SUMMARY
More than 98% of the mammalian genome is noncoding and interspersed transposable elements account for ~50% of noncoding space. Here, we demonstrate that a specific interaction between the Polycomb protein, EZH2, and RNA made from B2 SINE retrotransposons controls stress-responsive genes in mouse cells. In the heat shock model, B2 RNA binds stress genes and suppresses their transcription. Upon stress, EZH2 is recruited and triggers cleavage of B2 RNA. B2 degradation in turn upregulates stress genes. Evidence indicates that B2 RNA operates as “speed bump” against advancement of RNA Polymerase II, and temperature stress releases the brakes on transcriptional elongation. These data attribute a new function to EZH2 that is independent of its histone methyltransferase activity and reconcile how EZH2 can be associated with both gene repression and activation. Our study reveals that EZH2 and B2 together control activation of a large network of genes involved in thermal stress.
Keywords: B2 RNA, SINE, EZH2, Polycomb, transcription, heat shock, stress, RNA cleavage
INTRODUCTION
For more than half a century, genome size has been known to correlate poorly with organism size and complexity (Gall, 1981). Many flowering plants and amphibians have genome sizes (or C-value) that are ≫10-times larger than those of mammals. This so-called “C-value paradox” was thought to be solved by the discovery that only 1–2% of our genomes have protein-coding potential. The rest of the genome consists largely of repetitive DNA, with satellite DNA, retrotransposable elements, and DNA transposons accounting for ~50% of noncoding sequences (de Koning et al., 2011). For much of the past few decades, these poorly conserved elements have been considered “junk DNA” — remnants of evolution and genetic parasites that proliferate without constraint of purifying selection (Kramerov and Vassetzky, 2011). Emerging studies, however, have hinted at possible functions of junk DNA (Lunyak et al., 2007; Bourque et al., 2008; Ponicsan et al., 2010; Lowe and Haussler, 2012). According to the ENCODE project, >80% of noncoding DNA is developmentally transcribed (Consortium et al., 2007).
While several long noncoding RNAs (lncRNA) now have known in vivo functions (Rinn and Chang, 2012; Lee and Bartolomei, 2013; Li et al., 2016), transcripts made from repetitive elements have been associated with few, if any, specific functions. The B2 RNA may be one exception. B2 elements belong to a family of short interspersed nuclear element (SINE), is specific to mouse, is present in ~100,000 copies, and is transcribed by RNA polymerase III into a 180- to 200-base lncRNA with a 5′ tRNA-like sequence and A-rich 3′ end (Kramerov and Vassetzky, 2011). B2 expression changes significantly during development (Bachvarova, 1988) and its expression is highly induced by specific cellular stresses and diseases, such as viral infection (Singh et al., 1985), macular degeneration (Tarallo et al., 2012), and cancer (Moolhuijzen et al., 2010; Kaczkowski et al., 2016). Notably, B2 RNA has been shown to play a role in heat shock (Fornace and Mitchell, 1986; Li et al., 1999), during which B2 RNA is assembled into the pre-initiation complex of RNA polymerase II (POL-II) (Espinoza et al., 2004) and inhibitis transcription in vitro (Allen et al., 2004). B2 is also implicated as a boundary element between heterochromatin and euchromatin (Lunyak et al., 2007), as promoter activity to mammalian genes (Ferrigno et al., 2001), and as potential translational upregulators (Schein et al., 2016). Thus, the B2 SINE elements may be much more than “junk”, though their specific functional and mechanistic relationship to stress and disease is not currently known.
With this in mind, we became intrigued by the RNA-binding activity of Polycomb repressive complex 2 (PRC2)(Zhao et al., 2010), a histone methyltransferase complex consisting of four core subunits, EED, RBBP4/7, SUZ12, and the catalytic subunit EZH2. PRC2 mediates the trimethylation of histone H3 at lysine 27 (H3K27me3) and helps to establish repressive chromatin (Margueron and Reinberg, 2011). By RNA immunoprecipitation with deep sequencing (RIP-seq), we previously revealed an RNA interactome of >9,000 unique transcripts (Zhao et al., 2010). Some interacting transcripts are known to target PRC2 to specific loci, while the raison d’etre for other transcripts is still under investigation (Cifuentes-Rojas et al., 2014; Davidovich et al., 2015). EZH2-RNA interactions can be found at active genes as well (Davidovich et al., 2013; Kaneko et al., 2013). Interestingly, the EZH2 RIP-seq analysis also identified repeat RNAs (Zhao et al., 2010). However, because repeats pose technical challenges for sequence alignment (Treangen and Salzberg, 2012), we previously ignored the repeat fraction despite the enrichment of such transcripts. Here, we revisited the RIP-seq dataset and explored EZH2’s interaction with repetitive RNAs. The resulting findings reveal the importance of B2 RNA during the stress response and pinpoint an EZH2-dependent RNA cleavage event.
RESULTS
B2 RNA associates with EZH2 and exists as short fragments in vivo
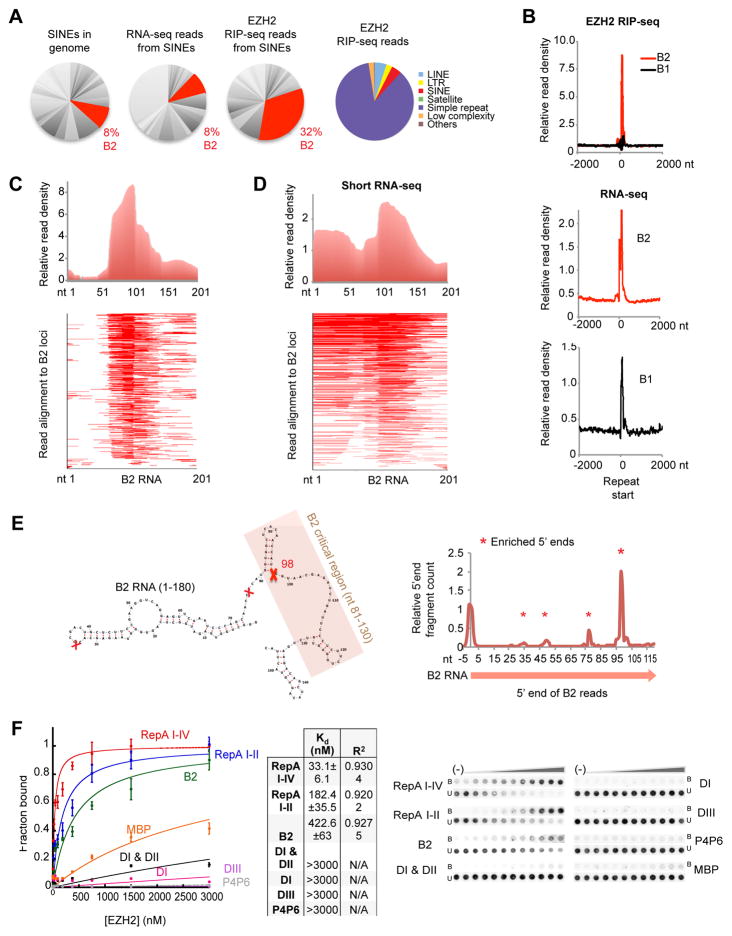
In the EZH2 RIP-seq dataset, reads derived from repetitive sequences comprised ~20% of total reads in mouse embryonic stem (ES) cells (Zhao et al., 2010)(Fig. 1A, right pie chart). While most repeat families were not enriched, SINEs accounted for ~4% of all RIP-seq reads. Within the SINE family, the B2 element was enriched 4-fold above its representation in the female ES transcriptome (32% versus 8%; Fig. 1A) and the nuclear ES transcriptome (32% versus 12%, data from (Kung et al., 2015)). Re-analysis of an independent EZH2 CLIP-seq data (Kaneko et al., 2013) confirmed enrichment of B2 RNA (Fig. S1A). B2 RNA was highly enriched in RIP-seq reads relative to B1, another type of SINE repeat, in spite of the fact that the RNAs have similar expression profiles in the mouse genome (Fig. 1B, bottom panels; Fig. S1)(Hasties, 1989). EZH2 therefore seems to have a preference for binding B2 RNA.
Figure 1. B2 RNA associates with EZH2 and exists as shorter species in vivo.
A) B2 representation (red) among SINEs, the ES cell transcriptome (RNA-seq), and the EZH2 interactome (RIP-seq). Right pie chart depicts SINEs in the EZH2 interactome, reproduced from (Zhao et al., 2010).
B) Top: Distribution of EZH2 RIP-seq reads around TSS (+/− 2000 bp) of B2 and B1 SINE elements represented as metagenes.
C) Distribution of EZH2 RIP-seq reads within B2. Upper panel: Across a metagene profile of all B2 elements aligned to their TSS. Absolute distances (nt) shown on x-axis. Lower panel: Alignment of EZH2 RIP-seq reads within B2 metagene. Because of single-end 50 nt sequencing, B2 species of >50 nt would appear as 50-nt reads.
D) Distribution of short RNA-seq reads within B2 (upper panel) and alignment within B2 metagene (lower panel).
E) Left: Map, structure, and critical domain of B2 RNA (Espinoza et al., 2007). Right: 5′ ends of short RNA-seq reads are plotted along the B2 locus (x-axis). Red X’s (left panel) and asterisks (right panel) mark sites of discontinuity.
F) Left: Binding isotherms of EZH2 from double-filter binding experiments. Middle: Table of Kd and R2 values for EZH2-B2 RNA interactions. Right: Filter binding assay for B2 RNA and EZH2. RepA I-IV and RepA I-II (positive controls), MBP and P4P6 (negative controls). Error bars within binding curves and standard deviations (SD) within the table represent three independent experiments. U, unbound; B, bound.
Examination of B2 read distributions revealed an intriguing non-uniform pattern. Instead of a homogeneous distribution across the ~200-nucleotide (nt) B2 element, there were at least two subpopulations, with a sharp discontinuity of reads at ~ nt 98 (Fig. 1C). This pattern suggested that B2 may also exist as subfragments in vivo. However, library generation and deep sequencing could have introduced biases. For example, only 36 bases were sequenced for each read (Zhao et al., 2010). To exclude technical artifacts as a basis for B2 discontinuities, we developed a short RNA-seq protocol that excluded an RNA fragmentation step and enriched for native transcripts in the 40- to 200-nt size range (Fig. S1B, C). This analysis in ES cells confirmed a discontinuity at nt 98 (Fig. 1D). To further exclude the possibility that these peaks arose from strong reverse transcription stops or other library preparation artifacts, we sequenced in vitro transcribed full-length B2 RNA. Throughout the library construction process, we did not observe inhomogeneities in RNA fragmentation (Fig. S1D) or reverse transcription (Fig. S1E), and short RNA-seq did not reveal cut sites (Fig. S1F). Second, we transfected a heterologous system, HeLa cells (which lack B2 RNA), with in vitro transcribed full-length B2 RNA (Fig. S1G), extracted total cellular RNA, and deeply sequenced. Again, B2 RNA was not cleaved (Fig. S1H). Together, these data argued against library preparation artifacts as a cause of the observed B2 RNA discontinuities (Fig. 1E).
The discontinuity captured our imagination, as it occurred within the 51-nt critical region of B2 (nt 81–131; shaded region, Fig. 1E) previously shown by deletional analysis to be necessary and sufficient to bind POL-II and prevent formation of the pre-initiation complex (Espinoza et al., 2007; Yakovchuk et al., 2009; Ponicsan et al., 2015). To map the precise location of the discontinuity, we aligned 5′ ends of reads from the short RNA-seq library to the B2 consensus and observed a strong peak at position 98 (Fig. 1E, “X”), with additional but smaller peaks at positions 77, 49, and 33. Thus, shorter forms of B2 RNA can indeed be detected in vivo. To determine whether EZH2 binds B2 RNAs directly, we produced affinity-purified, recombinant EZH2 in baculovirus-infected insect cells and performed filter-binding assays with in vitro-transcribed B2 RNA. The results demonstrated that the full-length (180 nt) B2 RNA interacted with EZH2 and did so with a dissociation constant (Kd) of 422.6 ± 63 nM (Fig. 1F). It has an affinity that is similar to that of a similar-sized positive control, RepA I-II — a 210-nt shortened form of Xist RepA containing four of eight repeats (Cifuentes-Rojas et al. 2014, and Fig. 1F). This affinity was much greater than that for the negative control P4P6 RNA, a 154-nt transcript from Tetrahymena (Kd>3000 nM) and also for the 300-nt MBP RNA from E. coli. Truncating B2 RNA also resulted in extremely low affinities for EZH2, with various domains — DI [nt 1–72], DI+D2 [nt 1–105], and DIII [nt 99–140] — all demonstrating Kd >3000 nM. These data demonstrate that B2 RNA directly interacts with EZH2 in vitro and confirm the binding interaction observed by RIP-seq in vivo.
B2 RNA is cleaved and degraded in the presence of EZH2
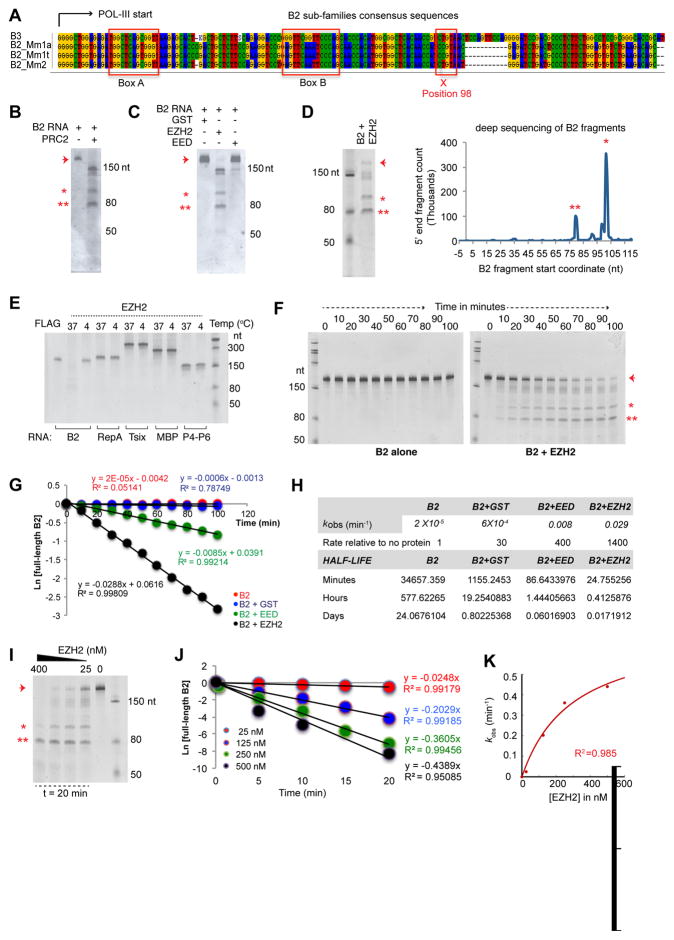
In principle, the discontinuity at position 98 could be due to an internal transcription start site (TSS) or to an RNA processing event. Examination of the B2 sequence revealed internal Box A and Box B sites characteristic of POL-III promoters and did not suggest additional TSS at position 98 or other cut sites (Fig. 2A), nor did conventional and short RNA-seq data suggest splice junctions. We instead suspected a specific endonucleolytic event. Intriguingly, whereas incubation of 200 nM in-vitro-transcribed B2 RNA [folded in 300 nM NaCl and supplemented with TAP100 buffer (incubation final concentrations: 5 nM Tris pH 7.9, 0.5 mM MgCl2, 0.02 mM EDTA, 0.01% NP40, 1% glycerol, 0.2 mM DTT)] did not reveal any instability, addition of 25 nM purified recombinant PRC2 resulted in RNA fragmentation to sizes similar to those observed in vivo (Fig. 2B). This endonucleolytic event was recapitulated by addition of the EZH2 subunit alone, and was negligible with GST protein or with another PRC2 subunit, EED (Fig. 2C).
Figure 2. EZH2 triggers cleavage of B2 RNA in vitro.
A) B2 sub-family consensus sequences.
B) Incubation (22°C, 13h) of B2 RNA (200 nM) with EZH2 (25 nM) results in B2 cleavage.
C) Incubation with 25 nM control proteins, GST and EED, does not result in significant cutting (22°C, 13h).
D) Cleaved RNA fragments (asterisks) are subjected to deep sequencing (x-axis: start coordinates for the sequenced reads).
E) Incubation of in vitro-transcribed RNAs (100 nM) with EZH2 (50 nM) results in cleavage only of B2. RNAs were mixed with EZH2 and incubated at 37°C or 4°C for 30 min. B2 was also incubated with FLAG peptide (50 nM) at 37°C as control.
F) Kinetic analysis of B2 cleavage in the presence of EZH2. 25 nM EZH2 was incubated with 200 nM B2 RNA (37°C for 0–100 min.) and run on a 6% TBE-Urea-PAGE.
G) Fraction of full-length B2 RNA at each time point from panel E (arrow) was plotted as a function of time (two independent experiments). Cleavage rate constants were then determined by a linear fit using the differential form of the rate equation for an irreversible, first-order reaction. The slope is the observed cleavage rate constant (kobs).
H) Table of calculated ob kobs and RNA half-lives for B2 in the presence of various test proteins.
I) Rate of B2 cleavage depends on the concentration of EZH2 protein. 50 nM B2 RNA is incubated with increasing concentrations of EZH2 in vitro (37°C, 20min) and run on a 6% TBE-Urea PAGE.
J) Kinetic analysis showing that the rate of B2 cleavage depends on the concentration of EZH2 (two independent experiments). 200 nM B2 RNA is incubated with increasing EZH2 concentrations (25–500 nM) at 37°C and the amount of remaining full-length B2 RNA is plotted as a function of time. Cleavage rate constants were then determined by a linear fit using the differential form of the rate equation for an irreversible, first-order reaction. The slope approximated observed rate constant (kobs).
K) kobs values from panel I are plotted as a function of EZH2 concentration. Arrowhead, full-length B2 RNA. Asterisks, cleaved B2 fragments. High R2, excellent fit of datapoints to the curve.
We performed deep sequencing of the RNA fragments to identify exact cleavage sites. Unlike in the no-protein control (Fig. S1F), several cleavage sites were observed in the presence of EZH2, including a major one at position 98 and minor ones at positions 77 and 33 (Fig. 2D), corresponding to the sharp discontinuities uncovered by EZH2 RIP-seq and our short RNA-seq analysis (Fig. 1C–E). EZH2’s effect was specific to B2 RNA, as RepA, Tsix, MBP, and P4-P6 were not cleaved under the same conditions (Fig. 2E). Thus, the in vivo activity can be recapitulated in vitro specifically using purified B2 RNA and EZH2 protein. These data demonstrate that full-length B2 RNA is subject to specific endonucleolytic cleavage.
We next studied the in vitro kinetics of B2 RNA processing. In the presence of 25 nM EZH2, cleaved products steadily accumulated between 0–100 minutes (Fig. 2F). Plotting the remaining full-length B2 RNA as a function of time (Fig. 2G), cleavage rate constants were determined by a linear fit using the differential form of the rate equation for an irreversible, first-order reaction (Fig. 2H). With either GST or no protein, we observed a low turnover rate (kobs = 2 × 10−5 min−1 and 6 × 10−4 min−1, respectively). The presence of EED mildly enhanced B2 cleavage at a modest rate of 8 × 10−3 min−1. On the other hand, the presence of EZH2 resulted in a 1,400-fold rate increase to a kobs of 0.029 (R2 > 0.99, indicating that the datapoints have an excellent fit to the curve). Without EZH2, full-length B2 has an extrapolated half-life of 24 days in vitro. In the presence of EZH2, its half-life was reduced to 24 minutes. Thus, the ribonucleolytic cleavages within B2 are accelerated considerably by contact with EZH2.
Cleavage rate also depended on EZH2 concentration. In the presence of 50 nM B2 RNA, increasingly higher processing rates were observed within 20 minutes as EZH2 concentration increased from 25 to 400 nM (Fig. 2I). Cleavage rate constants were again determined by fitting the data to a single-exponential function (Fig. 2J). At 25 nM EZH2, the observed rate constant, kobs, was 0.0248/min in the presence of 200 nM B2 RNA; at 125 nM EZH2, the kobs was 0.2029/min; at 250 nM and 500 nM EZH2, it increased further to 0.3605/min and 0.4389, respectively, without reaching saturation (Fig. 2J, K). Taken together, our data demonstrate that B2 RNA associates with EZH2 and induces a destabilization of B2 RNA and cleavage into multiple subfragments, both in vitro and in vivo.
Heat shock induces B2 cleavage in vivo
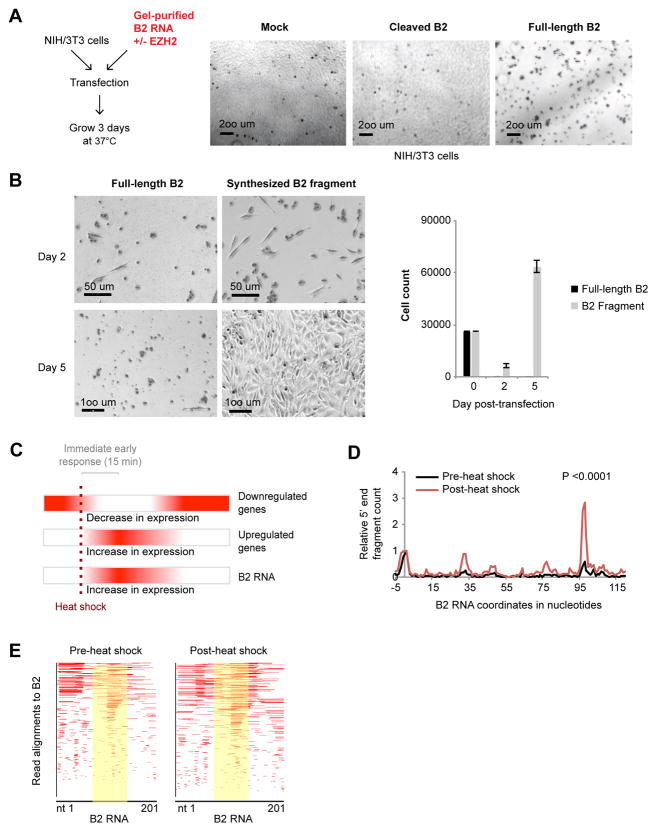
We asked whether degradation of B2 RNA is biologically relevant. First, we interrogated consequences of introducing excess B2 RNA into NIH/3T3 cells, the cell line used previously to study B2 effects (Allen et al., 2004). Surprisingly, transfection of purified full-length B2 RNA resulted in marked cell death within 2 days (Fig. 3A). However, when B2 RNA was pre-incubated with EZH2 to induce cutting, cytotoxicity was reduced and cells grew to confluence within 2–3 days (Fig. 3A). When this analysis was repeated using synthesized truncated B2 fragments (nt 99–140), rather than one cut from full-length B2, similar results were obtained: Starting with a transfection of 30,000 cells, full-length B2 RNA killed all cells within 2 days with no recovery after 5 days, whereas transfection of synthesized truncated B2 showed reduced cytotoxicity at 2 days and full recovery at 5 days (Fig. 3B). Thus, B2 RNA has biological activity in vivo and its cleavage neutralizes that activity.
Figure 3. Heat shock destabilizes B2 RNA in vivo.
A) Transfection of NIH3T3 cells with full-length B2 RNA or RNA pre-incubated with 25 nM EZH2 (37°C, 7h) and gel-purified. Mock represents transfection without any RNA. Photographed after 3 days.
B) NIH/3T3 cells transfected with either synthesized full-length or truncated B2 RNA, and allowed to recover for 2–5 days. Left, photograph; right, quantitation of cells on indicated day.
C) Diagram of the heat shock response and timecourse of up- and down-regulated genes. Red color represents high expression levels.
D) Short RNA-seq of NIH/3T3 cells pre- and post-H/S (45 °C for 15 minutes, two biological replicates). 5′ ends of reads are mapped to the B2 transcript and their relative number is plotted on the y-axis. Data are normalized to the +1 position reads. See also Fig. S1-I for different normalization method. KS test; P<0.0001.
E) Alignment of short RNA-seq reads from pre- and post-H/S cells to the B2 metagene (yellow: region of cleavage).
We set out to determine the nature of that activity. B2 RNA has been shown to block POL-II transcription during the heat shock response (Fornace and Mitchell, 1986; Li et al., 1999; Allen et al., 2004; Espinoza et al., 2004). Heat shock is a type of stress that puts cells at risk, and a rapid response is essential for survival (Chircop and Speidel, 2014). One immediate response is transcriptional downregulation of some cellular genes — an adaptation to suppress expression of unnecessary genes. An equally critical immediate response is transcriptional upregulation of so-called “immediate early genes” — genes upregulated within the first 15 minutes to buffer against cellular damage and aid in refolding or elimination of damaged cellular structures (Fig. 3C)(de Nadal et al., 2011). During this period, B2 RNA is also known to increase in expression (Fornace and Mitchell, 1986; Allen et al., 2004).
To determine whether B2 turnover bears connection to heat shock (H/S), we examined B2 integrity at 15 minutes after the initial application of a H/S stimulus (45°C) in NIH/3T3 cells. We performed short RNA-sequencing and compared the number of cut B2 fragments pre- and post-H/S. As B2 RNA levels also rose after H/S (Fig. 3C), we used the number of reads at position +1 as an internal normalization control (Fig. 3D). Alternatively, we normalized the number of cut sites to (i) total B2 RNA levels from the respective conventional RNA-seq performed in parallel, in order to exclude increased B2 expression as a confounding factor; and (ii) to tRNA levels which are POL-II-independent (Fig. S1-I). Both normalization methods yielded similar conclusions. Intriguingly, a major increase in cutting was observed at position 98 15 minutes post-H/S, as well as at position 77, regardless of the method of normalization (Fig. 3D, E, S1I). The difference in cutting was highly significant (Kolmogorov-Smirnov [KS] test; P<0.0001; see also shaded region of Fig. 3E). We conclude that B2 RNA has biological activity and temperature stress induces turnover of B2 RNA in vivo.
B2 RNA binds to heat shock-responsive genes
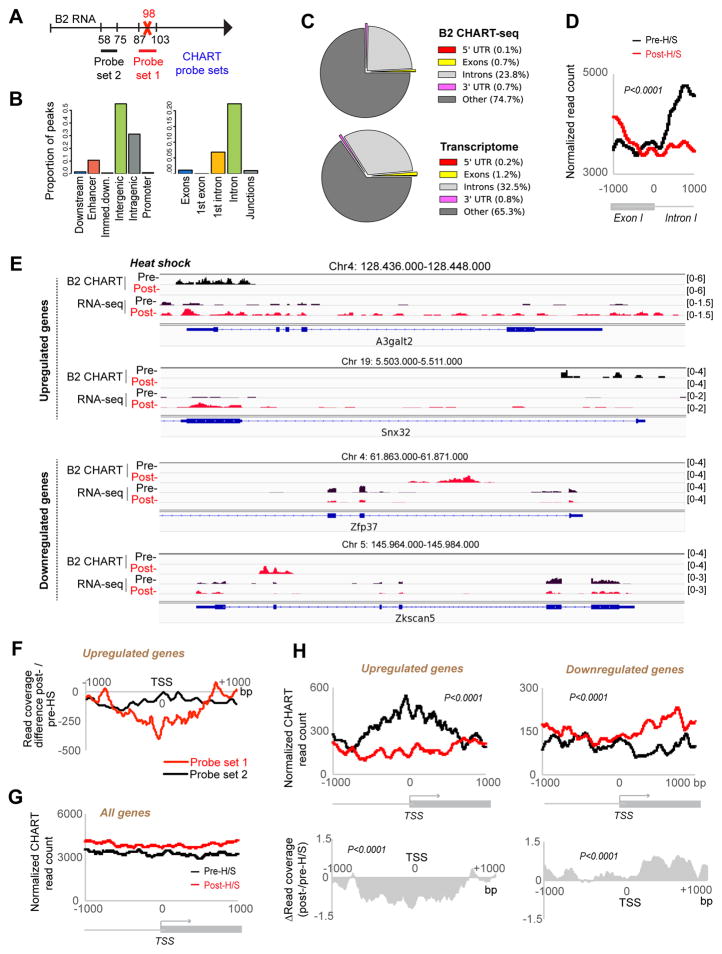
To understand the mechanism of action, we mapped genomic binding sites for B2 RNA using “capture hybridization analysis of RNA targets” with deep sequencing [CHART-seq (Simon, 2013; Simon et al., 2013)]. For capture probes, we designed complementary oligonucleotides to B2 RNA to pull down chromatin regions associated with B2 RNA (Fig. 4A). Probe set 1 comprised 17-base capture probes spanning nt 87–103 of B2 RNA and overlapping the major cut site, thereby enabling us to specifically identify target sites bound by intact B2 RNA. Given variability of the B2 sequence, the probe set was a cocktail that would capture SNP variants for the vast majority of B2’s in NIH/3T3 cells. CHART reads were then normalized to input DNA and to CHART reads obtained by a scrambled capture probe. Peaks were called using SICER (Xu et al., 2014) to identify statistically significant B2 targets sites throughout the genome (FDR < 0.05). CHART-seq was conducted on pre- and post-heat shock cells (pre-H/S and post-H/S, respectively), and biological replicates showed excellent correlation (Fig. S2A, B).
Figure 4. CHART-seq analysis of B2 RNA.
A) B2 CHART-seq analysis with Probe 1 (captures uncleaved B2) or Probe 2 (captures uncleaved and cleaved B2). Probe 1 pooled probe set captures majority of B2 SNP variants.
B) B2 binding peaks within indicated features of RefSeq genes.
C) Pie charts showing distributions of B2 CHART peaks in comparison to general transcriptome.
D) An exon/intron 1-focused metagene analysis of B2 CHART reads. KS test, P <0.0001.
E) B2 binding patterns for two H/S-upregulated and two H/S-downregulated genes, along with RNA-seq data. Pre- and post-H/S profiles shown. Scale shown in brackets.
F) Metagene analysis of changes in CHART-seq coverage after H/S using Probe sets 1 vs 2.
G) B2 binding across TSS-centered metagene profiles +/− 1000 bp of flanking sequence. Pre- and post-H/S traces for all genes (two biological replicates, FDR<0.05 estimation of noise to input signal, E-value of 1000).
H) Upper: B2 binding across TSS-centered metagene profiles +/− 1000 bp of flanking sequence. Pre- (black) and post- (red) H/S traces for upregulated genes and downregulated genes (Table S6)(two biological replicates, FDR<0.05 estimation of noise to input signal, E-value of 1000). Statistical significance (P) of the difference between pre- and post-H/S read counts determined by KS test. Lower: Corresponding relative changes in B2 binding after H/S. Relative change=ratio of post- to pre-H/S CHART reads. Positive and negative values represent increase and decrease in B2 binding, respectively.
Among 83,928 significant peaks altogether, 39,330 corresponded to nascent transcription from genomic B2 elements and served as positive controls (Fig. S2C). Because our goal is to identify B2 target sites, peaks localizing up to 9 kb of a B2 element (max. three times the average size of captured fragments) were excluded from further analysis. We examined the remaining 44,598 B2 RNA target sites. In pre-H/S cells, we observed 18,964 such sites (Table S1). After only 15 minutes of heat shock, the number of B2 target sites nearly doubled to 31,368 (Table S2). Interestingly, target sites were largely non-overlapping between the two conditions. Among 18,964 pre-H/S sites, 13,230 were present only before heat shock (mentioned as “Type I” sites; Table S3). Reciprocally, among 31,368 post-H/S sites, 25,634 were observed only after H/S (“Type II” sites; Table S4). A minority (5,734) occurred in both pre- and post-H/S cells (“Type III” sites; Table S5).
We then characterized the target sites and found that the vast majority of B2 binding sites were intergenic and intronic (Fig. 4B, C; pre-H/S shock shown), especially intron 1 (Fig. 4B). Some genes harbored multiple B2 binding sites. These observations held true for all three types of B2-binding targets (Tables S3–S5). With regards to the 1st intron, peaks often occurred at the exon-intron boundary and generally within 1,000 bp of the transcription start site (TSS), as shown by metagene analysis (Fig. 4D; KS test, P<0.0001) and by specific genic loci (Fig. 4E). It should be emphasized, however, that B2 binding sites could occur anywhere within the gene body. Furthermore, binding sites tend to be broad, frequently spanning multiple introns (Fig. 4E, S3) and thereby appearing different from discrete peaks typified by transcription factors.
We also performed CHART-seq with probe set 2, designed to hybridize to B2 RNA outside of the major cleavage site and to capture both cleaved and uncleaved B2 RNA (Fig. 4A). Using the same analysis pipeline for Probe 1, Probe 2 identified 9,406 pre-H/S and 9,394 post-H/S B2-binding sites, excluding B2 transcription sites (Tables S1–S2, probe 2). Among them, 5,439 pre-H/S and 5,461 post-H/S peaks either overlapped or were within 9 kb (three times the average size of captured fragments) to the peaks identified by Probe 1. Interestingly, whereas probe set 1 demonstrated a strong net negative change in B2 binding after H/S, probe set 2 revealed only a small, albeit statistically signifcant [P<0.001] decrease (Fig. 4F). This observation suggests that cleavage reduces RNA binding to genes, but the RNA does not fully disengage from chromatin during the first 15 minutes of H/S. This interpretation would be consistent with detectability of cleaved B2 fragments by EZH2 RIP-seq analysis (Fig. 1).
To determine how B2 binding affects gene expression, we performed RNA-seq analysis of NIH/3T3 cells before and after H/S and compared the results to B2 CHART-seq profiles. We observed that 1,636 genes were upregulated (log2fold-change ≥0.5; Table S6) and 1,438 genes were downregulated (log2fold-change ≤0.5; Table S6). Biological replicates were highly correlated (Pearson’s R = 0.9; Fig. S4A). Intriguingly, H/S-upregulated genes were enriched in the Type I subclass of B2 targets — i.e., they were bound by B2 RNA prior to H/S, and were freed from binding following H/S. In contrast, H/S-downregulated genes were enriched in the Type II subclass — i.e., they were free of B2 binding prior to H/S, but became B2-bound after H/S (Fig. 4E, S3). At two H/S-upregulated genes, A3galt2 and Snx32, B2 binding was high when the genes were expressed at low levels, but was lost after 15 minutes of H/S when the genes became upregulated. At two H/S-downregulated genes, Zfp37 and Zkscan5, B2 binding was not apparent before H/S, but became significant after H/S.
Metagene analysis confirmed these trends on a genome-wide scale. At 15 minutes post-H/S, most genes displayed no changes in B2 localization (Fig. 4G). However, H/S-upregulated genes (Table S6) showed a significant loss of B2 binding, while H/S-downregulated genes (Table S6) showed a significant increase (Fig. 4H). Among 2510 genes with Type I sites, 151 such genes lost B2 binding and were upregulated within 15 minutes of H/S (Fig. S4B, C). Of 3482 genes with Type II sites, 218 gained B2 binding and were downregulated during this timeframe (Fig. S4D, E). Thus, B2 RNA appears to regulate at least 369 genes (151 Type I + 218 Type II) during the immediate early response. Other Type and Type II targets genes might also be H/S-responsive but they may not respond within 15 min. Intriguingly, most of these dynamic B2 sites were intergenic (Tables S1–S2). Altogether, these data demonstrate that B2 RNA binds many genic and intergenic targets, that the binding pattern is rapidly (within 15 minutes) altered by H/S, and that B2 binding is anti-correlated with expression of H/S-responsive genes.
Cleavage of B2 RNA induces heat shock-responsive genes
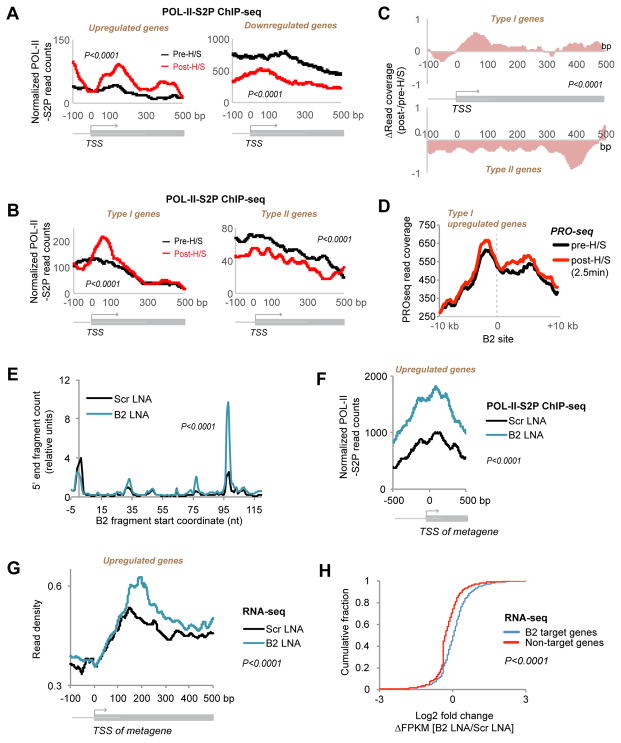
Given anti-correlation between B2 binding and target gene activity, the cleavability of B2 RNA raised the fascinating possibility that B2 degradation might lift suppression of POL-II at H/S-responsive genes. To test this idea, we performed ChIP-seq for the Serine-2 phosphorylated form of RNA POL-II (POL-II-S2P) to quantitate changes in density of elongating RNA polymerase relative to changes in B2 binding (Fig. 5, S5). As expected, genes upregulated by H/S (Table S6) showed increased POL-II density, whereas genes downregulated by H/S (Table S6) showed a decrease (Fig. 5A, KS test, P<0.0001). Among the subset of genes that bind B2 only before H/S (Type I), H/S resulted in a significant spike in POL-II density (KS test; P<0.0001) (Fig. 5B, C), coinciding with the loss of the B2 binding (Fig. 4H). Conversely, among genes that bind B2 only after H/S (Type II), H/S led to a significant decrease in POL-II density (KS test; P<0.0001) (Fig. 5B, C), coinciding with the gain of B2 binding (Fig. 4H). Thus, POL-II activity decreases when B2 binding appears and increases when B2 binding is lost.
Figure 5. Loss of B2 binding induces H/S-responsive genes.
A) Metagene analysis of changes in POL-II-S2P binding (ChIP-seq) at H/S-upregulated and -downregulated genes (two biological replicates, FDR<0.05 estimation of noise to input signal). Statistical significance (P) determined by KS test.
B) Metagene analysis of changes in POL-II-S2P binding at Type I (B2 binding in pre-H/S) and Type II (B2 binding in post-H/S) genes. Analysis performed as in (A).
C) Metagene analysis of relative changes in POL-II-S2P binding after H/S for Types I and II genes. Analysis performed as in Fig. 4H.
D) Metasite analysis showing distribution of PRO-seq reads (Mahat et al., 2016) around B2 binding sites (x=0) pre- and post-H/S. The shorter time frame post-H/S (2.5 min, instead of 15 min.) in the PRO-seq experiment might explain why there is incomplete release from B2 blockade.
E) B2 turnover induced by B2-specific LNA shown by short RNA-sequenced after 24h. 5′ ends of short RNA-seq reads are mapped to the B2 transcript and the relative number is plotted on the y-axis (KS test, P <0.0001). Values are normalized to position +1.
F) ChIP-seq analysis indicates that B2 LNA treatment recapitulates increased POL-II-S2P density across H/S-upregulated genes without application of H/S (KS test, P <0.0001).
G) Metagene analysis of RNA-seq data also demonstrates that B2 LNA treatment recapitulates upregulation of H/S-responsive genes in the absence of H/S (KS test, P <0.0001).
H) Cumulative frequency plot of log2 fold changes in FPKM in B2 LNA-treated cells versus Scr LNA-treated cells. Right shift for B2 target genes indicates a net positive change in gene expression after B2 cleavage without H/S. P <0.0001, KS test.
A large body of literature has linked H/S-induced transcriptional upregulation to release of a paused POL-II downstream of the TSS (Brown et al., 1996; Kwak et al., 2013; Kwak and Lis, 2013; Mahat et al., 2016), thereby implicating increased transcriptional elongation as a major mechanism of gene upregulation. To asked whether the POL-II pause sites might correspond to B2 sites, we compared our B2 CHART data to previously published PRO-seq data (Mahat et al., 2016). Intriguingly, metasite analysis revealed that the POL-II pause sites occurred just upstream of the B2 binding sites in pre-H/S cells (Fig. 5D). PRO-seq analysis also showed that, at 2.5 minutes post-H/S, POL-II demonstrated increased advancement past B2 sites (Fig. 5D). B2 RNA may therefore serve as a roadblock to elongating POL-II and contribute to the mechanism of polymerase pausing.
These data suggest that B2 RNA may be a key trigger of the H/S response. If so, turnover of B2 alone might be sufficient to release transcriptional pausing. To de-couple B2 turnover from H/S, we designed a B2-specific antisense oligonucleotide (ASO) using locked nucleic acid chemistry (LNA) to cleave B2 RNA. After 24 hours of treating NIH/3T3 cells (without H/S), we observed significantly elevated B2 cutting relative to a scrambled (Scr) LNA-treated sample (Fig. 5E, KS test; P<0.0001, Fig. S6A). B2 LNA treatment recapitulated the increase in POL-II density across H/S-responsive genes in the absence of H/S (Fig. 5F, KS test; P<0.0001). Concurrently, RNA-seq analysis showed activation of H/S-responsive genes (Fig. 5G, KS test; P<0.0001), and cumulative distribution plots revealed a significant right-shift in the change of read coverage (ΔFPKM) for B2 target genes relative to non-targets (Fig. 5H). Biological replicates for RNA-seq and POL-II-S2P ChIP-seq showed excellent reproducibility (Fig. S5, S6B, C). Additionally, upregulation of select genes was confirmed by RT-qPCR (Fig. S7A). We conclude that increased POL-II density and gene expression can be uncoupled from the H/S stimulus by ectopically inducing B2 degradation. Thus, B2 cleavage is central to the H/S response.
EZH2 is recruited to B2 target sites to promote the heat shock response
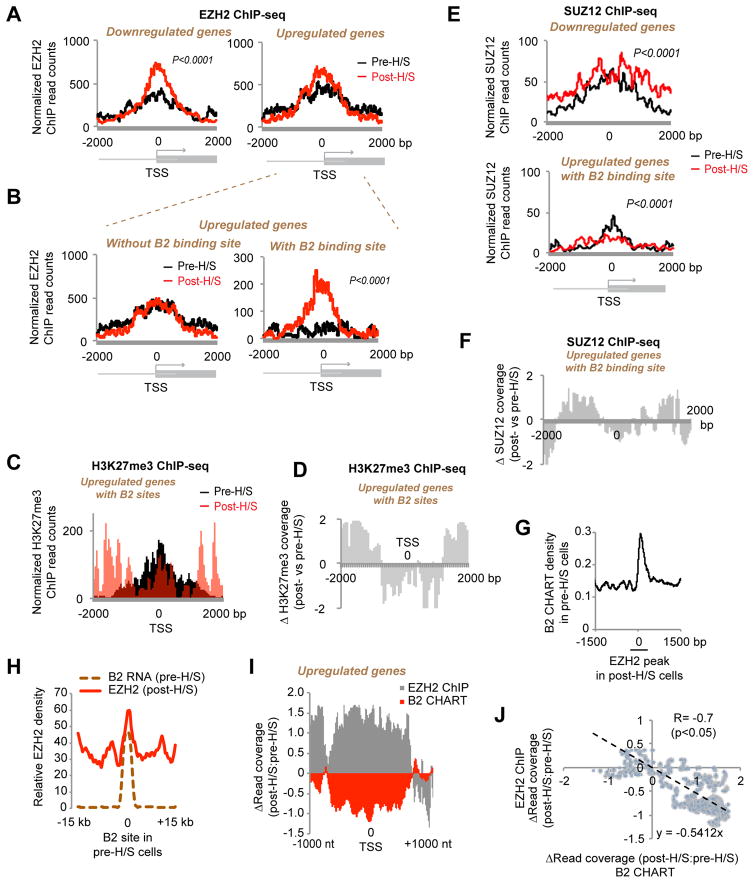
We were initially led to B2 RNA by its enriched representation in the EZH2 RIP-seq data (Fig. 1). We then observed that contact with EZH2 resulted in B2 RNA cleavage (Fig. 2) and that cut forms have reduced affinity for EZH2 in vitro (Fig. 1F). Together, these findings hinted at a role for EZH2 in the H/S response To test this idea, we performed EZH2 ChIP-seq in pre- and post-H/S NIH/3T3 cells, with biological replicates showing similar results (Fig. S5). Consistent with EZH2’s generally repressive role, we observed an EZH2 enrichment at the TSS of H/S-downregulated genes (Fig. 6A). Unexpectedly, EZH2 also appeared to be slightly increased at H/S-upregulated genes, though the increase was not statistically significant. However, the difference became highly significant when analysis was focused H/S-upregulated genes bound by B2 before H/S (Fig. 6B, KS test; P<0.0001). Those without a B2 site did not show increased EZH2 binding. These data showed that B2-bound H/S genes paradoxically gained EZH2 during activation. Interestingly, recruitment of EZH2 was not accompanied by trimethylation of H3K27. Rather, there was decreased H3K27me3 over the TSS (Fig. 6C, D), consistent with transcriptional upregulation.
Figure 6. EZH2 is recruited to B2 target genes to direct H/S activation.
A) Metagene analysis of changes in EZH2 binding (ChIP-seq) at H/S-upregulated and -downregulated genes. Two biological replicates (FDR<0.05 for sample signal to input noise). P<0.0001 (KS test) between pre- and post-H/S read count distribution for downregulated genes only.
B) EZH2 is recruited to H/S-responsive genes with B2 sites. Metagene analysis of changes in EZH2 binding (ChIP-seq) at H/S-upregulated genes with or without B2 binding sites (Type I versus Type II). P <0.0001 (KS test) for upregulated genes with B2 binding site.
C) H3K27me3 coverage is not significantly increased at TSS after EZH2 recruitment. Metagene analysis performed on subclass of H/S-upregulated Type I genes with EZH2 binding sites (either pre- or post-H/S).
D) Metagene analysis showing relative changes in H3K27me3 coverage after H/S for the subclass of upregulated genes shown in (C).
E) SUZ12 is not recruited to H/S-upregulated Type I genes after heat shock. Metagene analysis of changes in SUZ12 binding (ChIP-seq) at H/S-upregulated Type I genes with EZH2 binding sites. P <0.0001 (KS test).
F) Metagene analysis showing relative changes in SUZ12 coverage after H/S for genes in (E).
G) Meta-site analysis centered on EZH2 binding sites shows B2 binds in pre-H/S cells where EZH2 is gained after H/S. x=0 corresponds to EZH2 peak start of post-H/S cells.
H) Meta-site analysis centered on B2 binding sites shows that EZH2 binds where B2 is lost during H/S. x=0 corresponds to B2 peaks of pre-H/S cells.
I) Anti-correlation of B2 and EZH2 binding viewed in a metagene plot. Relative changes in either B2 or EZH2 coverage at upregulated genes are shown after H/S.
J) Linear anti-correlation between B2 coverage and EZH2 density. Change in B2 density (x-axis) plotted as a function of change in EZH2 density (y-axis). R = −0.7, P< 0.05. a function of change in EZH2 density (y-axis). R = −0.7, P< 0.05.
These observations suggest that EZH2 may be recruited for a purpose other than H3K27 trimethylation. It is known that EZH2’s histone methyltransferase activity is inert without additional PRC2 subunits (Margueron and Reinberg, 2011). From analysis of two biological replicates of SUZ12 ChIP-seq (Fig. S7B, C), we observed that SUZ12 increased for H/S-downregulated genes as expected (Fig. 6E top panel), consistent with increased EZH2 occupancy (Fig. 6A). Intriguingly, however, at H/S-upregulated genes (Type I), the increase in EZH2 recruitment was not accompanied by SUZ12 (compare Fig. 6E). In fact, SUZ12 coverage actually decreased in post-H/S cells (Fig. 6F). The discordance between EZH2 and SUZ12 was observed at H/S-upregulated Type I genes, but was not the case in general (EZH2, SUZ12, and H3K27me3 generally tracked together)(Fig. S7B, C). A SUZ12-independence agrees with the in vitro finding that EZH2 alone was sufficient to induce B2 cleavage (Fig. 2).
We conclude that EZH2’s role in H/S does not relate to its histone methyltransferase activity and instead suspected that EZH2 may activate H/S genes by destabilizing B2. Indeed, “meta-site” analysis from an EZH2-centric view (x=0 at EZH2 site) revealed that, after H/S, B2 binding was lost at sites of EZH2 recruitment (Fig. 6G). Conversely, a B2-centric view (x=0 at B2 sites) revealed that EZH2 was recruited where B2 binding was lost (Fig. 6H). There was a strong anti-correlation between change in B2 binding density and change in EZH2 coverage (Fig. 6I, J).
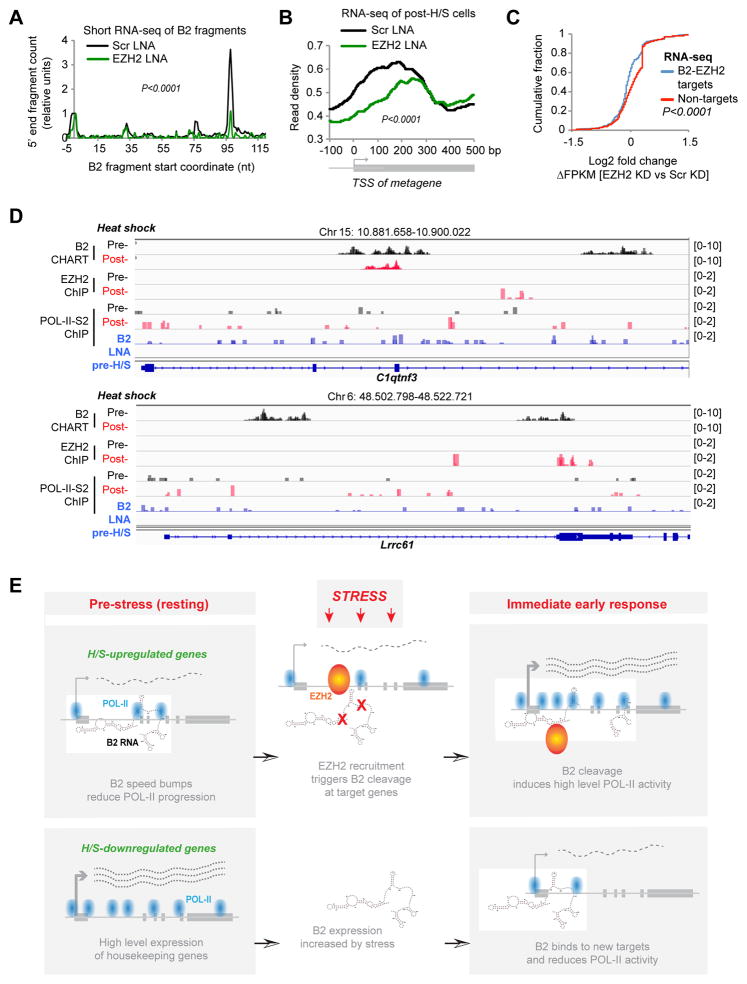
We then depleted EZH2 and assessed effects on B2 processing and H/S gene induction in vivo. EZH2-specific ASOs led to a significant knockdown (KD) of EZH2 (Fig. S7D), significantly decreased B2 cleavage at positions 98 and 77 in NIH3T3 cells (Fig. 7A), and a blunted activation of H/S-responsive genes in two biological replicates (Fig. 7B, Fig. S7E; specific examples shown in Fig. S7A). A plot of cumulative distributions showed decreased expression of genes that are both B2 and EZH2 targets (Fig. 7C). Thus, while cleaving B2 induces upregulation of H/S target genes (Fig. 5GH, S7A), depleting EZH2 precludes their upregulation (Fig. 7BC, S7A). Together, these experiments demonstrate that EZH2 is a H/S pro-induction factor that functions through turnover of B2 RNA.
Figure 7. The Speed Bump Model.
A) Depleting EZH2 for 24 hours reduces processing of B2 RNA in NIH/3T3 cells transfected with EZH2 or Scr LNAs. 5′ ends of short RNA-seq reads are mapped to the B2 transcript and the relative number of 5′ ends is plotted on the y-axis (P <0.0001, KS test) and normalized to position +1.
B) EZH2 is required for the heat shock response. Metagene analysis of RNA-seq data demonstrates that EZH2 depletion reduces expression of H/S-upregulated genes (P <0.0001, KS test for pre- post-HS distributions).
C) Cumulative distribution plot of log2 fold changes in FPKM in EZH2 LNA- versus Scr LNA-treated cells after H/S. Left shift for B2-EZH2 target genes indicates a net negative change in gene expression after depletion of EZH2. P <0.0001, KS test.
D) Binding patterns for B2 RNA, EZH2, and POL-II-S2P at two specific genes.
E) The Speed Bump Model. Upper panels, H/S-upregulated genes: In unstressed cells, B2 RNA binds target genes, serves as POL-II speed bump, and dampens gene expression. Upon stress, EZH2 is recruited and triggers B2 degradation, thereby enabling more transcriptional elongation. Bottom panels, H/S-downregulated genes bind B2 after stress induction and are reduced in expression.
The dynamic interplay between B2 RNA, EZH2, and POL-II activity can be appreciated by examination of specific H/S-inducible loci (Fig. 7D). In resting cells, C1qtnf3 was transcribed at low levels, as reflected by low POL-II-S2P coverage (0.257) and RNA-seq values (FPKM=0.009), B2 RNA binding was high, and EZH2 binding was not detectable. Upon H/S, EZH2 appeared within intron 3, while B2 binding decreased in introns 2 and 3. Concurrently, we observed increased POL-II-S2P coverage within the gene body (FPKM=0.308) and a 2.4-fold transcriptional upregulation (FPKM=0.022). [N.B: The H/S genes respond in a graded rather than all-or-none manner (Brown et al., 1996; Kwak et al., 2013; Chircop and Speidel, 2014)]. Similarly, at Lrrc61, B2 binding disappeared when EZH2 binding appeared after H/S, at which time POL-II-S2P coverage increased 2-fold (FPKM=0.093 to 0.192). These changes were measurable within 15 minutes of H/S. Ectopically cleaving B2 RNA (using LNAs) recapitulated the H/S response in the absence of stimulus (Fig. 5E, 7D). For C1qtnf3, B2 LNA treatment resulted in a ~2-fold increase of POL-II-S2P coverage (FPKM=0.481) and a 3-fold increase in RNA levels (FPKM=0.027). For Lrrc61, there was a 2.3-fold increase of POL-II-S2 coverage (FPKM=0.213) and a 1.36-fold increase in transcription (FPKM=0.420). We conclude that EZH2 and B2 play pivotal roles during the H/S response and that contact-induced B2 elimination triggers activation of H/S-inducible genes
DISCUSSION
Environmental stress is an everyday reality for all organisms. A rapid and effective response is essential for survival in the face of acute stress, such as extreme temperature, chemical toxin, radiation, and infection. Activation of stress response genes protects cells from conditions that would normally be lethal, and a failure to mount an effective or controlled stress response can lead to a variety of diseases, including cancer and autoimmunity. Cancer therapeutic agents often target the stress/heat shock pathway to block cancer cell proliferation, but cancer cells frequently respond by mutating stress-control genes (Chircop and Speidel, 2014). A better understanding of the stress response would therefore be beneficial towards human health.
Here, we have used the heat shock model to study the stress response and discovered a central regulatory role for EZH2 and B2 transcript (Fig. 7E). Intriguingly, the key triggering event is a specific EZH2-B2 interaction that results in B2 RNA cleavage. During the immediate early period (15 min. after temperature shift), 369 B2 target genes are upregulated as a consequence. It should be noted that, just like the well-studied HSF-1 (Budzynski et al., 2015; Duarte et al., 2016), the EZH2-B2 interaction controls only a subset of H/S-response genes. B2 sites do not appear in the gene bodies of some classic H/S-inducible genes, such as Hsp70 and Hsp90. Therefore, B2 RNA and HSF-1 may control different subsets of H/S genes.
Our data indicate that B2 acts as transcriptional “speed bumps” for POL-II elongation (Fig. 7E). In unstressed cells, B2 RNA binds broadly in 5′UTRs and intronic regions to control the rate of POL-II progression. Upon thermal stress, EZH2 is rapidly recruited to many H/S-responsive genes and triggers endonucleolytic cleavage of B2 RNA. The nature of the endonucleolytic activity is currently not known. Although lacking canonical features of an RNAse, EZH2 may in fact be directly responsible. Regardless, cleavage of B2 RNA is sufficient to induce the H/S-responsive genes. Cut B2 fragments have dramatically reduced affinities for EZH2 (ΔKd 423 nM to >3000 nM; Fig. 1F) and are measurably released from target genes during the immediate early period (Fig. 4), thereby removing POL-II speed bumps and enabling a larger percent of elongating POL-II to reach the 3′ termini of target genes. Our model is consistent with the large body of literature attributing gene upregulation during the immediate early phase of the heat shock response to release of a “paused” polymerase downstream of the TSS (Brown et al., 1996; Kwak et al., 2013; Kwak and Lis, 2013; Mahat et al., 2016). Intriguingly, the pause sites for POL-II elongation occur just proximal to sites of B2 binding (Fig. 5D). A mechanism based on an RNA speed bump that can be rapidly removed by a nuclease activity would enable a swift cellular response to stress on the order of minutes.
Our study ascribes a new function to EZH2 that is independent of its well-known histone methyltransferase activity. It also provides an explanation for the paradox that EZH2-RNA interactions can be found at both active and inactive genes (Zhao et al., 2010; Davidovich et al., 2013; Kaneko et al., 2013) and lends further credence to the idea that EZH2 interacts with RNA in a highly specific manner (Cifuentes-Rojas et al., 2014; Sarma et al., 2014; Davidovich et al., 2015). Whereas EZH2-mediated gene silencing is defined by the H3K27me3 mark (Margueron and Reinberg, 2011), gene activation by the EZH2 instead depends on turnover of bound B2 RNA. Thus, frequent mutation of EZH2 (Margueron and Reinberg, 2011) and misexpression of Alu/B2 elements (Moolhuijzen et al., 2010; Chircop and Speidel, 2014; Kaczkowski et al., 2016) in cancer cells may reflect this critical role of EZH2 and B2. Although our work was conducted in mammalian cells, EZH2 may also function during stress in other organisms (Siebold et al., 2010; Kleinmanns and Schubert, 2014; Basenko et al., 2015).
In the context of heat shock, EZH2 appears to operate independently of SUZ12, both in vitro (Fig. 2) and in vivo (Fig. 6). This is interestingly, given that EZH2 and SUZ12 are currently thought to be inseparable. Indeed, EZH2 cannot function as histone methyltransferase without other PRC2 subunits (Margueron and Reinberg, 2011). Consistent with this, EZH2’s effect on B2 and gene induction does not involve H3K27me3 (Fig. 6). Indeed, EZH2 may have a repressive effect in the context of PRC2, but an independent activating effect when operating outside of PRC2. Our model is also consistent with a previous ChIP-seq study in which SUZ12 knockdown minimally perturbed expression of active genes associated with continued EZH2 binding (Davidovich et al., 2013). We currently do not know how B2 RNA is directed to bind H/S genes and how EZH2 is specifically recruited during stress. Given its role in directing PRC2 to Xist RNA, the RNA/DNA helicase, ATRX, or other chaperones may guide the specificity (Sarma et al., 2014).
Notably, heat shock normally leads to two distinct transcriptional responses – upregulation of stress response genes (Table S6) and downregulation of housekeeping genes, among others (Table S6). While we have focused primarily on the EZH2-B2 dynamic for the former set, our data suggest that B2 plays an equally important role for the latter (Fig. 4E, 7E, S3). Repression of non-essential genes is an adaptation to conserve cellular resources during stress. Existing studies have demonstrated a role for B2 RNA in repression of two housekeeping genes, including ActinB and Hk2 (Fornace and Mitchell, 1986; Li et al., 1999; Allen et al., 2004; Espinoza et al., 2004). Our B2 CHART data now provide a genomic view for downregulated genes and reveal a large number of genes targeted by B2, whose expression increases, immediately after heat shock (Fig. 4H; Tables S2, S4, S6). Previous studies showed that B2 incorporates into POL-II in vitro and blocks formation of the pre-iniation complex at promoters. In view of our present findings, we suggest that B2 has effects on both transcriptional initiation and elongation in vivo. The continued presence of EZH2 at H/S-upregulated genes may explain their resistance to increased B2 expression immediately following heat shock.
In conclusion, we have shown that a specific interaction between EZH2 and B2 triggers the heat shock response via an RNA elimination event. Our findings arose from a re-evaluation of existing RIP-seq data to include the repeat fraction. In the analysis of large epigenomic datasets, repetitive elements are customarily dismissed and ignored as artifacts. Our study, however, suggests that repetitive elements play essential in vivo roles and prompts further examination of junk DNA as crucial epigenomic regulators.
METHODS AND RESOURCES
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to, and will be fulfilled by the corresponding author Jeannie T. Lee (lee@molbio.mgh.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
NIH/3T3 and HeLa cells were cultured in DMEM supplemented with Glutamax (Life Technologies) supplemented with 10% fetal bovine serum and 1% Penicillin/Streptomycin. Before heat shock stimulus cells were trypsinized and resuspended in 5ml complete medium in a 15ml falcon tube. Subsequently, cells were either placed in 37°C (control cells, pre-H/S condition) or in 45°C (treated cells, post-H/S condition) for 15 min. Time points mentioned throughout this work have as a starting point the moment of the start of the heat shock stimulus. After the end of this 15 minutes period, cells were centrifuged shortly (2min) and cell pellets were directly resuspended into Trizol (Thermofischer) for the RNA-seq analysis or fixated with 1% formaldehyde for the ChIP-seq and CHART-seq analysis.
METHOD DETAILS
Cell culture and nucleic acid transfections
For LNA transfections against B2 RNA we used the HiPerfect transfection reagent (Qiagen) and the sequence of the LNAs used were as follows: LNA11: 5′-GTTACGGATGGTTGTG-3′ and LNA12: 5′-TGTAGCTGTCTTCAG-3′. The scramble LNA sequence was 5′-CACGTCTATACACCAC-3′. In detail, the LNAs were diluted to 100uM and incubated with 1.35ul of the transfection reagent in a final volume of 10ul for 15–20 min at room temperature (RT). Subsequently the transfection mix was transferred to 2ml of recently trypsinized cells in full culture medium containing 5 × 105 cells (final LNA concentration 500nM). A fluorophore conjugated LNA was also transfected to test transfection efficiency. Subsequently cells were plated and incubated at 37°C for 24 hours before testing. In the meanwhile, after 1h from plating, a subset of cells was subjected to FACS analysis and transfection rate was estimated to 90% of live cells. For LNA transfections against EZH2 we used the following LNA ASO sequence: 5′-TTCTTCTTCTGTGCAG-3′. Transfections were performed with HiPerfect as mentioned above but for a final LNA concentration of 25nM. For RNA transfections of the B2 RNA and its fragments we used the TransMessenger Transfection Reagent (Qiagen). In brief, 16 pmol of RNA in Buffer EC was incubated for 5 min at RT with 2ul enhancer, and subsequently 8ul transfection reagent was added to a total reaction of 100ul and incubated for 10 min at RT before addition to recently trypsinized cells in culture medium without serum. 2.6 × 104 transfected cells were plated and incubated at 37°C for 30 min before adding an equal volume of complete medium (with serum). After 2 hours, a subset of these cells were washed with PBS twice and RNA was extracted using Trizol and analyzed with qPCR against B2 RNA to confirm B2 overexpression. Cells were counted during the subsequent days using a Nexcelom Cellometer.
RNA in vitro transcription and RNA-protein incubations
Proteins were prepared as previously described (Cifuentes-Rojas et al 2014). Briefly, GST was expressed in E.coli BL21 transformed with pGEX4T1. Cell extracts were prepared by three freeze-thaw cycles in lysis buffer [PBS, 1% TX-100, 1mM DTT and protease inhibitors]; sonicated and pelleted debris and supernatant was collected for further purification by Glutathione agarose affinity purification. After binding to the beads, protein was eluted in 50mM Tris8, 10mM reduced glutathione, 1mM DTT, 0.1%Tween-20 and dialyzed in 10mM Tris7.5, 100mM NaCl, 5mM MgCl2, 0.1mM ZnSO4, 10% glycerol, 0.1% Tween-20.
Mouse EZH2 and EED subunits were expressed in Sf9 insect cells using the bac-to-bac system (Invitrogen). pFastBac1 expression vectors containing an N-terminal Flag-epitope tagged EZH2, and EED, were obtained from the Kingston Lab (Massachusetts General Hospital-Harvard Medical School). Protein extracts were prepared by three freeze-thaw cycles in lysis buffer [50mM Tris-HCl pH 8.0, 300mM NaCl, 1% NP-40, 0.3% Triton-X, 4mM EDTA, 1mM MgCl2, 1mM DTT, 20% Glycerol and complete protease inhibitors (Roche)] M2 anti-Flag beads (Sigma) were used for all purifications. After binding and washes, proteins were eluted with 1 hr incubations with 0.2μg/ml 3X-Flag peptide (Sigma) and were concentrated using Amicon ultra concentrators (Millipore).
For RNA in vitro transcription we used the AmpliScribe T7 High Yield Transcription Kit (Epicentre) applying a 3h incubation at 42°C and using a template resulting to the following B2 RNA sequence: 5′-GGGGCTGGTGAGATGGCTCAGTGGGTAAGAGCACCCGACTGCTCTTCCGAAGGTCCGGAGTTCAAATCCCAGCAACCACATGGTGGCTCACAACCATCCGTAACGAGATCTGACTCCCTCTTCTGGAGTGTCTGAAGACAGCTACAGTGTACTTACATATAATAAATA AATAAATCTTTAAAAAAAAA - 3′. DNA template for the transcription was a PCR amplified product derived from a gBlock (IDT) using as forward primer the T7: 5′-TAATACGACTCACTATAG and as reverse primer for B2 RNA PCR template the following sequence 5′-TTTTTTTTTAAAGATTTATTTATTTATTATATGTAAGTACA.
For smaller B2 RNA fragments the respective templates were constructed based on the above sequence and the nucleotide (nt) numbering mentioned in the text. In detail, domain I RNA was from +1nt to +72nt, domain I+II RNA from +1nt to +105nt, and domain III from +99 to +140nt. The quality of the transcribed RNA was tested running a 6% UREA PAGE gel as well as through small RNA-seq library construction and next generation sequencing (see below). RNAs were purified using the ZymoResearch RNA clean kit. Incubations, unless mentioned differently in the text were performed with 200 nM in-vitro-transcribed B2 RNA folded with 300mM NaCl and supplemented with TAP buffer (final reaction concentrations: 5nM Tris pH 7.9, 0.5mM MgCl2, 0.02mM EDTA, 0.01% NP40, 1% glycerol, 0.2mM DTT). For RNA folding the RNA was incubated for 1 min at 50°C and cooled down with a rate of 1°C/10sec. We monitored the disappearance of full-length B2 RNA by denaturing PAGE, followed by SYBR Green II staining for 30 minutes. Cleavage time-courses were quantified using ImageJ (NIH). The fraction of full-Length B2 RNA present at each time point was measured by taking the ratio of the size-corrected intensity of full-length B2 RNA and dividing by the sum of size-corrected intensities for all gel bands. Cleavage data were fit using Kaleidagraph (Synergy) to the differential form of the rate equation for an irreversible, first-order reaction to derive an observed cleavage rate constant, kobs.
Double-Filter Binding Assays
Binding reactions were assembled with 1 μl of 1.000 cpm/μl (0.1 nM final concentration) folded RNA and purified protein at the shown concentrations in binding buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 5 mM MgCl2, 10 μg/ml BSA, 0.05% NP40, 1 mM DTT, 20 U RNaseOUT [Invitrogen], and 5% glycerol) in 30 μl. A total of 50 ng/μl yeast tRNA (Ambion catalog number AM7119) was used as a nonspecific competitor. After 30 min at 30°C, the reactions were filtered through nitrocellulose (PROTRAN, Schleicher & Schuell) and Hybond-N+ (GE Healthcare) membranes using a Minifold I system (Whatman), washed with 600 μl washing buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1.5 mM MgCl2, 0.05% NP40, 1 mM DTT), dried, exposed to a phosphor screen, and scanned after 2 hr in a Typhoon Trio (GE Healthcare Life Sciences). Data were quantified by Quantity One Software (Biorad). Protein was titrated at a constant RNA concentration and isotherms were plotted as the fraction of RNA molecules bound, [RNA]bound/[RNA]total, as a function of [protein]total. Nitrocellulose-filter-retention efficiencies were calculated and corrected to the non-specific retention of free RNA by the nitrocellulose filter (Cifuentes-Rojas et al., 2014). Equilibrium dissociation constants, Kd, were obtained by fitting the binding data to a one-site binding model by nonlinear regression using Graphpad Prism.
Genomewide analysis of RNA and protein genomic binding sites
At least two biological replicates were analyzed for whole B2 CHART and ChIP experiments. The B2 CHART was modified from the original CHART protocols (Simon et al., 2011). In detail, 12 millions cells were crosslinked with 1% formaldehyde for 10 min at room temperature. Crosslinking was then quenched with 0.125 M glycine for 5 min and washed with PBS 3 times. Snap freezing cells could be stored at −80°C. Crosslinked cells were re-suspended in 2 ml of sucrose buffer (0.3 M sucrose, 1% Triton-X-100, 10 mM HEPES pH 7.5, 100 mM KOAc, 0.1 mM EGTA), dounced 20 times with a tight pestle, and kept on ice for 10 min. The following steps were using polystyrene tubes, glass pipettes, and DNA LoBind microtubes (Eppendorf) to avoid cell clumps sticking onto the walls of tubes or pipettes. Nuclei were collected by centrifugation at 1.500g for 10 min on top of a cushion of 5 ml glycerol buffer (25% glycerol, 10 mM HEPES pH7.5, 1 mM EDTA, 0.1 mM EGTA, 100 mM KOAc). Nuclei were further crosslinked with 3% formaldehyde for 30 min at room temperature. After washing three times with ice-cold PBS, nuclei were extracted once with 50mM HEPES pH7.5, 250 mM NaCl, 0.1mM EGTA, 0.5% N-lauroylsarcosine, 0.1% sodium deoxycholate, 5mM DTT, 100 U/ml SUPERasIN (Invitrogen) for 10 min on ice, and centrifuged at 400g for 5 min at 4°C. Nuclei were resuspended in 1.2 ml of sonication buffer (50 mM HEPES pH 7.5, 75 mM NaCl, 0.1 mM EGTA, 0.5% N-lauroylsarcosine, 0.1% sodium deoxycholate, 5 mM DTT, 10 U/ml SUPERasIN, and sonicated in microtubes using Covaris E220 sonicator at 10% duty cycle, 200 bursts per cycle, 105 peak intensity power for 5 min. The major size of chromatin fragments was around 3–4 kb. Fragmented chromatin was subjected to hybridization immediately. Hybridization, washing and elution were performed as follows. In brief, beads were blocked with 500 ng/ul yeast total RNA, and 1 mg/ml BSA for 1 hour at 37°C, and respuspended in 1X hybridization buffer. 360 μl of 2X hybridization buffer (750 mM NaCl, 1% SDS, 50 mM Tris pH 7.0, 1 mM EDTA, 15% Formamide, 1 mM DTT, PMSF, protease inhibitor, and 100 U/ml Superase-in) was added into 180 μl lysates, and then this 1X hybridization lysate was pre-cleaned by 60 μl of blocked beads at room temperature for 1 hour. After removal of the beads, B2 probes (labeled with 3′ biotin-TEG, 18 pmol) for B2 RNA were added into the 1X hybridization lysate and incubate at room temperature for overnight. Given the variability of the different B2 repeats, we used a pool of probes that correspond to the majority of the sequence variations within the target region presented at Fig. 4a. As control we used also a negative probe that does not show any sequence similarity to the used probes with he following sequence: 5-GCACGTCTATACACCACT-3′. 120 ul of blocked beads were added into lysates and incubated at RT for two hours. Beads:biotin-probes:RNA:chromatin adducts were captured by magnets, washed once with 1X hybridization buffer at 37°C for 30 min, washed four times at 37°C for 5 min with SDS wash buffer (2X SSC, 1% SDS, 1 mM DTT, 1 mM PMSF), and then washed once for 5 min at room temperature with 0.1% NP40 buffer (150 mM NaCl, 50 mM Tris pH8.0, 3 mM MgCl2, 10 mM DTT, 0.1% NP40). DNA was then eluted in 100 μl twice for 20 min in 100 μl of 0.1% NP40 buffer with 200 U/ml RNase H (NEB) at room temperature and purified further using phenol-chloroform extraction.
Before ChIP analysis, 3 millions cells were crosslinked as above and sheared chromatin was prepared using the ChIP-IT Express kit (Active motif) in a 135ul volume using the following conditions in a Covaris E220 sonicator: 2% duty cycle, 200 bursts per cycle, 105 peak intensity power for 5 min. Chromatin immunoprecipitations were performed in 100ul reaction volumes using the same kit as with chromatin shearing and the following antibodies for 14 hours incubation times: EZH2 (D2C9, 5246S Cell signaling technology), H3K27me3 (39155, active motif), SUZ12 (39357, active motif), RNA POL II phospho S2 (from the ab103968 panel, abcam). Eluted DNA was further purified with phenol-chloroform.
Purified DNA was subjected to further fragmentation in a Covaris E220 sonicator using 10% duty cycle, 200 bursts per cycle, 175 peak intensity power for 5 min in 125ul. Subsequently, we used the NEBNext ChIP-seq library Prep Master MIX set (NEB) with the following modifications: For ChIP-seq the EndRepair of ChIP DNA was performed only for 15min in a 10.5ul total volume (using 1ul buffer and 0.5ul enzyme) followed by no cleanup but dA-Tailing in a reaction scaled to 100ul for 15 min. Subsequently we performed double size selection 0.2X-2.5X before adaptor (0.3uM) ligation for 30 min and USER enzyme incubation for another 30 min. Ligation reaction was cleaned using 1.4 sample-bead ratio and the final library was size selected and clean with Ampure beads twice using 1x and 0.5x–0.9x ratios. In addition, the PCR reaction had an extension time of 1 min and 30 sec. For CHART-seq the end repair was scaled to 150ul, while the dA-tailing was performed at 25.5ul total volume. After adaptor ligation it was size selected with 0.6x–1.2x bead-sample ratio, while after the PCR it was cleaned twice with 1x and 0.9x Ampure beads and quantified using the qPCR KAPPA kit.
Genomewide analysis of long and short RNAs
RNA used for short RNA-seq and RNA-seq libraries was prepared as follows: Total RNA from cells was extracted using Trizol and 4 ug of total RNA was subjected to ribosomal RNA depletion using the ribominus V2 kit (Life technologies). Incubation of the RNA with the probe was done for 40 min instead of 20 min. RNA depleted RNA was separated into two fractions of short (<200) and longer RNAs using the mirVana separation kit (Life technologies) with the following modifications: After addition of the lysis/binding buffer and the miRNA homogenate additive solution, 100% EtOH at 1/3 of the volume was added and the mix was passed through the filter to bind long RNAs. The flow through was collected and 100% EtOH at 2/3 of the flow through volume was added and passed through a new filter column to bind short RNAs. Elution of the long and short RNAs from each column respectively was done per manufacturer instructions. Eluted RNAs were concentrated in both cases using the RNeasy MinElute Spin Columns (Qiagen) and tested for its size and quality using an Agilent Bioanalyzer RNA kit. For short RNA library construction, ribo-depleted short RNAs were subjected to PNK 3′-phosphoryl removal for 1 hour at 37C. Subsequently we used the NEBnext small RNA library construction kit (NEB) with the following modifications: Incubation of the 3′adaptor was performed for 2 hours, and the libraries at the end were not subjected to double size selection with the Ampure beads but with 1.2X size selection. For sequencing of the in vitro B2 fragments no ribosomal depletion was applied
For the longer RNAs we used the NEBNext Ultra directional RNA library kit (NEB) with an RNA fragmentation of 10 min at 95C and with the following modifications: First strand synthesis at 42C was done for 50 min and the End Prep of cDNA library was followed by an Ampure Beads selection of 1.8x and ligation of the adapters using the 5x quick ligation buffer and Quick T4 DNA ligase (NEB) for 30 min. Incubation with the USER enzyme was done before the PCR amplification for 30 min, followed by a double size selection of 0.5x–1x, while the final library was size selected using Ampure beads at a 1x sample-beads ratio. Libraries were evaluated using the Bioanalyser high sensitivity DNA kit (Agilent) and quantitated using the qPCR KAPPA kit (Kappa).
Quantitative gene expression analysis
cDNA synthesis was performed using the SuperScript III first strand synthesis system (Life Technologies) and random primers (10ng/ul) (Promega) based on manufacturer’s recommendations. For the quantitative real time PCR we used the iTaq Universal SYBR Green Supermix and the primers mentioned as a separate excel sheet of the Supplementary Table S6 with the following conditions (denat. 95°C/15 sec, anneal 54°C/30sec, ext. 66°C/30 sec). tRNALeu or Actin were used as housekeeping genes as described before (Allen et al., 2004; Wagner et al., 2013).
Bioinformatics analysis
Raw RIP-seq, CHART-seq and ChIP-seq reads and the respective sequenced input reads were mapped using bwa.0.5.5 against mm9 genome (Li and Durbin, 2010) (default parameters). Using the bamToBed utility from the bedtools analysis suite (Quinlan and Hall, 2010) the resulting sam files were converted to bed files and enriched genomics regions against the input were filtered using SICER (Xu et al., 2014) with a window parameter of 300 and a gap parameter of 300 and an FDR 0.05. Subsequently, CHART-seq reads of the B2 probe were filtered further by omitting reads captured by the negative CHART probe Metagene profiles were constructed using the Babraham NGS analysis suite Seqmonk (http://www.bioinformatics.babraham.ac.uk/projects/seqmonk/) employing normalized cumulative distributions filtered in case of CHART-reads against the positions of B2 elements (9KB range). In detail, every peak close to a B2 element within the range of 9kb, which is three times the average CHART fragment size, was excluded from further analysis. B2 element coordinates are based on the UCSC genome browser RepeatMasker track (as of Dec. 14). Normalization was performed based on the total number of mapped reads. Gene and mRNA coordinates are based on the RefSeq annotation track of Seqmonk (as of Dec.14). Scatterplots, selection of regions overlapping B2 and EZH2 sites and hierarchical clustering were also performed using Seqmonk. Peak annotation was done using Galaxy and PAVIS (Huang et al., 2013).
For PRO-seq analysis BigWig files from (Mahat et al., 2016) were converted to mm9 bed files using UCSC Genome Browser Utilities and bedops (doi: 10.1093/bioinformatics/bts277) and read distributions were analyzed using Seqmonk as described above. Analysis of distribution of EZH2 CLIP-seq reads (from Kaneko et al., 2013) was done as in RIP-seq reads.
Short RNA reads were trimmed from adapters in both ends using cutadapt (DOI: http://dx.doi.org/10.14806/ej.17.1.200) for the following adapter sequences: AAGATCGGAAGAGCACACGTCT. Subsequently reads were mapped using bwa and converted to bed files using the bamToBed utility of the analysis suite bedtools (Quinlan and Hall, 2010). Then, using a perl script the 5′ ends co-ordinates of the reads included in the bed files were extracted into a separate text file. These 5′end were subsequently plotted against a metagene representing the absolute distance between start of B2 repeats and downstream sequences using the trend plot module of Seqmonk and constructing a cumulative read distribution around the B2 elements start position. Reads alignments were performed using Seqmonk’s aligned probes plot function. Raw RNA-seq reads were trimmed using cutadapt for the following adapter sequences: AAGATCGGAAGAGCACACGTCT and AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGT for read 1 and read 2, respectively. Subsequently they were mapped against mm9 reference transcriptome using tophat (Trapnell et al., 2009) with the following parameters: bowtie1 -r -100 -N 20 --read-gap-length 10 --segment-mismatches 3 --read-edit-dist 20. Metagene profiles were plotted with Seqmonk using the prob trend plot relative read density function. Transcriptional start site was defined using the TSS Eponine track from Seqmonk (Down and Hubbard, 2002). Δread coverage was calculated (CoveragePostH/S- CoveragePreH/S)/((CoveragePostH/S + CoveragePreH/S)/2). Genome browser screenshots were derived using the IGV viewer.
QUANTIFICATION AND STATISTICAL ANALYSIS
Read counts in deep sequencing experiments were estimated using the Seqmonk quantification module and RNA differential expression was performed using Seqmonk’s intensify difference function for a p value less than 0.05. For the statistical analysis of the read distributions we applied the Kolmogorov–Smirnov test, using Prism6 (Graphpad). Quantification of the RT-qPCR data was done by constructing a standard curve for each gene and the housekeeping genes and normalizing the gene values to those of the housekeeping genes. For the statistical analysis of the RT-qPCR data is judged to be statistically significant when p < 0.05 by two-tailed Student’s t test (n=4 for figure S7A).
DATA AND SOFTWARE AVAILABILITY
Datasets for short-RNA-seq, RNA-seq, ChIP-seq and CHART-seq have been deposited in GEO under accession number GSE82255.
Supplementary Material
Figure S1. The short RNA-seq method.
Figure S1 relates to Figures 1,2, and 3.
A) Distribution of EZH2 CLIP-seq reads (from Kaneko et al., 2013) around the start site (+/− 2000 bp) of B1 and B2 repeats.
B) In order to study the B2 RNA fragments, we employed a short RNA-seq protocol that enables the unbiased study of non-polyadenylated RNAs between 40–200 nucleotides. No RNA fragmentation step is included. rRNAs, mRNAs, miRNAs, and other small RNAs are depleted by this method.
C) Representative bioanalyzer trace of short RNAs isolated using the short RNA-seq protocol.
D) Bioanalyzer trace of in vitro transcribed B2 RNA at various steps of library construction (input, after CIP treatment, and after PNK treatment). No RNA degradation is evident.
E) Bioanalyzer trace of the cDNA pool after reverse transcription of B2 RNA (from panel S1D) during library construction. The full length B2 cDNA is highly enriched and no smaller cDNAs are observed.
F) Deep sequencing of the library constructed from B2 cDNA (from Figure S1E). As in Fig. 1E, 5′ ends of our short RNA-seq reads are plotted along the B2 locus (x-axis). Note the absence of cutting at position 98.
G) qRT-PCR of B2 RNA after transfection of full-length B2 RNA (+) into HeLA cells. Mock transfection (−) used a negative control RNA, P4-P6.
H) Plot of the 5′ end of B2 RNAs after short RNA-seq preparation from HeLa cells. Note the absence of cutting at position 98.
I) Short RNA-seq of NIH/3T3 cells before and after heat shock (45 °C for 15 minutes). Two biological replicates yielded similar results. 5′ ends of short RNA-seq reads are mapped to the B2 transcript and the relative number of 5′ ends is plotted on the y-axis. The 5′ end counts are normalized to the levels of full length B2 RNAs from long RNA-seq of the respective samples to account for any possible changes in the general B2 levels during heat shock (KS test; P<0.0001). The values are further normalized to the total tRNA levels, which are not transcribed by POL-II, as an internal loading control. A graph of the same data normalized to the +1 position is shown in Figure 3D, with accompanying read alignment display in Figure 3E. In the corresponding Figure 3D, we normalized +1 position. Notably, both methods of presentation show increased cutting at +98 after H/S (KS test; P<0.0001).
Figure S2. Correlation between biological replicates of the B2 CHART-seq experiment.
Figure S2 relates to Figure 4.
A, B) Log2 normalized read counts from replicates 1 and 2 for the B2 CHART-seq analysis of pre-H/S (A) and post-H/S (B) cells in 10kb windows. Pearson’s R is indicated. Colors represent point density (blue: low, red: high)
C) Metagene plot of B2 CHART read density at B2 elements, the site of nascent transcription. As expected, B2 RNA is enriched at the site of transcription. These loci served as positive control and are excluded from further analysis.
Figure S3. B2 CHART-seq profiles at four loci, with biological replicates.
Figure S3 relates to Figure 4.
IGV screenshots of two biologlical replicates for B2 binding patterns at two H/S-upregulated and two H/S-downregulated genes. Pre- and post-H/S profiles are shown. Paired data are shown at the same scale (numbers in brackets, right) for comparison. Replicates of RNA-seq data are shown below CHART tracks.
Figure S4. Transcriptomic analysis before and after heat shock in relation to B2 CHART analysis.
Figure S4 relates to Figure 4.
A) Correlation between RNA-seq biological replicates. Log2 normalized read counts from replicates 1 and 2 for the RNA-seq analysis of pre- and post-H/S cells for RefSeq regions. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
B–E) Upper panels: Numbers and percentages of genes with Type I (panels B, C) or Type II B2 sites (panels D, E). Genes are defined as gene body +/− 3kb. Bottom panels: Heatmaps representing density of B2 CHART coverage in pre- and post-H/S cells. Hierarchical clustering revealed two main clusters – genes that gain versus lose B2 coverage with H/S. Genes are defined as gene body +/− 3kb of flanking DNA. Upregulated genes are analyzed in panel C. Downregulated genes are analyzed in panel E.
Figure S5. Correlation between biological replicates of POL-II-S2P, EZH2, and H3K27me3 ChIP-seq experiments.
Figure S5 relates to Figures 5 and 6.
Log2 normalized read counts from replicates 1 and 2 for POL-II-S2P, EZH2, and H3K27me3 ChIP-seq experiments for pre-H/S and post-H/S cells. Each dot represents a RefSeq gene. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
Figure S6. Correlation between biological replicates of POL-II-S2P ChIP-seq and RNA-seq experiments from cells treated with B2 LNA.
Figure S6 relates to Figure 5.
A) Alignment of short RNA-seq reads to the B2 metagene. Cells were treated with either Scr LNA or B2 LNA treatment within the B2 metagene between nt 1–201. The Scr LNA plot is the same experiment shown in Figure 3E.
B, C) Correlation plots showing Log2 normalized read counts from replicates 1 and 2 of the POL-II-S2P ChIP-seq (B) or RNA-seq (C) experiments. Cells were treated for 24 hr with B2 LNA to induce B2 cleavage without H/S. Each dot represents a RefSeq gene. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
Figure S7. Effects of EZH2 depletion during the heat shock response.
Figure S7 relates to Figures 6 and 7.
A) RT-qPCR for the differences in RNA expression levels observed after treatment with B2, EZH2, or Scr LNA as indicated. Averages of 4 biological replicates shown. P values are determined by t-test.
B) Metasite analysis centered on EZH2 binding sites shows that EZH2, SUZ12, and H3K27me3 peaks coincide with each other in general across the genome, as expected, in post-H/S cells. x=0 corresponds to EZH2 peaks.
C) EZH2, SUZ12, and H3K27me3 peaks generally correlate with each other across the genome, as expected. Shown are correlation plots of Log2 normalized ChIP read counts for the indicated epitopes. Each dot represents a ChIP-seq peak/site for the indicated epitope in post-H/S cells. Pearson’s R is indicated. Colors represent point density (blue: low, red: high). Two biological replicates for each epitope.
D) Significant EZH2 knockdown by LNA transfection. P =0.04, as determined by t-test.
E) Correlation plots showing Log2 normalized read counts from RNA-seq biological replicates 1 and 2 after EZH2 knockdown. Each dot represents a RefSeq gene. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
Table S-1 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis, listing all peaks present in pre-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated. Peaks identified by Probes 1 and 2 listed separately in sheets 1 and 2, respectively.
Table S-2 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis, listing all peaks present in post-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated. Peaks identified by Probe 1 are listed in a separate sheet from peaks captured by Probe 2.
Table S-3 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis using Probe 1), listing peaks present only in pre-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated.
Table S-4 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis using Probe 1, listing all peaks present only in post-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated.
Table S-5 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis using Probe 1, listing all peaks present in both pre- and post-H/S NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated.
Table S-6 relates to Figures 3, 4 and the “Quantitative gene expression analysis” methods section.
Upregulated and downregulated genes are listed in separate sheets.
Column A: Heat shock-upregulated or -downregulated gene shown by RNA-seq analysis of NIH/3T3 cells. Column B: Chromosome position and mm9 start and stop coordinates of the gene in Column A. Column C: Log2 fold-change of the gene in post-H/S cells relative to pre-H/S state.
Acknowledgments
We thank the Lee Lab for many excellent discussions. Special thanks go to J. Froberg for MiSeq experiments, W. Press for optimizing transfection conditions, B. Del Rosario for GST protein, and R. Kingston for sharing the pfastbac plasmids encoding FLAG-EZH2 and -EED. This study was funded by the German Research Foundation to A.Z, National Institutes of Health R01-GM090278 to J.T.L. and K99-GM115868-02 to C.C.R. J.T.L. is also an Investigator of the HHMI.
Footnotes
AUTHOR CONTRIBUTIONS
A.Z.: Conception and design, bioinformatics analysis, data generation, and performing of the experiments, validation, data curation, formal analysis, manuscript writing. CCR: Generation of protein and RNA, consultation on design of in vitro experiments, double filter binding assays. HPC: Contribution of CHART experiments, RNA-seq data. AJH: Kinetics analysis. JTL: Conception and design, supervision, validation, data curation, formal analysis, manuscript writing.
References
- Allen TA, Von Kaenel S, Goodrich JA, Kugel JF. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol. 2004;11:816–821. doi: 10.1038/nsmb813. [DOI] [PubMed] [Google Scholar]
- Bachvarova R. Small B2 RNAs in mouse oocytes, embryos, and somatic tissues. Developmental biology. 1988;130:513–523. doi: 10.1016/0012-1606(88)90346-6. [DOI] [PubMed] [Google Scholar]
- Basenko EY, Sasaki T, Ji L, Prybol CJ, Burckhardt RM, Schmitz RJ, Lewis ZA. Genome-wide redistribution of H3K27me3 is linked to genotoxic stress and defective growth. Proc Natl Acad Sci U S A. 2015;112:E6339–6348. doi: 10.1073/pnas.1511377112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque G, Leong B, Vega VB, Chen X, Lee YL, Srinivasan KG, Chew JL, Ruan Y, Wei CL, Ng HH, et al. Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res. 2008;18:1752–1762. doi: 10.1101/gr.080663.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Imbalzano AN, Kingston RE. Activator-dependent regulation of transcriptional pausing on nucleosomal templates. Genes Dev. 1996;10:1479–1490. doi: 10.1101/gad.10.12.1479. [DOI] [PubMed] [Google Scholar]
- Budzynski MA, Puustinen MC, Joutsen J, Sistonen L. Uncoupling Stress-Inducible Phosphorylation of Heat Shock Factor 1 from Its Activation. Molecular and cellular biology. 2015;35:2530–2540. doi: 10.1128/MCB.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chircop M, Speidel D. Cellular stress responses in cancer and cancer therapy. Frontiers in oncology. 2014;4:304. doi: 10.3389/fonc.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Rojas C, Hernandez AJ, Sarma K, Lee JT. Regulatory interactions between RNA and polycomb repressive complex 2. Mol Cell. 2014;55:171–185. doi: 10.1016/j.molcel.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium EP. Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C, Wang X, Cifuentes-Rojas C, Goodrich KJ, Gooding AR, Lee JT, Cech TR. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol Cell. 2015;57:552–558. doi: 10.1016/j.molcel.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C, Zheng L, Goodrich KJ, Cech TR. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol. 2013;20:1250–1257. doi: 10.1038/nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning AP, Gu W, Castoe TA, Batzer MA, Pollock DD. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7:e1002384. doi: 10.1371/journal.pgen.1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nature reviews Genetics. 2011;12:833–845. doi: 10.1038/nrg3055. [DOI] [PubMed] [Google Scholar]
- Down TA, Hubbard TJ. Computational detection and location of transcription start sites in mammalian genomic DNA. Genome Res. 2002;12:458–461. doi: 10.1101/gr.216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte FM, Fuda NJ, Mahat DB, Core LJ, Guertin MJ, Lis JT. Transcription factors GAF and HSF act at distinct regulatory steps to modulate stress-induced gene activation. Genes Dev. 2016;30:1731–1746. doi: 10.1101/gad.284430.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza CA, Allen TA, Hieb AR, Kugel JF, Goodrich JA. B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nat Struct Mol Biol. 2004;11:822–829. doi: 10.1038/nsmb812. [DOI] [PubMed] [Google Scholar]
- Espinoza CA, Goodrich JA, Kugel JF. Characterization of the structure, function, and mechanism of B2 RNA, an ncRNA repressor of RNA polymerase II transcription. RNA. 2007;13:583–596. doi: 10.1261/rna.310307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno O, Virolle T, Djabari Z, Ortonne JP, White RJ, Aberdam D. Transposable B2 SINE elements can provide mobile RNA polymerase II promoters. Nature genetics. 2001;28:77–81. doi: 10.1038/ng0501-77. [DOI] [PubMed] [Google Scholar]
- Fornace AJ, Jr, Mitchell JB. Induction of B2 RNA polymerase III transcription by heat shock: enrichment for heat shock induced sequences in rodent cells by hybridization subtraction. Nucleic Acids Res. 1986;14:5793–5811. doi: 10.1093/nar/14.14.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Chromosome structure and the C-value paradox. J Cell Biol. 1981;91:3s–14s. doi: 10.1083/jcb.91.3.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasties N. Genetic Variants and Strains of the Laboratory Mouse. Oxford: Oxford University Press; 1989. Highly repeated DNA families in the genome of Mus musculus. [Google Scholar]
- Huang W, Loganantharaj R, Schroeder B, Fargo D, Li L. PAVIS: a tool for Peak Annotation and Visualization. Bioinformatics. 2013;29:3097–3099. doi: 10.1093/bioinformatics/btt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkowski B, Tanaka Y, Kawaji H, Sandelin A, Andersson R, Itoh M, Lassmann T, Hayashizaki Y, Carninci P, Forrest AR, et al. Transcriptome Analysis of Recurrently Deregulated Genes across Multiple Cancers Identifies New Pan-Cancer Biomarkers. Cancer research. 2016;76:216–226. doi: 10.1158/0008-5472.CAN-15-0484. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Son J, Shen SS, Reinberg D, Bonasio R. PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells. Nat Struct Mol Biol. 2013;20:1258–1264. doi: 10.1038/nsmb.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinmanns JA, Schubert D. Polycomb and Trithorax group protein-mediated control of stress responses in plants. Biological chemistry. 2014;395:1291–1300. doi: 10.1515/hsz-2014-0197. [DOI] [PubMed] [Google Scholar]
- Kramerov DA, Vassetzky NS. SINEs. Wiley Interdiscip Rev RNA. 2011;2:772–786. doi: 10.1002/wrna.91. [DOI] [PubMed] [Google Scholar]
- Kung JT, Kesner B, An JY, Ahn JY, Cifuentes-Rojas C, Colognori D, Jeon Y, Szanto A, del Rosario BC, Pinter SF, et al. Locus-specific targeting to the X chromosome revealed by the RNA interactome of CTCF. Mol Cell. 2015;57:361–375. doi: 10.1016/j.molcel.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H, Fuda NJ, Core LJ, Lis JT. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science. 2013;339:950–953. doi: 10.1126/science.1229386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H, Lis JT. Control of transcriptional elongation. Annual review of genetics. 2013;47:483–508. doi: 10.1146/annurev-genet-110711-155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Bartolomei MS. X-Inactivation, Imprinting, and Long Noncoding RNAs in Health and Disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Spearow J, Rubin CM, Schmid CW. Physiological stresses increase mouse short interspersed element (SINE) RNA expression in vivo. Gene. 1999;239:367–372. doi: 10.1016/s0378-1119(99)00384-4. [DOI] [PubMed] [Google Scholar]
- Li W, Notani D, Rosenfeld MG. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nature reviews Genetics. 2016;17:207–223. doi: 10.1038/nrg.2016.4. [DOI] [PubMed] [Google Scholar]
- Lowe CB, Haussler D. 29 mammalian genomes reveal novel exaptations of mobile elements for likely regulatory functions in the human genome. PLoS One. 2012;7:e43128. doi: 10.1371/journal.pone.0043128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunyak VV, Prefontaine GG, Nunez E, Cramer T, Ju BG, Ohgi KA, Hutt K, Roy R, Garcia-Diaz A, Zhu X, et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science. 2007;317:248–251. doi: 10.1126/science.1140871. [DOI] [PubMed] [Google Scholar]
- Mahat DB, Salamanca HH, Duarte FM, Danko CG, Lis JT. Mammalian Heat Shock Response and Mechanisms Underlying Its Genome-wide Transcriptional Regulation. Mol Cell. 2016;62:63–78. doi: 10.1016/j.molcel.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolhuijzen P, Kulski JK, Dunn DS, Schibeci D, Barrero R, Gojobori T, Bellgard M. The transcript repeat element: the human Alu sequence as a component of gene networks influencing cancer. Functional & integrative genomics. 2010;10:307–319. doi: 10.1007/s10142-010-0168-1. [DOI] [PubMed] [Google Scholar]
- Ponicsan SL, Kugel JF, Goodrich JA. Genomic gems: SINE RNAs regulate mRNA production. Curr Opin Genet Dev. 2010;20:149–155. doi: 10.1016/j.gde.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponicsan SL, Kugel JF, Goodrich JA. Repression of RNA Polymerase II Transcription by B2 RNA Depends on a Specific Pattern of Structural Regions in the RNA. Noncoding RNA. 2015;1:4–16. doi: 10.3390/ncrna1010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma K, Cifuentes-Rojas C, Ergun A, Del Rosario A, Jeon Y, White F, Sadreyev R, Lee JT. ATRX directs binding of PRC2 to Xist RNA and Polycomb targets. Cell. 2014;159:869–883. doi: 10.1016/j.cell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein A, Zucchelli S, Kauppinen S, Gustincich S, Carninci P. Identification of antisense long noncoding RNAs that function as SINEUPs in human cells. Sci Rep. 2016;6:33605. doi: 10.1038/srep33605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold AP, Banerjee R, Tie F, Kiss DL, Moskowitz J, Harte PJ. Polycomb Repressive Complex 2 and Trithorax modulate Drosophila longevity and stress resistance. Proc Natl Acad Sci U S A. 2010;107:169–174. doi: 10.1073/pnas.0907739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MD. Capture hybridization analysis of RNA targets (CHART) Current protocols in molecular biology/edited by Frederick M Ausubel [et al] 2013;Chapter 21(Unit 21):25. doi: 10.1002/0471142727.mb2125s101. [DOI] [PubMed] [Google Scholar]
- Simon MD, Pinter SF, Fang R, Sarma K, Rutenberg-Schoenberg M, Bowman SK, Kesner BA, Maier VK, Kingston RE, Lee JT. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature. 2013;504:465–469. doi: 10.1038/nature12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Carey M, Saragosti S, Botchan M. Expression of enhanced levels of small RNA polymerase III transcripts encoded by the B2 repeats in simian virus 40-transformed mouse cells. Nature. 1985;314:553–556. doi: 10.1038/314553a0. [DOI] [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen TJ, Salzberg SL. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nature reviews Genetics. 2012;13:36–46. doi: 10.1038/nrg3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner SD, Yakovchuk P, Gilman B, Ponicsan SL, Drullinger LF, Kugel JF, Goodrich JA. RNA polymerase II acts as an RNA-dependent RNA polymerase to extend and destabilize a non-coding RNA. EMBO J. 2013;32:781–790. doi: 10.1038/emboj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Grullon S, Ge K, Peng W. Spatial clustering for identification of ChIP-enriched regions (SICER) to map regions of histone methylation patterns in embryonic stem cells. Methods Mol Biol. 2014;1150:97–111. doi: 10.1007/978-1-4939-0512-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovchuk P, Goodrich JA, Kugel JF. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci U S A. 2009;106:5569–5574. doi: 10.1073/pnas.0810738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, Song JJ, Kingston RE, Borowsky M, Lee JT. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The short RNA-seq method.
Figure S1 relates to Figures 1,2, and 3.
A) Distribution of EZH2 CLIP-seq reads (from Kaneko et al., 2013) around the start site (+/− 2000 bp) of B1 and B2 repeats.
B) In order to study the B2 RNA fragments, we employed a short RNA-seq protocol that enables the unbiased study of non-polyadenylated RNAs between 40–200 nucleotides. No RNA fragmentation step is included. rRNAs, mRNAs, miRNAs, and other small RNAs are depleted by this method.
C) Representative bioanalyzer trace of short RNAs isolated using the short RNA-seq protocol.
D) Bioanalyzer trace of in vitro transcribed B2 RNA at various steps of library construction (input, after CIP treatment, and after PNK treatment). No RNA degradation is evident.
E) Bioanalyzer trace of the cDNA pool after reverse transcription of B2 RNA (from panel S1D) during library construction. The full length B2 cDNA is highly enriched and no smaller cDNAs are observed.
F) Deep sequencing of the library constructed from B2 cDNA (from Figure S1E). As in Fig. 1E, 5′ ends of our short RNA-seq reads are plotted along the B2 locus (x-axis). Note the absence of cutting at position 98.
G) qRT-PCR of B2 RNA after transfection of full-length B2 RNA (+) into HeLA cells. Mock transfection (−) used a negative control RNA, P4-P6.
H) Plot of the 5′ end of B2 RNAs after short RNA-seq preparation from HeLa cells. Note the absence of cutting at position 98.
I) Short RNA-seq of NIH/3T3 cells before and after heat shock (45 °C for 15 minutes). Two biological replicates yielded similar results. 5′ ends of short RNA-seq reads are mapped to the B2 transcript and the relative number of 5′ ends is plotted on the y-axis. The 5′ end counts are normalized to the levels of full length B2 RNAs from long RNA-seq of the respective samples to account for any possible changes in the general B2 levels during heat shock (KS test; P<0.0001). The values are further normalized to the total tRNA levels, which are not transcribed by POL-II, as an internal loading control. A graph of the same data normalized to the +1 position is shown in Figure 3D, with accompanying read alignment display in Figure 3E. In the corresponding Figure 3D, we normalized +1 position. Notably, both methods of presentation show increased cutting at +98 after H/S (KS test; P<0.0001).
Figure S2. Correlation between biological replicates of the B2 CHART-seq experiment.
Figure S2 relates to Figure 4.
A, B) Log2 normalized read counts from replicates 1 and 2 for the B2 CHART-seq analysis of pre-H/S (A) and post-H/S (B) cells in 10kb windows. Pearson’s R is indicated. Colors represent point density (blue: low, red: high)
C) Metagene plot of B2 CHART read density at B2 elements, the site of nascent transcription. As expected, B2 RNA is enriched at the site of transcription. These loci served as positive control and are excluded from further analysis.
Figure S3. B2 CHART-seq profiles at four loci, with biological replicates.
Figure S3 relates to Figure 4.
IGV screenshots of two biologlical replicates for B2 binding patterns at two H/S-upregulated and two H/S-downregulated genes. Pre- and post-H/S profiles are shown. Paired data are shown at the same scale (numbers in brackets, right) for comparison. Replicates of RNA-seq data are shown below CHART tracks.
Figure S4. Transcriptomic analysis before and after heat shock in relation to B2 CHART analysis.
Figure S4 relates to Figure 4.
A) Correlation between RNA-seq biological replicates. Log2 normalized read counts from replicates 1 and 2 for the RNA-seq analysis of pre- and post-H/S cells for RefSeq regions. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
B–E) Upper panels: Numbers and percentages of genes with Type I (panels B, C) or Type II B2 sites (panels D, E). Genes are defined as gene body +/− 3kb. Bottom panels: Heatmaps representing density of B2 CHART coverage in pre- and post-H/S cells. Hierarchical clustering revealed two main clusters – genes that gain versus lose B2 coverage with H/S. Genes are defined as gene body +/− 3kb of flanking DNA. Upregulated genes are analyzed in panel C. Downregulated genes are analyzed in panel E.
Figure S5. Correlation between biological replicates of POL-II-S2P, EZH2, and H3K27me3 ChIP-seq experiments.
Figure S5 relates to Figures 5 and 6.
Log2 normalized read counts from replicates 1 and 2 for POL-II-S2P, EZH2, and H3K27me3 ChIP-seq experiments for pre-H/S and post-H/S cells. Each dot represents a RefSeq gene. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
Figure S6. Correlation between biological replicates of POL-II-S2P ChIP-seq and RNA-seq experiments from cells treated with B2 LNA.
Figure S6 relates to Figure 5.
A) Alignment of short RNA-seq reads to the B2 metagene. Cells were treated with either Scr LNA or B2 LNA treatment within the B2 metagene between nt 1–201. The Scr LNA plot is the same experiment shown in Figure 3E.
B, C) Correlation plots showing Log2 normalized read counts from replicates 1 and 2 of the POL-II-S2P ChIP-seq (B) or RNA-seq (C) experiments. Cells were treated for 24 hr with B2 LNA to induce B2 cleavage without H/S. Each dot represents a RefSeq gene. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
Figure S7. Effects of EZH2 depletion during the heat shock response.
Figure S7 relates to Figures 6 and 7.
A) RT-qPCR for the differences in RNA expression levels observed after treatment with B2, EZH2, or Scr LNA as indicated. Averages of 4 biological replicates shown. P values are determined by t-test.
B) Metasite analysis centered on EZH2 binding sites shows that EZH2, SUZ12, and H3K27me3 peaks coincide with each other in general across the genome, as expected, in post-H/S cells. x=0 corresponds to EZH2 peaks.
C) EZH2, SUZ12, and H3K27me3 peaks generally correlate with each other across the genome, as expected. Shown are correlation plots of Log2 normalized ChIP read counts for the indicated epitopes. Each dot represents a ChIP-seq peak/site for the indicated epitope in post-H/S cells. Pearson’s R is indicated. Colors represent point density (blue: low, red: high). Two biological replicates for each epitope.
D) Significant EZH2 knockdown by LNA transfection. P =0.04, as determined by t-test.
E) Correlation plots showing Log2 normalized read counts from RNA-seq biological replicates 1 and 2 after EZH2 knockdown. Each dot represents a RefSeq gene. Pearson’s R is indicated. Colors represent point density (blue: low, red: high).
Table S-1 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis, listing all peaks present in pre-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated. Peaks identified by Probes 1 and 2 listed separately in sheets 1 and 2, respectively.
Table S-2 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis, listing all peaks present in post-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated. Peaks identified by Probe 1 are listed in a separate sheet from peaks captured by Probe 2.
Table S-3 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis using Probe 1), listing peaks present only in pre-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated.
Table S-4 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis using Probe 1, listing all peaks present only in post-heat shock NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated.
Table S-5 relates to Figure 4.
Column A: Statistically significant peaks of B2 RNA binding from CHART-seq analysis using Probe 1, listing all peaks present in both pre- and post-H/S NIH/3T3 cells. Column B: Chromosome on which peak resides. Columns C, D: Start and end positions (in order) on the chromosome using coordinates from mm9 genome assembly. Column E: Closest or overlapping gene. Column F: ENSEMBL ID of closest or overlapping gene. Column G: Description of gene. Columns H, I: Indicate whether gene is overlapping. If not, the distance (in bp) upstream or downstream is indicated.
Table S-6 relates to Figures 3, 4 and the “Quantitative gene expression analysis” methods section.
Upregulated and downregulated genes are listed in separate sheets.
Column A: Heat shock-upregulated or -downregulated gene shown by RNA-seq analysis of NIH/3T3 cells. Column B: Chromosome position and mm9 start and stop coordinates of the gene in Column A. Column C: Log2 fold-change of the gene in post-H/S cells relative to pre-H/S state.