Abstract
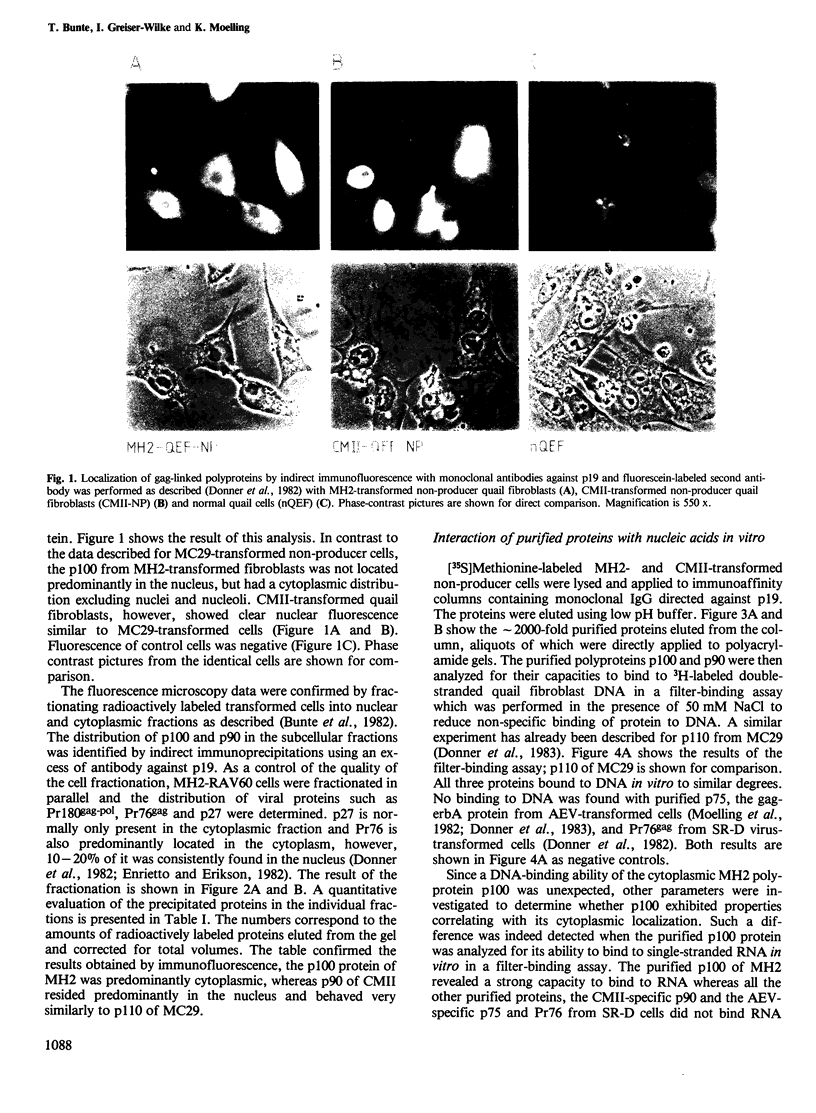
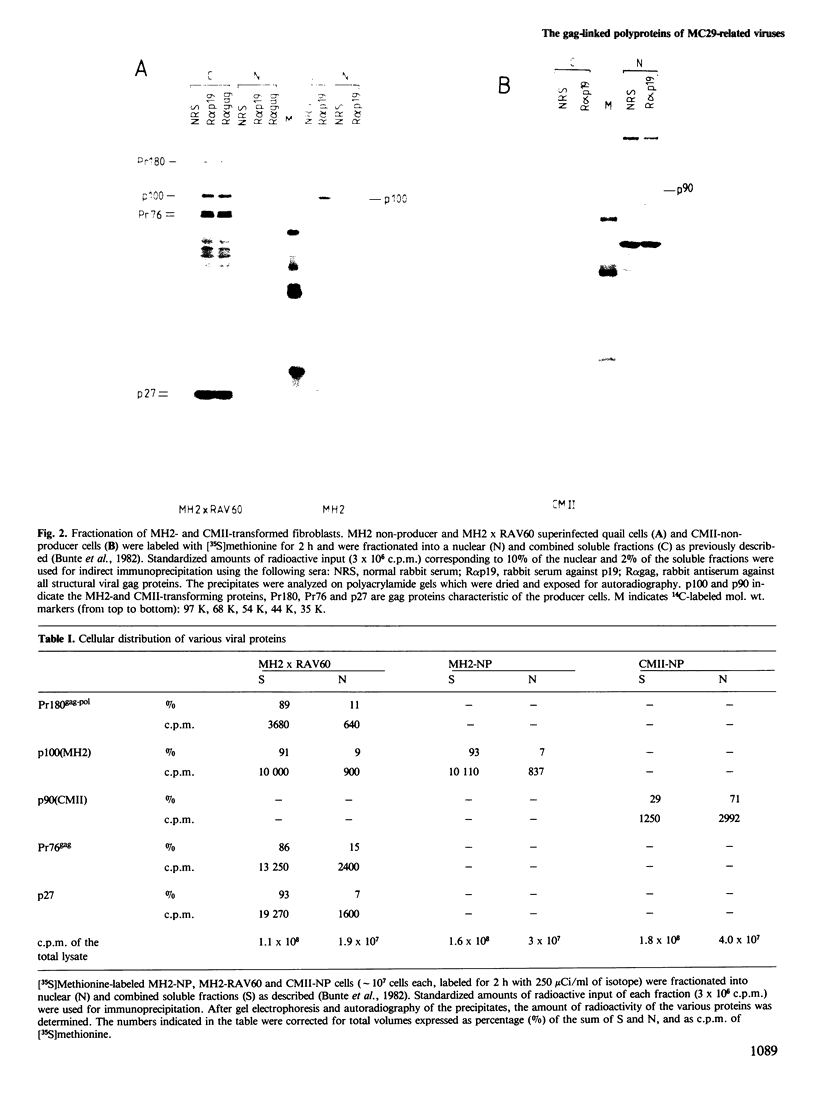
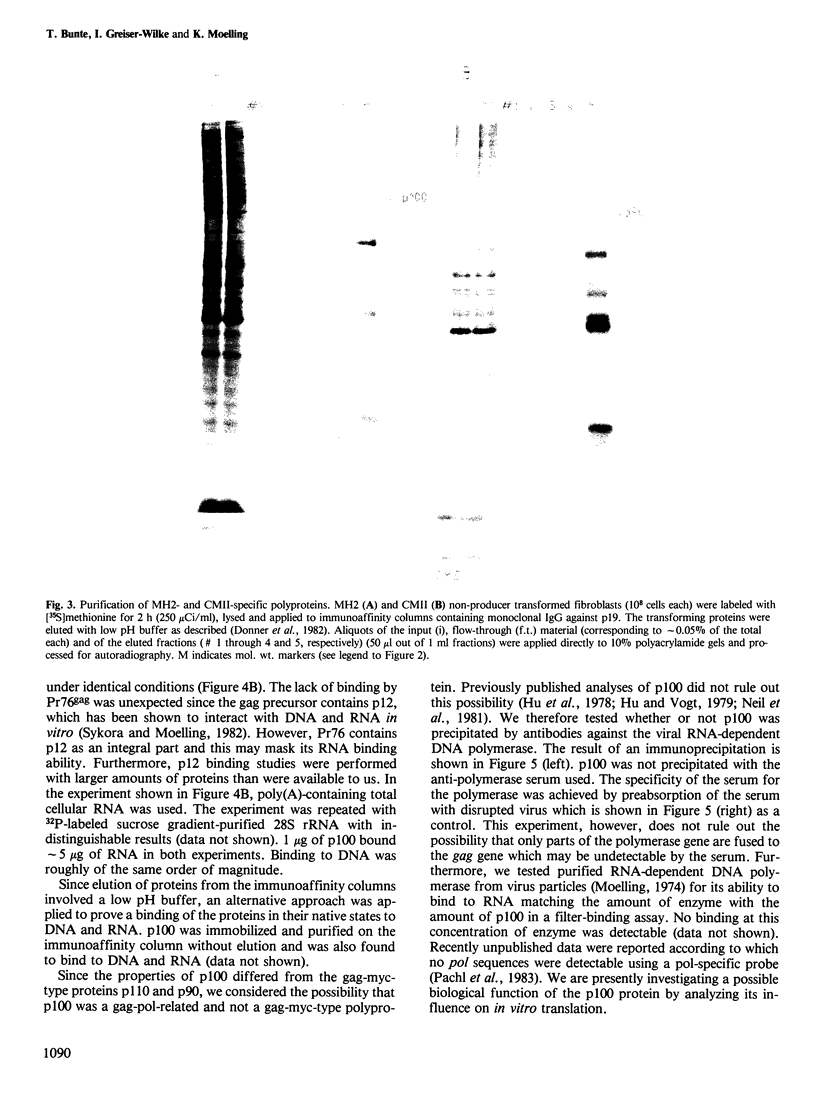
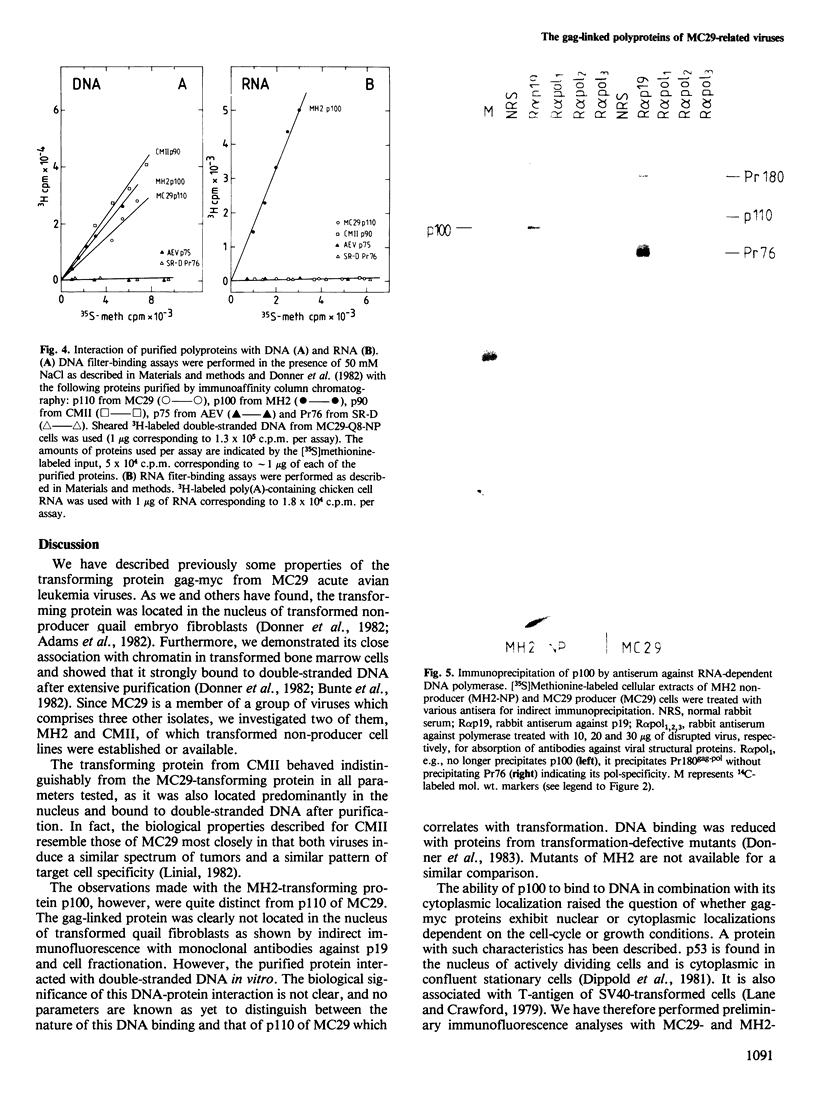
The acute avian leukemia viruses MH2 and CMII belong to the group of avian myelocytomatosis viruses, the prototype virus of which is MC29. This group of viruses is characterized by myc-specific oncogenes which are presumably expressed as gag-myc polyproteins. These polyproteins are synthesized in non-producer cells transformed by MH2 and CMII and have mol. wts. of 100 000 (p100) and 90 000 (p90), respectively. Monoclonal antibodies against the N terminus of gag, p19, were used to localize the protein in MH2- and CMII-transformed non-producer fibroblasts. Immunofluorescence and cell fractionation indicated that greater than 90% of p100 from MH2 was located in the cytoplasm, whereas greater than 70% of p90 from CMII resided in the nucleus. Isolation of p100 and p90 by immunoaffinity chromatography resulted in an approximately 2000-fold purification of the two polyproteins. Both of them, as well as p110 of MC29, bound to double-stranded DNA of chick fibroblasts in vitro. However, only the MH2-specific polyprotein p100 bound to RNA in vitro. Such a binding was not observed for p90 or p110, or for the purified gag precursor Pr76. Another polyprotein, gag-erbA, from avian erythroblastosis virus, which is also located in the cytoplasm, did not bind to RNA. Our results indicate that the CMII-specific polyprotein p90 behaved indistinguishably from the p110 of MC29. However, the MH2-specific polyprotein p100 exhibited unique and novel properties which were distinct from a gag-myc-type protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bunte T., Greiser-Wilke I., Donner P., Moelling K. Association of gag-myc proteins from avian myelocytomatosis virus wild-type and mutants with chromatin. EMBO J. 1982;1(8):919–927. doi: 10.1002/j.1460-2075.1982.tb01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W. G., Jay G., DeLeo A. B., Khoury G., Old L. J. p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1695–1699. doi: 10.1073/pnas.78.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrietto P. J., Erikson R. L. Localization of viral structural proteins in the cytoplasm and nucleus of Rous-associated virus-2-infected chicken embryo fibroblasts. J Virol. 1982 Nov;44(2):658–665. doi: 10.1128/jvi.44.2.658-665.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Stéhelin D. Avian leukemia viruses. Oncogenes and genome structure. Biochim Biophys Acta. 1982 Jun 28;651(4):245–271. doi: 10.1016/0304-419x(82)90014-2. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Owada K. M., Moelling K. Isolation of monoclonal antibodies against avian oncornaviral protein p19. J Virol. 1981 Jul;39(1):325–329. doi: 10.1128/jvi.39.1.325-329.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J. Transforming proteins of avian retroviruses. J Gen Virol. 1981 Jan;52(Pt 1):1–14. doi: 10.1099/0022-1317-52-1-1. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Vogt P. K. Avian oncovirus MH2 is defective in Gag, Pol, and Env. Virology. 1979 Jan 30;92(2):278–284. doi: 10.1016/0042-6822(79)90132-6. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linial M. Two retroviruses with similar transforming genes exhibit differences in transforming potential. Virology. 1982 Jun;119(2):382–391. doi: 10.1016/0042-6822(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Moelling K. Characterization of reverse transcriptase and RNase H from friend-murine leukemia virus. Virology. 1974 Nov;62(1):46–59. doi: 10.1016/0042-6822(74)90302-x. [DOI] [PubMed] [Google Scholar]
- Moelling K., Owada M. K., Greiser-Wilke I., Bunte T., Donner P. Biochemical characterization of transformation-specific proteins of acute avian leukemia and sarcoma viruses. J Cell Biochem. 1982;20(1):63–69. doi: 10.1002/jcb.240200107. [DOI] [PubMed] [Google Scholar]
- Pachl C., Biegalke B., Linial M. RNA and protein encoded by MH2 virus: evidence for subgenomic expression of v-myc. J Virol. 1983 Jan;45(1):133–139. doi: 10.1128/jvi.45.1.133-139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J., Bister K. Phosphorylation of specific sites in the gag-myc polyproteins encoded by MC29-type viruses correlates with their transforming ability. EMBO J. 1982;1(9):1111–1116. doi: 10.1002/j.1460-2075.1982.tb01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Saule S., Sergeant A., Torpier G., Raes M. B., Pfeifer S., Stehelin D. Subgenomic mRNA in OK10 defective leukemia virus-transformed cells. J Virol. 1982 Apr;42(1):71–82. doi: 10.1128/jvi.42.1.71-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Bister K., Moscovici C., Fanshier L., Gonda T., Bishop J. M. Avian retroviruses that cause carcinoma and leukemia: identification of nucleotide sequences associated with pathogenicity. J Virol. 1980 Mar;33(3):962–968. doi: 10.1128/jvi.33.3.962-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora K. W., Moelling K. Properties of the avian viral protein p12. J Gen Virol. 1981 Aug;55(Pt 2):379–391. doi: 10.1099/0022-1317-55-2-379. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]