Abstract
Study Design
Systematic review and meta-analysis.
Objective
The goal of this study was to (i) assess the risk of neurological injury after anterior cervical spine surgery (ACSS) with and without intraoperative neuromonitoring (ION) and (ii) evaluate differences in the sensitivity and specificity of ION for ACSS.
Summary of Background Data
Although ION is used to detect impending neurological injuries in deformity surgery, it’s utility in ACSS remains controversial.
Methods
A systematic search of multiple medical reference databases was conducted for studies on ION use for ACSS. Studies that included posterior cervical surgery were excluded. Meta-analysis was performed using the random-effects model for heterogeneity. Outcome measure was postoperative neurological injury.
Results
The search yielded 10 studies totaling 26,357 patients. The weighted risk of neurological injury after ACSS was 0.64% (0.23–1.25). The weighted risk of neurological injury was 0.20% (0.05–0.47) for ACDFs compared with 1.02% (0.10–2.88) for corpectomies. For ACDFs, there was no difference in the risk of neurological injury with or without ION (odds ratio, 0.726; confidence interval, CI, 0.287–1.833; P = 0.498). The pooled sensitivities and specificities of ION for ACSS are 71% (CI: 48%–87%) and 98% (CI: 92%–100%), respectively. Unimodal ION has a higher specificity than multimodal ION [unimodal: 99% (CI: 97%–100%), multimodal: 92% (CI: 81%–96%), P = 0.0218]. There was no statistically significant difference in sensitivities between unimodal and multimodal [68% vs. 88%, respectively, P = 0.949].
Conclusion
The risk of neurological injury after ACSS is low although procedures involving a corpectomy may carry a higher risk. For ACDFs, there is no difference in the risk of neurological injury with or without ION use. Unimodal ION has a higher specificity than multimodal ION and may minimize “subclinical” intraoperative alerts in ACSS.
Keywords: anterior cervical discectomy and fusion, anterior cervical spinal surgery, cervical spine, corpectomy, electrophysiological monitoring, meta-analysis, motor evoked potential, neurologic injury, neuromonitoring, somatosensory evoked potential
Neurological injuries are known complications of spine surgery. To decrease the risk of these adverse events, intraoperative neuromonitoring (ION) is used to detect impending neurological injury. Somatosensory evoked potentials (SSEPs)and motor-evoked potentials (MEPs) are the two most commonly used ION modalities to monitor spinal cord function. SSEPs monitor spinal cord function through the ascending sensory pathway whereas MEPs provide a direct measure of the corticospinal motor tract function.
Although ION has been shown to decrease the risk of neurological injury in deformity surgery, its utility in anterior cervical spine surgery (ACSS) remains controversial.1–8 Proponents of ION for ACSS claim that it improves patient safety and functional outcome whereas opponents refute this claim by citing increased cost and the lack of correlation between ION abnormalities and postoperative neurological deficits especially with anterior cervical discectomy and fusions (ACDFs).9–12 The goal of this meta-analysis was to (i) assess the risk of neurological injury after ACSS with and without ION and (ii) evaluate differences in the sensitivity and specificity of ION for ACSS.
MATERIALS AND METHODS
Search Strategy
A systematic search of Medline, Embase, Cochrane Reviews, SCOPUS, and Web-of-Science was performed to identify studies that reported on ION use for ACSS. The search criteria utilized were “cervical AND monitoring,” “cervical AND neuromonitoring,” “cervical AND intra-operative neuromonitoring,” “cervical AND electrophysiological monitoring,” “cervical AND motor evoked potential,” and “cervical AND somatosensory evoked potential.” The search strategy was developed to include all study designs. English-language full text manuscripts or abstracts were reviewed. After review of all relevant reports, the references of articles selected for review were further assessed to identify studies that were not captured in our initial database search.
Selection of Studies
Studies that reported ION use for ACSS were included. Studies that involved cranial surgery, posterior cervical spinal surgery, or nonspinal surgery were excluded. Case reports, studies that utilized spinal cord evoked potential only and studies that did not report patient outcomes, were also excluded.
Data Extraction
Two reviewers (R.A. and S.Z.) reviewed and extracted data from studies that fulfilled all inclusion and exclusion criteria. The following variables were extracted from each study: year of study, type of study, level of evidence, demographic data, type of surgery, indication for surgery, type of neuromonitoring modality, neuromonitoring alert criteria, risk of neurological injury, ION sensitivity, specificity, false positive rate, and false negative rate. Primary outcome measure used was neurologic injury (defined as any evidence of worsening postoperative neurologic status from baseline).
Assessment of Level of Evidence and Methodological Quality of the Studies
Two reviewers (S.P. and R.A.) conducted a quality assessment for each of the studies selected for final analysis. Level of evidence ratings were assigned to each study using the criteria set forth by Wright et al13 (Table 1).11,14–22 Quality assessment of all the selected reports was made by using the 12-point Methodological Index for Nonrandomized Studies (MINORS) criteria (Table 2,11,14–22 Appendix A and B, http://links.lww.com/BRS/B184). This criteria has been previously reported to have high test-retest, external and internal validity, and interobserver reliability.23 The risk of bias (RoB) of comparative studies was assessed using the Cochrane Back Review Group tool.24 If studies met at least six of the 12 criteria, the study was regarded as low RoB. If five or less of the 12 criteria were met, the study was labeled as high RoB. For noncomparative studies, RoB was assessed using a modified 5-point assessment score as previously described.25 Inconsistencies in methodologic quality assessment were reconciled through discussion with a third author (S.Z.).
TABLE 1.
Study Information and Demographics
| Study | Year | Type of Study | Level of Evidence | Dates of Surgery | Mean Age, Yrs (Range) | % Male | Number of Institutions | Type of Surgery | Indication for Surgery |
|---|---|---|---|---|---|---|---|---|---|
| Bose et al14 | 2004 | Retrospective | IV | 23 month period | 46 (24–82) | NR | 1 | ACDF only | R, M, T, Tu, O |
| Bouchard et al15 | 1996 | Retrospective | IV | 1986–1991 | NR | 1 | ACDF + Corpectomy | R, M | |
| Gokaslan et al16 | 1997 | Retrospective | IV | NR | 48.6 (18–86) | NR | NR | ACDF only | R, M |
| Lee et al17 | 2006 | Retrospective | IV | 2000–2004 | NR | NR | 1 | ACDF only and ACDF + Corpectomy | R, M, T, Tu, O |
| Sebastian et al18 | 1997 | Prospective | IV | NR | 47.2 (21 – 80) | 45.5 | 1 | ACDF only and ACDF + Corpectomy | R, M, T |
| Smith et al19 | 2007 | Retrospective | III | 1995–2004 | NR | NR | 1 | ACDF only | R, T, Tu, O |
| Taunt et al20 | 2005 | Retrospective | IV | 1995–2002 | 48.6 (14–84) | NR | 1 | ACDF only | R, M, T, O |
| Xu et al21 | 2011 | Retrospective | IV | 2007–2010 | 47.6 +/− 11.1 | NR | 1 | ACDF + Corpectomy | R, M |
| Cole et al22 | 2014 | Retrospective | III | 2006–2010 | NR | NR | National database | ACDF only | NR |
| Khan et al23 | 2006 | Retrospective | IV | 1994–2004 | 55.7 (NR) | 52.8 | 1 | ACDF + Corpectomy | R, M, O |
Relevant and available characteristics of each study are present.
I indicates infection; M, myelopathy; NR, not reported; O, other; R, radiculopathy; T, trauma; Tu, tumor.
TABLE 2.
Risk of Bias and Quality Assessment of the Noncomparative Studies
| Study | Risk of Bias | MINORS Score* |
|---|---|---|
| Bose et al14 | Low | 10/16 |
| Bouchard et al15 | Low | 9/16 |
| Gokaslan et al16 | Low | 10/16 |
| Lee et al17 | Low | 9/16 |
| Sebastian et al18 | Low | 11/16 |
| Smith et al19 | Low | 14/24 |
| Taunt et al20 | Low | 9/16 |
| Xu et al21 | Low | 9/16 |
| Cole et al22 | Low | 18/24 |
| Khan et al23 | Low | 10/16 |
The global ideal MINORS score is 16 for noncomparative studies and 24 for comparative studies.
Data Analysis
Data analysis was performed using the random-effects model with inverse variance weighting. The principal study measures were summary estimates and 95% confidence intervals (CI). Results among studies were compared with 95% CIs and corresponding forest plots. Meta-analysis was used to estimate the risk of neurological injury in procedures involving ACDF with or without corpectomy, ACDF alone, and in ACDF with and without ION. The number of adverse neurologic events was divided by the patient cohort size to determine the incidence of injury in each study. A Q statistic and I2 value were calculated to assess for heterogeneity between individual studies in each meta-analysis group. I2 heterogeneity less than 25% generally indicates consistent results and homogenous studies, whereas 25% to 75% indicates moderate heterogeneity, and greater than 75% indicates severe heterogeneity, as reported by Delong et al.26 Bivariate analysis was performed for sensitivity and specificity of ION. Mixed-effect logistic regression was used to compare the sensitivity and specificity of unimodal and multimodal ION. A P-value of <0.05 was considered to be statistically significant.
Calculations for the meta-analysis were performed with StataMP v. 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX) and forest plots and ROC curves were generated using Review Manager v5.3 [Review Manager (RevMan) (Computer program) Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014].
RESULTS
Studies
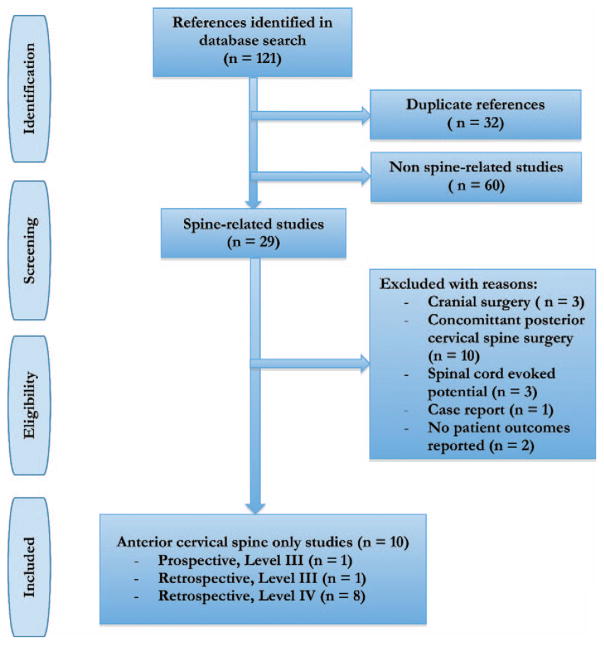
Overall, the search yielded 121 citations of which 111 were excluded. Thirty-two duplicate studies were excluded and 60 studies were excluded because they were not spine-related based on abstract review. The full text of the remaining 29 studies was then assessed, and 19 studies were excluded with reasons, leaving the final included studies (Figure 1). Ten studies fulfilled all inclusion and exclusion criteria and were included in this systematic review.11,14–22
Figure 1.
Flowchart for included studies.
Five of the 10 studies analyzed a uniform cohort of patients undergoing ACDF alone,11,14,16,19,21 whereas the remaining five studies analyzed patients undergoing either ACDF alone or ACDF with corpectomy (Table 1).15,17,18,20,22 Six studies analyzed patients monitored with a unimodal technique;11,15,16,18,19,22 five of which used SSEP,11,15,18,19,22 and one of which used MEP16 (Table 3).11,14–22
TABLE 3.
Neuromonitoring Information
| Study | Year | Monitoring Modality | Type of Monitoring | SSEP Nerve | SSEP Alert Criterion: Amplitude Decrease | SSEP Alert Criterion: Latency Increase | MEP Muscle Group | MEP Alert Criterion: Amplitude Decrease |
|---|---|---|---|---|---|---|---|---|
| Bose et al14 | 2004 | Multimodal | SSEP and MEP | U, Ti | 30%–50% | NR | APB, DI, TA, AH | 50% |
| Bouchard et al15 | 1996 | Unimodal | SSEP | M, Ti | NR | NR | NR | NR |
| Gokaslan et al16 | 1997 | Unimodal | MEP/epidural electrode | NR | NR | NR | NR | NR |
| Lee et al17 | 2006 | Multimodal | MEP, SSEP and EMG | NR | 60% | NR | D, Tr, ECR, DI, TA, AH | 60% (10 min) |
| Sebastian et al18 | 1997 | Unimodal | SSEP | M | 50% | 10% | NR | NR |
| Smith et al19 | 2007 | Unimodal | SSEP | M, U, Ti, P | 50% | 10% | NR | NR |
| Taunt et al20 | 2005 | Unimodal | SSEP | M, Ti | NR | NR | NR | NR |
| Xu et al21 | 2011 | Multimodal | SSEP and MEP | M, U, Ti | 50% | 10% | APB, TA, AH | “Significant reduction” |
| Cole et al22 | 2014 | NR | NR | NR | NR | NR | NR | NR |
| Khan et al23 | 2006 | Unimodal | SSEP | M, U, Ti, P | 50% | 10% | NR | NR |
Relevant and available monitoring characteristics of each study are present.
AH indicates abductor hallucis; APB, abductor pollicis brevis; D, deltoid; DI, 1st dorsal interosseuous; M, median; MEP, motor evoked potential; NR, not reported; P, peroneal; SSEP, somatosensory evoked potential; TA, tibialis anterior; Ti, tibial; Tr, triceps; U, ulnar.
Indications for ACSS were strictly limited to myelopathy or radiculopathy in three studies,15,16,20 whereas the remaining studies included a small percentage of patients treated for infection, tumor, trauma, and ossified posterior longitudinal ligament (Table 1).14,17–19,21,22
Quality Assessment of Included Studies
Two studies were published as level III evidence19,21 and eight studies were published as level IV evidence (Table 1).11,14–18,22 The MINORS score of the noncomparative studies ranged from 9 to 11, with an average score of 9.63 and a standard deviation (SD) of 0.74 (Table 2). The MINORS score of the comparative studies ranged from 14 to 18, with an average score of 16 and a SD of 2.83 (Table 3). All studies included in this review were of low methodologic quality but with low RoB.
Demographics
There were a total of 26,357 patients (range, 16–22,768 per study).11,14–22 One study, drawn from a national database, had 22,768 patients.21 Six studies reported mean patient age with a resulting weighted mean of 51.3 years and a range of 14 to 86 years.11,14,16,18,19,22 Two studies reported the sex of their patient population, which were 52% male22 and 45% male,18 respectively (Table 1).
Monitoring Technique
Of the studies which used SSEP monitoring, seven used either the median nerve or ulnar nerve for upper extremity monitoring and the tibial or peroneal nerves for lower extremity monitoring.11,14,15,18–20,22 One study used only upper extremity SSEP monitoring.18 Two studies did not report the technique for SSEP monitoring.17,21 Criteria for a significant alert were a decrease in amplitude of 50% or an increase in latency of 10% in three studies,18,20,22 a 30% to 50% decrease in amplitude in one study,14 and a 60% decrease in amplitude in one study (Table 3).17
Of the studies which reported their MEP monitoring technique, various combinations of the upper extremity muscle groups including abductor pollicis brevis, first dorsal interosseous, extensor carpi radialis, triceps, and deltoid were monitored, and the lower extremity muscle groups including abductor hallucis and anterior tibialis were monitored.14,17,20 To define a significant alert, one study used a cutoff of 50% decrease in amplitude,14 one used a cutoff of 60% decrease in amplitude over 10 minutes,17 and one used an undefined, “significant” reduction in amplitude (Table 3).20
Risk of Neurological Injury
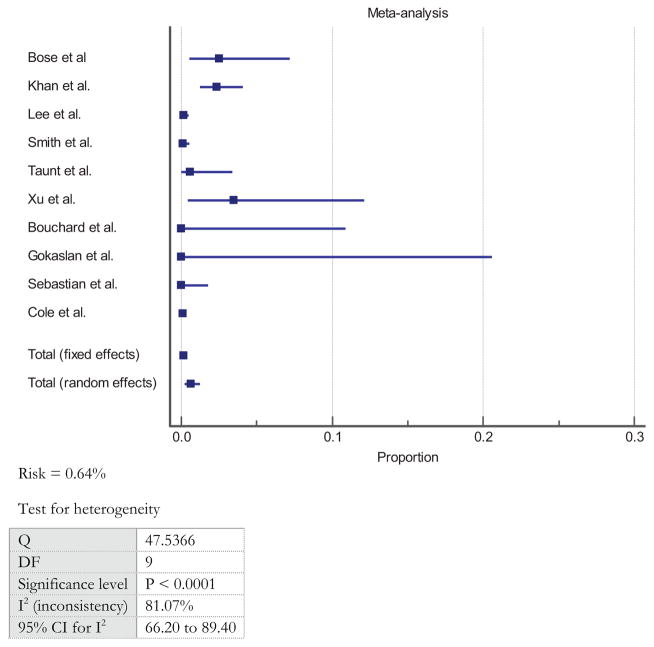
There were 49 events of neurological injury among 26,357 patients from all studies, leading to an overall unweighted risk of adverse event of 0.19% (95% CI: 0.13–0.24). Weighted per study, the overall weighted risk of neurological injury after ACSS was 0.64% (95% CI: 0.23–1.25) (Figure 2).11,14–22 The studies demonstrated severe heterogeneity with a Q value of 47.5366 and I2 value of 81.07%.11,14–22 In the five studies that analyzed a uniform cohort of patients undergoing ACDF alone,11,14,16,19,21 the weighted risk of neurological injury was 0.20% (95% CI: 0.05–0.47) compared with 1.02% (95% CI: 0.10–2.88) in studies that involved corpectomies (there is insufficient data to perform a comparative statistical analysis between both groups).15,17,18,21,22
Figure 2.
Overall weighted risk of neurological injury after ACSS.
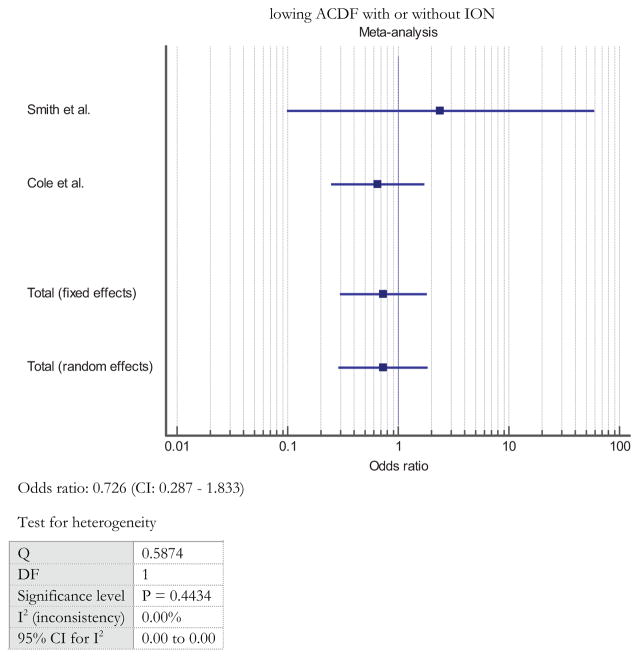
Two studies compared the risk of neurological injury in ACDF patients with or without ION.19,21 From these groups, there was no statistically significant difference in the risk of neurological injury with or without ION (OR: 0.726, 95% CI: 0.287–1.833, P = 0.498) (Figure 3). The studies demonstrated consistent results and homogeneity with a Q value of 0.584 and I2 value of 0.00%.
Figure 3.
Risk of neurological injury after ACDF with or without ION.
Intraoperative Neuromonitoring Sensitivity and Specificity
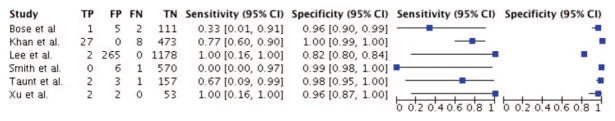
Six studies reported complete data on sensitivity and specificity.11,14,17,19,20,22 Using bivariate analysis, the pooled sensitivities and specificities of these six studies on ION for ACSS were 71% (95% CI: 48%–87%) and 98% (CI: 92%–100%), respectively (Table 4,11,14–22 Figure 4). Weighted per study, the positive predictive value for ION in ACSS was 24.2% and the negative predictive value was 99.6%.
TABLE 4.
Sensitivity and Specificity
| Study | Year | Number of Patients | Total Incidence of Neurologic Injury (%) | Neurologic Injury Risk With ION (%) | Neurologic Injury Risk Without ION (%) | Sensitivity | Specificity | False Negative | False Positive |
|---|---|---|---|---|---|---|---|---|---|
| Bose et al14 | 2004 | 119 | 3 (2.5) | NR | NR | 0.33 | 0.96 | 0.67 | 0.04 |
| Bouchard et al15 | 1996 | 32 | 0 (0) | NR | NR | N/A | 1.00 | N/A | 0.00 |
| Gokaslan et al16 | 1997 | 16 | 0 (0) | NR | NR | N/A | 1.00 | N/A | 0.00 |
| Lee et al17 | 2006 | Total: 1445 Corpectomy: 483 |
Total: 2 (0.01) Corpectomy: 2 (0.4) |
NR | NR | 1.00 | 0.82 | 0.00 | 0.18 |
| Sebastian et al18 | 1997 | Total: 210 Corpectomy: 80 |
Total: 0 (0) Corpectomy: 0 (0) |
NR | NR | N/A | 0.81 | N/A | 0.19 |
| Smith et al19 | 2007 | 1039 | 1 (0.09) | 1/577 (0.17) | 0/462 (0) | 0.00 | 0.99 | 1.00 | 0.01 |
| Taunt et al20 | 2005 | 163 | 1 (0.6) | NR | NR | 0.67 | 0.98 | 0.33 | 0.02 |
| Xu et al21 | 2011 | 57 | 2 (3.5) | NR | NR | 0.67 | 0.96 | 0.00 | 0.04 |
| Cole et al22 | 2014 | 22768 | 28 (0.12) | 5/5700 (0.09) | 23/17068 | N/A | N/A | N/A | N/A |
| Khan et al23 | 2006 | 508 | 12 (2.4) | NR | NR | 0.77 | 1.00 | 0.23 | 0.00 |
Data for each study’s individual sensitivity and specificity analysis.
The values represent the total sensitivity and specificity for all patients included in the study, the total value in “Number of Patients.”
ION indicates intraoperative neuromonitoring; NR, not reported.
Figure 4.
Sensitivity and specificity of ION for ACSS.
To compare data for unimodal versus multimodal ION, the six studies with complete sensitivity and specificity data were pooled using bivariate analysis.11,14,17,19,20,22 There was high specificity in both unimodal (specificity: 99%, 95% CI: 97%–100%) and multimodal (specificity: 92%, 95% CI: 81%–96%) ION; the difference was significant (P = 0.0218). Both modalities had lower sensitivities, and the difference between unimodal (sensitivity: 68%, 95% CI: 39%–88%) and multimodal (sensitivity: 88%, 95% CI: 4%–100%) was not significant (P = 0.949) (Table 5).
TABLE 5.
Sensitivity and Specificity of Unimodal Versus Multimodal ION for ACSS
| Multimodal ION | Unimodal ION | P | |
|---|---|---|---|
| Sensitivity (CI) | 0.88 (0.04–1.0) | 0.68 (0.39–0.88) | 0.9495 |
| Specificity (CI) | 0.92 (0.81–0.96) | 0.99 (0.97–1.0) | 0.0218 |
CI indicates confidence interval; ION, intraoperative neuromonitoring.
DISCUSSION
Intraoperative neuromonitoring has been used since the 1970 s for spinal surgeries.9 Before the widespread use of ION, the Stagnara wake-up test served as the only way to intraoperatively assess neurologic function.27 ION is now used in a variety of spine surgeries including tumor resection, deformity surgery, trauma, and degenerative spine surgery.1,28 A substantial body of literature shows that ION can aid with early detection of neurological injuries that occur with traction, compression, or ischemia of the spinal cord during deformity correction.29–32
For cervical spine surgeries such as ACDF, the routine use of ION remains controversial and there is no consensus on the optimal neuromonitoring modality to use. Epstein et al10 was one of the first surgeons to argue in favor of ION for ACSS. In their series, 100 patients that had cervical spine surgery with SSEPs were compared with 218 historical control patients that had no SSEPs. Four percent of the patients in the non-monitored group developed quadriplegia compared with none of the patients in the monitored group. Improved outcomes with ION in the study were attributed to early detection of impending neurological injury by SSEPs.
The study by Epstein et al10 has been refuted, notably for an outdated and elevated rate of neurological injury at 4% that they published in their historical control group. Although ION is recognized to be sensitive for diagnosing potential neurological injury, a national practice guideline in 2009 gave no recommendation in support of routine use of ION for ACSS for degenerative conditions because of lack of specificity, lack of demonstrated clinical improvement, and conflicting class I evidence on ION parameters.33 In light of these guidelines, authors have argued that ION for degenerative ACSS has little utility when examined from a medical, cost-benefit, or medico-legal standpoint.34 Specifically, in an economic analysis study of 720 patients that had cervical decompression and reconstruction without ION, Traynelis et al12 reported no persistent postoperative neurological deficits in their series. Based on their estimate, the use of ION would have cost an hourly rate of $633.32 and incurred a total of $1,024,754 in 2011 US dollars for reimbursement at the 2011 Medicare rate.
In light of this controversial topic, we sought to answer two questions. First, what is the incidence of neurological injuries after ACSS, with and without a corpectomy, and with and without ION, from the available literature? Second, what is the sensitivity and specificity of unimodal and multimodal ION for ACSS?
Through this meta-analysis, we found that there was a low rate of neurological injury after ACSS (0.63%), and lower rate after ACDF alone (0.2%), which is in agreement with the range of 0% to 4% reported in the literature.11,17,9,35,36 Furthermore, we found that corpectomies may be higher risk with a neurological injury rate of 1.02%.
In addition, the studies examined in this analysis generally found limited utility for ION for ACDFs as there was no difference in the risk of neurological injury with or without ION. Taunt et al11 reported on 163 patients that had ACDFs with continuous SSEPs. Three (1.8%) false positives were noted in which intraoperative SSEP alerts did not correlate with postoperative neurological deterioration. In a series comparing 577 monitored patients to 462 unmonitored patients that had ACDFs, Smith et al19 reported no new postoperative neurological deficits in the unmonitored group compared with one deficit in the monitored group despite normal SSEP signals.
Because of the fact that SSEPs only monitor the ascending sensory pathways of the spinal cord, some authors have advocated for the additional use of MEPs to monitor the ventral corticospinal tract especially in ACSS. Lee et al17 published on a series of 1445 patients that had ACSS with MEPs, SSEPs, and electromyography. Eight surgeries needed to be aborted because of persistent MEP/SSEP amplitude loss. However, none of these eight patients developed a new postoperative neurological deficit. Conversely, Li et al37 did report a low sensitivity for unimodal monitoring with SSEPs (37.5%) and MEPs (62.5%), but a high sensitivity with multimodal ION (100%) with no false positive or false negative events in their retrospective series. Therefore, there have been contradictory findings from these two studies on the utility of multimodal ION for ACSS. In this study, when comparing unimodal ION with multimodal ION, there was a significantly higher specificity for detecting neurologic injury with unimodal ION. This finding demonstrates that unimodal monitoring may help minimize “subclinical” intraoperative alerts.
LIMITATIONS
There are limitations inherent to this meta-analysis as it is subject to the cumulative weaknesses of the included studies (eight level IV and two level III). This review includes predominantly retrospective studies which are considered to be of low methodologic quality. Unfortunately, there is no sufficient body of evidence in the literature involving prospective studies and randomized controlled trials. These retrospective studies were included to amass sufficient data for comparison, as only one prospective study was available in our review of the literature.18 A recent study by Martins et al38 analyzed the quality of systematic reviews in low back surgery, and found a high rate of studies that did not reach a “very good” or “excellent” rating, highlighting the difficulties inherent in reviewing the available spine literature systematically, including the challenges in identifying randomized controlled trials.
Another limitation of this study is the significant amount of heterogeneity that exists in the included studies with regards to the type of procedure (i.e., ACDFs with and without corpectomy), different types of neuromonitoring modalities and techniques, and variability in criteria for defining significant ION alerts, all of which would affect the overall sensitivity and specificity of ION reported.
Lastly, not all studies provide detailed demographic data, which makes it difficult to identify a suitable population to apply the findings of this study to. Several studies included surgeries for trauma, tumor, and infection; these indications often carry worse prognosis and have different risks of neurologic injury compared with degenerative conditions. However, the percentage of patients that fell into these categories was very low.
Despite these limitations, this study provides important aggregate data, identifying needed avenues for future research that will aid in making informed choices on the indications for ION in ACSS.
CONCLUSION
The risk of neurological injury after ACSS is low. For ACDFs, there is no difference in the risk of neurological injury with or without ION use. Unimodal ION has a higher specificity than multimodal ION and may minimize “subclinical” intraoperative alerts.
Key Points.
A systematic review and meta-analysis was performed of available literature on intraoperative neuromonitoring during ACSS.
The overall risk of neurological injury after ACSS is low but may be higher when involving corpectomy.
There is no difference in the risk of neurological injury with or without ION use in anterior cervical discectomy and fusions.
Unimodal ION has a higher specificity than multimodal ION and may minimize “subclinical” intraoperative alerts.
Footnotes
No relevant financial activities outside the submitted work.
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal’s Web site (www.spinejournal.com).
No funds were received in support of this work.
Level of Evidence: 3
References
- 1.Dawson EG, Sherman JE, Kanim LE, et al. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine. 1991;16:S361–4. [PubMed] [Google Scholar]
- 2.Diab M, Smith AR, Kuklo TR. Neural complications in the surgical treatment of adolescent idiopathic scoliosis. Spine. 2007;32:2759–63. doi: 10.1097/BRS.0b013e31815a5970. [DOI] [PubMed] [Google Scholar]
- 3.Eggspuehler A, Sutter MA, Grob D, et al. Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J. 2007;16:S188–96. doi: 10.1007/s00586-007-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes HJ, Allen PW, Waller CS, et al. Spinal cord monitoring in scoliosis surgery. Experience with 1168 cases. J Bone Joint Surg Br. 1991;73:487–91. doi: 10.1302/0301-620X.73B3.1670455. [DOI] [PubMed] [Google Scholar]
- 5.Kamerlink JR, Errico T, Xavier S, et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine. 2010;35:240–5. doi: 10.1097/BRS.0b013e3181c7c8f6. [DOI] [PubMed] [Google Scholar]
- 6.Nuwer MR, Emerson RG, Galloway G, et al. Evidence-based guideline update: intraoperative spinal monitoring with somato-sensory and transcranial electrical motor evoked potentials*. J Clin Neurophysiol. 2012;29:101–8. doi: 10.1097/WNP.0b013e31824a397e. [DOI] [PubMed] [Google Scholar]
- 7.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2005;2:725–32. doi: 10.3171/spi.2005.2.6.0725. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang Q, Wang S, Zhang J, et al. How to make the best use of intraoperative motor evoked potential monitoring? Experience in 1162 consecutive spinal deformity surgical procedures. Spine. 2014;39:E1425–32. doi: 10.1097/BRS.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 9.Engler GL, Spielholz NJ, Bernhard WN, et al. Somatosensory evoked potentials during Harrington instrumentation for scoliosis. J Bone Joint Surg Am. 1978;60:528–32. [PubMed] [Google Scholar]
- 10.Epstein NE, Danto J, Nardi D. Evaluation of intraoperative somatosensory-evoked potential monitoring during 100 cervical operations. Spine. 1993;18:737–47. doi: 10.1097/00007632-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Taunt CJ, Jr, Sidhu KS, Andrew SA. Somatosensory evoked potential monitoring during anterior cervical discectomy and fusion. Spine. 2005;30:1970–2. doi: 10.1097/01.brs.0000176321.02963.72. [DOI] [PubMed] [Google Scholar]
- 12.Traynelis VC, Abode-Iyamah KO, Leick KM, et al. Cervical decompression and reconstruction without intraoperative neurophysiological monitoring. J Neurosurg Spine. 2012;16:107–13. doi: 10.3171/2011.10.SPINE11199. [DOI] [PubMed] [Google Scholar]
- 13.Wright JG, Einhorn TA, Heckman JD. Grades of recommendation. J Bone Joint Surg Am. 2005;87:1909–10. doi: 10.2106/JBJS.8709.edit. [DOI] [PubMed] [Google Scholar]
- 14.Bose B, Sestokas AK, Schwartz DM. Neurophysiological monitoring of spinal cord function during instrumented anterior cervical fusion. Spine. 2004;4:202–7. doi: 10.1016/j.spinee.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard JA, Bohlman HH, Biro C. Intraoperative improvements of somatosensory evoked potentials: correlation to clinical outcome in surgery for cervical spondylitic myelopathy. Spine (Phila Pa 1976) 1996;21:589–94. doi: 10.1097/00007632-199603010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gokaslan ZL, Samudrala S, Deletis V, et al. Intraoperative monitoring of spinal cord function using motor evoked potentials via transcutaneous epidural electrode during anterior cervical spinal surgery. J Spinal Disord. 1997;10:299–303. [PubMed] [Google Scholar]
- 17.Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916–22. doi: 10.1097/01.brs.0000228724.01795.a2. [DOI] [PubMed] [Google Scholar]
- 18.Sebastian C, Raya JP, Ortega M, et al. Intraoperative control by somatosensory evoked potentials in the treatment of cervical myeloradiculopathy. Results in 210 cases. Eur Spine J. 1997;6:316–23. doi: 10.1007/BF01142677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PN, Balzer JR, Khan MH, et al. Intraoperative somatosensory evoked potential monitoring during anterior cervical discectomy and fusion in nonmyelopathic patients—a review of 1,039 cases. Spine J. 2007;7:83–7. doi: 10.1016/j.spinee.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Xu R, Ritzl EK, Sait M, et al. A role for motor and somatosensory evoked potentials during anterior cervical discectomy and fusion for patients without myelopathy: Analysis of 57 consecutive cases. Surg Neurol Int. 2011;2:133. doi: 10.4103/2152-7806.85606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole T, Veeravagu A, Zhang M, et al. Intraoperative neuromonitoring in single-level spinal procedures: a retrospective propensity score-matched analysis in a national longitudinal database. Spine. 2014;39:1950–9. doi: 10.1097/BRS.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 22.Khan MH, Smith PN, Balzer JR, et al. Intraoperative somatosensory evoked potential monitoring during cervical spine corpectomy surgery: experience with 508 cases. Spine (Phila Pa 1976) 2006;31:E105–13. doi: 10.1097/01.brs.0000200163.71909.1f. [DOI] [PubMed] [Google Scholar]
- 23.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 24.Furlan AD, Pennick V, Bombardier C, et al. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. 2009;34:1929–41. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 25.Nellensteijn J, Ostelo R, Bartels R, et al. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J. 2010;19:181–204. doi: 10.1007/s00586-009-1155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLong WB, Polissar N, Neradilek B. Timing of surgery in cauda equina syndrome with urinary retention: meta-analysis of observational studies. J Neurosurg Spine. 2008;8:305–20. doi: 10.3171/SPI/2008/8/4/305. [DOI] [PubMed] [Google Scholar]
- 27.Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973;93:173–8. doi: 10.1097/00003086-197306000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Magit DP, Hilibrand AS, Kirk J, et al. Questionnaire study of neuromonitoring availability and usage for spine surgery. J Spinal Disord Tech V. 2007;20:282–9. doi: 10.1097/01.bsd.0000211286.98895.ea. [DOI] [PubMed] [Google Scholar]
- 29.Dinner DS, Luders H, Lesser RP, et al. Intraoperative spinal somatosensory evoked potential monitoring. J Neurosurg. 1986;65:807–14. doi: 10.3171/jns.1986.65.6.0807. [DOI] [PubMed] [Google Scholar]
- 30.Bieber E, Tolo V, Uematsu S. Spinal cord monitoring during posterior spinal instrumentation and fusion. Clin Orthop Relat Res. 1988;229:121–4. [PubMed] [Google Scholar]
- 31.Jones SJ, Edgar MA, Ransford AO, et al. A system for the electrophysiological monitoring of the spinal cord during operations for scoliosis. J Bone Joint Surg Br. 1983;65:134–9. doi: 10.1302/0301-620X.65B2.6826615. [DOI] [PubMed] [Google Scholar]
- 32.Brown RH, Nash CL, Jr, Berilla JA, et al. Cortical evoked potential monitoring. A system for intraoperative monitoring of spinal cord function. Spine. 1984;9:256–61. [PubMed] [Google Scholar]
- 33.Resnick DK, Anderson PA, Kaiser MG, et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J Neurosurg Spine. 2009;11:245–52. doi: 10.3171/2009.2.SPINE08730. [DOI] [PubMed] [Google Scholar]
- 34.Harel R, Knoller N, Regev G, et al. The value of neuromonitoring in cervical spine surgery. Surg Neurol Int. 2014;5:120. doi: 10.4103/2152-7806.138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn TB. Neurologic complications of anterior cervical interbody fusion. Spine. 1982;7:536–9. doi: 10.1097/00007632-198211000-00004. [DOI] [PubMed] [Google Scholar]
- 36.James WS, Rughani AI, Dumont TM. A socioeconomic analysis of intraoperative neurophysiological monitoring during spine surgery: national use, regional variation, and patient outcomes. Neurosurg Focus. 2014;37:E10. doi: 10.3171/2014.8.FOCUS14449. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Gorji R, Allott G, et al. The usefulness of intraoperative neurophysiological monitoring in cervical spine surgery: a retrospective analysis of 200 consecutive patients. J Neurosurg Anesthesiol. 2012;24:185–90. doi: 10.1097/ANA.0b013e318255ec8f. [DOI] [PubMed] [Google Scholar]
- 38.Martins DE, Astur N, Kanas M, et al. Quality assessment of systematic reviews for surgical treatment of low back pain: an overview. The spine journal: official journal of the North American Spine Society. 2016;16:667–75. doi: 10.1016/j.spinee.2016.01.185. [DOI] [PubMed] [Google Scholar]