Model A: Temporal O3 + Temporal Tmin + Temporal PM2.5
-
-
Model B: Spatio-temporal O3 + Temporal Tmin + Temporal PM2.5
-
-
Model C: Spatio-temporal O3 + Spatio-temporal Tmin + Aspatial PM2.5
-
-
Model D: Spatio-temporal O3 + Spatio-temporal Tmin + Spatio-temporal PM2.5
Temporal therefore refers to the model or specific exposure estimates that include only the temporal variation component. Spatio-temporal includes both the temporal component (i.e. the estimate for the case day) adjusted spatially per the case residential address.
Analyses were conducted using R statistical package (version 2.14.0; R Development Core Team 2013).
Sensitivity analyses
To test the sensitivity of model results to covariate formulation, we re-fit all models with 4 and 5 df and with 7-day average co-pollutant exposure estimates. In previous analyses (Sheffield et al. 2015), we observed no difference in case-crossover model results using the same temporal exposure data for temperature covariates at 3–5 df, or by replacing RH with dew point. Additionally, we re-fit all models with the subset of cases geocoded using the highest positional accuracy address locator (inpatient n = 2,251; outpatient n = 11,121). Because NOx is associated with ground-level O3 formation (as nitrogen oxides react with ambient ozone, producing localized ozone “scavenging” in very dense urban areas), treating NO2 as a confounder may not be correct in this city-level domain, and as such we chose not to include NO2 exposures in our main models, but do explore its effect in sensitivity analyses.
All study protocols were reviewed and approved by the Institutional Review Boards of the University of Pittsburgh and Mount Sinai School of Medicine.
RESULTS
Our analyses included 2,353 asthma inpatient hospitalizations and 11,719 outpatient ED visits (Table 1). Overall, characteristics of in- and outpatient case events were similar. Case events were most common in the youngest age range (5–9 years), and among male children. Table 2 reports summary statistics for aspatial and spatio-temporal exposure estimates.
Table 1.
Population characteristics. Note that age groupings represent biologically-relevant categories for asthma etiology (Sheffield et al. 2015).
| Inpatient (n = 2,353) | Outpatient (n = 11,719) | |||
|---|---|---|---|---|
|
| ||||
| N | % | N | % | |
|
| ||||
| Age categories | ||||
| 5 – 9 yrs. | 1246 | 52.3 | 5809 | 49.6 |
| 10 – 13 yrs. | 645 | 27.4 | 3253 | 27.7 |
| 14 – 17 yrs. | 462 | 19.6 | 2657 | 22.7 |
|
| ||||
| Sex | ||||
| Female | 1037 | 44.1 | 5108 | 43.6 |
| Male | 1316 | 55.9 | 6611 | 56.4 |
|
| ||||
| Borough of residence | ||||
| Bronx | 845 | 35.9 | 4241 | 36.2 |
| Brooklyn | 764 | 32.5 | 3189 | 27.2 |
| Manhattan | 281 | 11.9 | 2152 | 18.4 |
| Queens | 405 | 17.4 | 1911 | 16.3 |
| Staten Island | 54 | 2.3 | 226 | 1.9 |
|
| ||||
| Month of asthma event | ||||
| June | 989 | 42.0 | 5004 | 42.7 |
| July | 568 | 24.1 | 3178 | 27.1 |
| August | 796 | 33.8 | 3537 | 30.2 |
|
| ||||
| Year of asthma event | ||||
| 2005 | 423 | 18.0 | 2132 | 18.2 |
| 2006 | 395 | 16.8 | 1863 | 15.9 |
| 2007 | 328 | 13.9 | 1634 | 13.9 |
| 2008 | 264 | 11.2 | 1516 | 12.9 |
| 2009 | 432 | 18.4 | 1756 | 15.0 |
| 2010 | 260 | 11.1 | 1428 | 12.2 |
| 2011 | 251 | 10.1 | 1390 | 11.9 |
Table 2.
Temporal and spatio-temporal air pollution and temperature exposure estimates
| Inpatient (n = 2,353) | |||||
|---|---|---|---|---|---|
|
| |||||
| Mean | STD | Min | Max | IQR | |
|
| |||||
| Ozone (case-day) (ppb) | |||||
| Temporal | 30.4 | 9.4 | 5.0 | 60.0 | 13.0 |
| Spatio-temporal | 29.0 | 9.1 | 4.6 | 60.3 | 12.5 |
|
| |||||
| Tmin (case-day) (°F) | |||||
| Temporal | 66.5 | 5.7 | 50.5 | 81.4 | 7.8 |
| Spatio-temporal | 67.4 | 5.8 | 50.2 | 82.9 | 8.1 |
|
| |||||
| PM2.5 (4-day average) (pg/m3) | |||||
| Temporal | 13.8 | 9.1 | 1.0 | 52.5 | 11.6 |
| Spatio-temporal | 14.9 | 10.1 | 1.0 | 58.5 | 12.7 |
|
| |||||
| Outpatient (n = 11,719) | |||||
|
| |||||
| Mean | STD | Min | Max | IQR | |
|
| |||||
| Ozone (case-day) (ppb) | |||||
| Temporal | 30.4 | 9.5 | 5.0 | 60.0 | 13.0 |
| Spatio-temporal | 28.7 | 9.1 | 3.2 | 64.4 | 12.5 |
|
| |||||
| Tmin (case-day) (°F) | |||||
| Temporal | 66.4 | 5.8 | 50.5 | 81.4 | 7.8 |
| Spatio-temporal | 67.4 | 5.9 | 49.7 | 84.0 | 8.3 |
|
| |||||
| PM2 5 (4-day average) (pg/m3) | |||||
| Temporal | 13.6 | 8.8 | 1.0 | 52.5 | 11.2 |
| Spatio-temporal | 14.9 | 9.8 | 0.9 | 73.2 | 12.4 |
Table 2 summarizes distributions for temporal and spatio-temporal exposure distributions, across in- and outpatient populations. Table 3 reports correlations among air pollution and temperature exposure estimates among in- and outpatient cases, respectively. In both populations, temporal and spatio-temporal exposure estimates were highly-correlated (rho = 0.99). Temporal and spatio-temporal ozone and Tmin exposures were correlated at 0.38 and 0.36, respectively, in both in- and outpatient populations. Temporal and spatio-temporal ozone exposure estimates were more highly correlated with PM2.5 than with Tmin, in both case populations. Temporal and spatio-temporal ozone estimates were less correlated with NO2 (rho = 0.29 – 0.22 among inpatient cases; 0.27 – 0.19 among outpatient cases).
Table 3.
Pearson correlation coefficients across exposure metrics
| Inpatient (n = 2,353) | ||||||
|---|---|---|---|---|---|---|
| Ozone (temporal) | PM2.5 (temporal) | Tmin (temporal) | Ozone (spatio-temporal) | PM2.5 (spatio-temporal) | Tmin (spatio-temporal) | |
| Ozone (temporal) | 1.00 | |||||
| PM2.5 (temporal) | 0.60 | 1.00 | ||||
| Tmin (temporal) | 0.38 | 0.46 | 1.00 | |||
| Ozone (spatio-temporal) | 0.99 | 0.60 | 0.37 | 1.00 | ||
| PM2.5 (spatio-temporal) | 0.60 | 0.99 | 0.46 | 0.57 | 1.00 | |
| Tmin (spatio-temporal) | 0.37 | 0.46 | 0.99 | 0.36 | 0.46 | 1.00 |
| Outpatient (n = 11,719) | ||||||
| Ozone (temporal) | PM2.5 (temporal) | Tmin (temporal) | Ozone (spatio-temporal) | PM2.5 (spatio-temporal) | Tmin (spatio-temporal) | |
| Ozone (temporal) | 1.00 | |||||
| PM2.5 (temporal) | 0.58 | 1.00 | ||||
| Tmin (temporal) | 0.38 | 0.45 | 1.00 | |||
| Ozone (spatio-temporal) | 0.99 | 0.57 | 0.37 | 1.00 | ||
| PM2.5 (spatio-temporal) | 0.57 | 0.99 | 0.45 | 0.54 | 1.00 | |
| Tmin (spatio-temporal) | 0.38 | 0.45 | 0.99 | 0.36 | 0.46 | 1.00 |
Spatial patterns of ozone ratios for ED visits and hospitalizations reflect the lower surface concentrations in the city core (Manhattan), and higher concentrations in the outer boroughs, as shown in Figure 1. As such, applying spatial ratios to daily regulatory concentrations resulted in lower spatio-temporal than temporal-only O3 exposure estimates, for approximately 90% of cases. Compared to ozone, PM2.5 and NO2 surface concentrations are higher in the city core, and more spatially-varying across boroughs.
Figure 1.
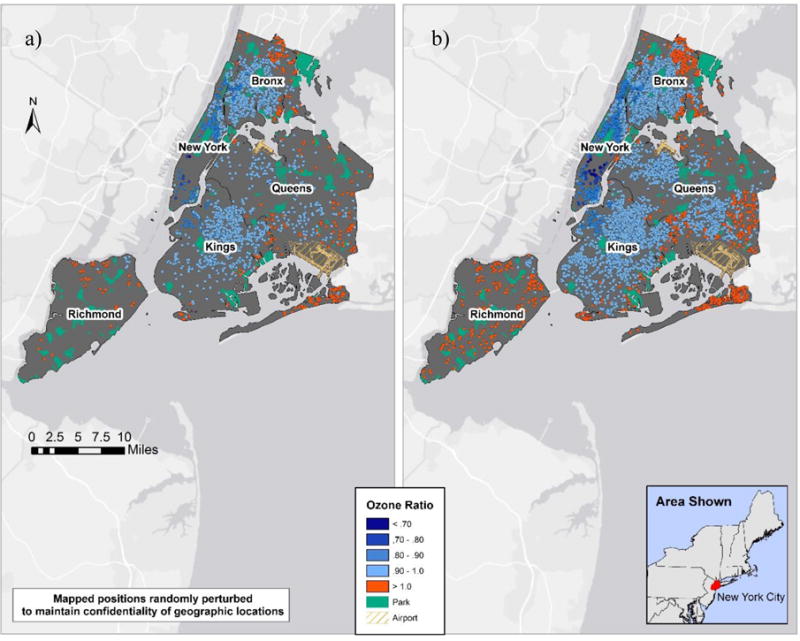
Spatial ozone ratios for a) Inpatient (n = 2,353; range = 0.69–1.13) and b) Outpatient (n = 11,719; range = 0.57–1.16).
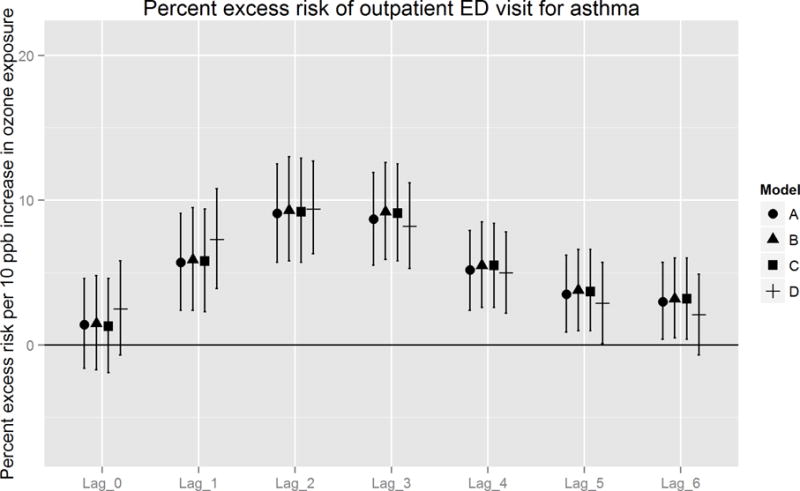
Figures 2A and 2B report estimated percent excess risk of an event per 10 ppb increase in ozone exposure for models A – D, ordered from the fully temporal model (A), to the fully spatially-resolved model (D). Overall, trends in association across lag days were similar between in- and outpatient cases, with the strongest excess risk observed from one to three days following exposure (i.e., lags 1 – 3). For inpatient cases, highest percent excess risks were consistently observed on lag day 2, for which the fully spatio-temporal (model D) excess risk estimates were higher (+ 2.0%) than temporal (model A) estimates. Excess risk estimates did not, however, differ significantly between these models [13.0% (95% CI: 6.0 – 20.6) vs. 11.0% (95% CI: 3.6 – 18.6), respectively]. For outpatient cases, strongest effects were also observed on lag day 2, at slightly lower magnitude and more narrow 95% CIs (due to higher statistical power) compared to inpatient cases. The difference in magnitude of excess risk estimates between models D and A was small (+ 0.3%) and not statistically significant [9.4% (95% CI 6.3 – 12.7) vs. 9.1% (95% CI 5.7 – 12.5), respectively].
Figure 2.
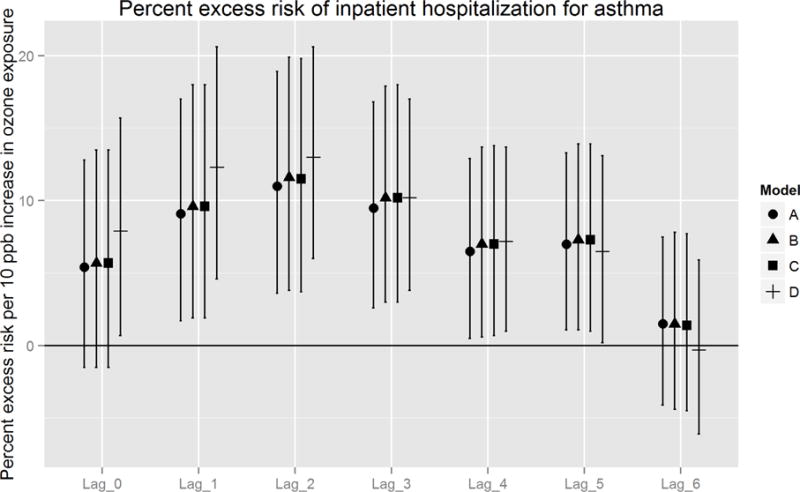
A. Percent excess risk of inpatient hospitalization for asthma, per 10 ppb increase in ozone exposure: comparison of models A – D.
B. Percent excess risk of outpatient ED visit for asthma, per 10 ppb increase in ozone exposure: comparison of models A – D.
When we considered the sequential incorporation of spatial information into each covariate, between-model differences were consistent, but not statistically significant. The largest increase in effect estimates occurred after substituting spatio-temporal PM2.5 (model C to D). These differences were most pronounced on lag days 0 to 2, with the greatest increase on lag 1 of 3.2 and 1.6%excess risks among inpatient and outpatient populations, respectively. In contrast, substituting spatio-temporal Tmin (model B to C) produced differences in percent excess risk < 0.1. In addition, we observed small (≤ 1 % excess risk) but consistent gains in precision between model A and D on lag days 2 and 3.
In sensitivity analyses, all model results were robust to adjustment for NO2 as a co-pollutant confounder, to varying the functional form of co-pollutant covariates (i.e., three to five d.f.), and were consistent among the subset of cases geocoded using the most accurate geocoding method. Models using 7-day co-pollutant averages showed significant effects over shorter periods: lag 1 – 3 for inpatient, and lag 1 – 4 for outpatient, compared to 4-day averages in main models.
DISCUSSION
We incorporated residential-level spatial exposure contrasts into a case-crossover design, to reduce exposure misclassification in estimating short-term effects of ambient ozone on asthma exacerbation. We found significant positive associations on lag days 1 to 5, with the strongest effects on lag days 1 to 3, on which percent excess risk ranged from 10.2 – 13.0% among inpatient populations, and 7.3 – 9.4% among outpatient populations, in fully-adjusted spatio-temporal models. Our modeling approach enables a refined understanding of potential measurement error in temporal-only versus spatio-temporal air pollution exposure assessments.
Our comparison of temporal vs. spatio-temporal models found generally similar percent excess risks for short-term ozone exposures, with slightly higher estimates on lag days 1 and 2 (+ 3.2 and 2.0 for inpatients, and + 1.6 and 0.3 for outpatients). The greatest (albeit small) change in effect estimates followed the substitution of spatio-temporal adjustment covariate PM2.5 (model D), into the model including spatio-temporal forms of ozone and other covariates. One potential explanation is that intra-urban concentration gradients within NYC are more spatially-varying for PM2.5 and NO2, compared to O3. Because the ozone gradients in dense urban areas is driven by scavenging, NOx and VOC availability, and transformation rates, the highest ozone concentrations occur in the outer-most, sparsely-populated areas of NYC, and in areas downwind of the metropolitan core. As such, adding spatial information on O3 into case-crossover studies may yield greater exposure contrasts across much larger and varied, regional-scale domains. Likewise, our results indicate that this approach for integrating temporal and spatial exposures in city-level studies may be particularly useful for reducing exposure misclassification for pollutants with more spatial variability.
Interestingly, we found that addition of spatial covariates resulted in a larger change in effect estimates for inpatient child asthma cases than for ED visits. This difference could be driven by underlying population differences, if younger children have more severe asthma and spend more time at home, improving precision in the spatio-temporal exposure estimates. Alternatively, this population may have greater susceptibility to ozone, given more severe or more poorly-controlled disease.
Comparing our findings with the few studies on spatio-temporal ozone variation in relation to asthma exacerbation is challenging, due to differing exposure metrics (e.g., per 10 ppb vs. IQR-increase; single-day lags vs. multi-day moving averages), case definitions (e.g., age categories, ICD-9 codes), and study area characteristics (e.g., spatial variation in ozone; scavenging). In a similar case-crossover study examining summertime all-ages asthma hospitalization cases in NYC, Jones et al. (2013) compared daily O3 and residential census tract-level O3 variation [predicted by EPA’s Stochastic Human Exposure and Dose Simulation (SHEDS) model], and observed a high correlation between temporal and spatio-temporal exposure estimates, and no difference between hazard ratios (HR) using each exposure metric (HR = 1.029 per IQR, 95% CI: 1.01 – 1.05). We sought further spatial refinement by using near-residence (300 m buffer) spatio-temporal estimates, and also found high correlations with temporal exposure estimates, but did observe small though non-significant between-model differences. In a separate study utilizing temporal (Poisson) case-crossover design to evaluate the association between 8-hr max ozone and asthma ED visits among children (aged 5 – 17), Strickland et al. (2010) observed elevated relative risk [RR = 1.08 (1.04–1.12)] during warm season months. Because an IQR for the 3-day moving average in ozone, in their Atlanta study area, was reported as 29 ppb, this increased risk of 8% appears substantially lower than our finding of 7.3% and 9.4% excess risk per 10 ppb on lag days 1 and 2, respectively, potentially due to unmeasured spatial exposure variation.
Limitations
Our exposure assignment has multiple limitations. First, near-residence exposure estimates do not capture the day-to-day activity patterns of individuals (e.g., travel to school), and we cannot know the degree of exposure misclassification induced, or whether it is non-differential. In our integration of spatial and temporal exposure data, we assumed similar spatial variation in air pollution and temperature across study years, while the spatial data reflect seasonal (O3) and annual (temperature, NO2 and PM2.5) averages for 2008–2010. While spatial temperature data used overnight temperatures to reflect the most robust spatial pattern over time, season-specific patterns in maximum daytime temperatures vary and may represent different physiologic risk for acute health events, compared to minimum temperature. The choice of buffer size for exposure assignment is another potential source of error measurement, common in air pollution epidemiology. However, prior work with NO2 and PM2.5 exposure assessment using NYCCAS data evaluated the effect of buffer size (address point, 300 m, and 800 m), and observed that health effects analyses would not be sensitive to buffer size within this near-residence range (Ross et al. 2013). Though between-season differences in NO2 and PM2.5 are substantially less than for ozone (NYC DOHMH 2009a, 2009b), we were not able to use summer seasonal average co-pollutant data. We were unable to account for the potential contribution of pollen to respiratory outcomes due to lack of data; limiting our analyses to June – August, however, effectively excluded peak spring pollen periods, which occur March – May in NYC (Sheffield et al. 2011). Finally, we were unable to account for potential “avoidance behaviors,” wherein people may change their behaviors (and resultant exposures) in response to air quality information (e.g., public alerts); controlling for these behavioral changes has been shown to increase observed strengths of associations (Neidell and Kinney 2010), presumably by reducing exposure misclassification.
Strengths
The case-crossover design inherently controlled for individual-level time-invariant confounders (e.g., age, sex), and enabled incorporation of both spatial and temporal exposure information. A unique contribution of our study is the inclusion of spatial variation in temperature as an adjustment covariate. Our results were robust to a range of sensitivity analyses, and demonstrated consistent patterns between in- and outpatient case populations. Although our approach for reducing exposure misclassification did not reveal significant differences in effect estimates when incorporating spatial information on exposures, we developed a useful approach for case-crossover studies to distinguish relative contributions of information on spatial and temporal exposure variation.
CONCLUSIONS
Incorporating spatial information on variation in ambient ozone exposures slightly increased excess risk estimates in our study. As ozone normally varies over much larger spatial scales than that observed in NYC, further work is necessary to evaluate potential reductions in exposure misclassification for populations spanning wider geographic areas, and for other pollutants.
Supplementary Material
Highlights.
We examined ozone exposure and child asthma ED visits and hospitalizations.
We compared temporal-only and spatio-temporal exposure estimates.
We observed significant excess risk on lag days 1 through 5, highest risk on lag day 2.
Spatial exposure variation produced small increases in excess risk estimates.
Acknowledgments
The authors are grateful to Zev Ross (Zev Ross Spatial Analysis) for methods consultation, and to Jiang Zhou (formerly of University of Pittsburgh) for work on early statistical models.
This research was funded by US NIH (NIEHS) grants R21ES021429, R01ES019955 and K23ES024127 (PS); US EPA grant RD-83457601-0.
Abbreviations
- EPAAQS
US Environmental Protection Agency Air Quality System
- ED
Emergency Department
- LUR
land use regression
- NO2
nitrogen dioxide
- NOAA
National Oceanic and Atmospheric Administration
- NYC
New York City
- NYCCAS
New York City Community Air Survey
- O3
ozone
- PM2.5
fine particulate matter
- RH
relative humidity
- SPARCS
New York State Department of Health Statewide Planning and Research Cooperative System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009 Mar;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001–2004. Environ Res. 2010;110(3):294–301. doi: 10.1016/j.envres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Babin SM, Burkom HS, Holtry RS, Tabernero NR, Stokes LD, Davies-Cole JO, et al. Pediatric patient asthma-related emergency department visits and admissions in washington, DC, from 2001–2004, and associations with air quality, socio-economic status and age group. Environ Health. 2007;6:9. doi: 10.1186/1476-069X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A. 2008;71(3):238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Selection bias and confounding in case-crossover analyses of environmental time-series data. Epidemiology. 2001;12(6):654–661. doi: 10.1097/00001648-200111000-00013. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology. 1999;10(5):539–544. [PubMed] [Google Scholar]
- Carracedo-Martinez E, Taracido M, Tobias A, Saez M, Figueiras A. Case-crossover analysis of air pollution health effects: A systematic review of methodology and application. Environ Health Perspect. 2010;118(8):1173–1182. doi: 10.1289/ehp.0901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Asthma Facts: CDC’s National Asthma Control Program Grantees. 2014 http://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdfed.
- Clougherty JE, et al. Intra-urban spatial variability in wintertime street-level concentrations of multiple combustion-related air pollutants: the New York City Community Air Survey (NYCCAS) J Expos Sci Environ Epidemiol. 2013;23(3):232–240. doi: 10.1038/jes.2012.125. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Jun W, Tjoa T, Gullerserian SK, Nickerson B, Gillen DL. Asthma morbidity and ambient air pollution: effect modification by residential traffic-related air pollution. Epidemiology. 2014;25(1):48–57. doi: 10.1097/EDE.0000000000000016. [DOI] [PubMed] [Google Scholar]
- Dong GH, Chen T, Liu MM, Wang D, Ma YN, Ren WH, et al. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: Northeast Chinese children health study. PLoS One. 2011;6(7):e22470. doi: 10.1371/journal.pone.0022470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman GT, Mulholland JA, Russell AG, Srivastava A, Strickland MJ, Klein M, Waller LA, Tolbert PE, Edgerton ES. Ambient Air Pollution Measurement Error: Characterization and Impacts in a Time-Series Epidemiologic Study in Atlanta. Environ Sci Technol. 2010;44:7692–7698. doi: 10.1021/es101386r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Lanki T, Tiittanen P, Niemi JV, Loh M, Pekkanen J. Ozone and cause-specific cardiorespiratory morbidity and mortality. J Epidemiol Community Health. 2010;64(9):814–820. doi: 10.1136/jech.2009.087106. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Ma R, Pope CA, 3rd, Krewski D, Newbold KB, Thurston G, Shi Y, Finkelstein N, Calle EE, Then MJ. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiol. 2005;16(6):727–36. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- Johnson M, Macneill M, Grgicak-Mannion A, Nethery E, Xu X, Dales R, et al. Development of temporally refined land-use regression models predicting daily household-level air pollution in a panel study of lung function among asthmatic children. J Expo Sci Environ Epidemiol. 2013;23(3):259–267. doi: 10.1038/jes.2013.1. [DOI] [PubMed] [Google Scholar]
- Jones RR, Özkaynak H, Nayak SG, Garcia V, Hwang S, Lin S. Associatrions between summertime ambient pollutants and respiratory morbidity in New York City: Comparison of results using ambient concentrations versus predicted exposures. J Expos Sci Environ Epidemiol. 2013;23:616–626. doi: 10.1038/jes.2013.44. [DOI] [PubMed] [Google Scholar]
- Lemke LD, Lamerato LE, Xu X, Booza JC, Reiners JJ, Raymond DM, III, Krouse HJ. Geospatial relationships of air pollution and acute asthma events across the Detroit–Windsor international border: Study design and preliminary results. J Expos Sci Environ Epidemiol. 2014;24(4):346–357. doi: 10.1038/jes.2013.78.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- Matte TD, Ross Z, Kheirbek I, Eisl H, Johnson S, Gorczynski JE, et al. Monitoring intraurban spatial patterns of multiple combustion air pollutants in New York City: Design and implementation. J Expo Sci Environ Epidemiol. 2013;23(3):223–231. doi: 10.1038/jes.2012.126. [DOI] [PubMed] [Google Scholar]
- Maynard D, Coull BA, Gryparis A, Schwartz J. Mortality Risk Associated with Short-Term Exposure to Traffic Particles and Sulfates. Environ Health Perspect. 2007;115(5):751–755. doi: 10.1289/ehp.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York City Department of Health and Mental Hygiene (NYC DOHMH) New York City Community Air Survey (NYCCAS): Results from Summer Monitoring 2009. 2009a Available: http://www.nyc.gov/html/doh/html/environmental/community-air-survey.shtml.
- New York City Department of Health and Mental Hygiene (NYC DOHMH) New York City Community Air Survey (NYCCAS): Results from Winter Monitoring 2008–2009. 2009b Available: http://www.nyc.gov/html/doh/html/environmental/community-air-survey.shtml.
- NOAA. Climate Data Online - Select Area. 2014a http://www7.ncdc.noaa.gov/CDO/cdoselect.cmd?datasetabbv=GSOD&countryabbv=&georegionabbv=
- NOAA. NOAA’s National Weather Service - Binghamton, NY - Relative Humidity. 2014b http://www.erh.noaa.gov/bgm/tables/rh.shtml.
- Özkaynak H, Baxter LK, Burke J. Evaluation and application of alternative air pollution exposure metrics in air pollution epidemiology studies. J Expos Sci Environ Epidemiol. 2013:23. doi: 10.1038/jes.2013.50. [DOI] [PubMed] [Google Scholar]
- Potter PC. Current guidelines for the management of asthma in young children. Allergy Asthma Immunol Res. 2010;2(1):1–13. doi: 10.4168/aair.2010.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross Z, Ito K, Johnson S, Yee M, Pezeshki G, Clougherty JE, et al. Spatial and temporal estimation of air pollutants in New York City: Exposure assignment for use in a birth outcomes study. Environ Health. 2013;12:51. doi: 10.1186/1476-069X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: Evidence of association and effect modification. Environ Res. 2011;111(3):418–424. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Sajani ZS, Hanninen O, Marchesi S, Lauriola P. Comparision of different exposure settings in a case-crossover study on air pollution and daily mortality: counterintuitive results. J Expo Sci Environ Epidelmol. 2011;21:385–394. doi: 10.1038/jes.2010.27. [DOI] [PubMed] [Google Scholar]
- Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Sheffield PE, Zhou J, Shmool JLC, Clougherty JE. Ambient ozone exposure and children’s acute asthma in New York City: a case-crossover analysis. Environ Health. 2015 doi: 10.1186/s12940-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield PE, Weinberger KR, Ito K, Matte TD, Mathes RW, Robinson GS, Kinney PL. The association of tree pollen concentration peaks and allergy medication sales in New York City: 2003–2008. ISRN Allergy. 2011;2011:537194. doi: 10.5402/2011/537194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125(2):367–373.e5. doi: 10.1016/j.jaci.2009.10.061. doi. [DOI] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182(3):307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. Excessive heat events guidebook. 2006 [accessed 2014 July 20]; Available from: www.epa.gov/heatisland/about/heatguidebook.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


