Abstract
The present study examined the efficacy of 5 MHz low-intensity focused ultrasound (LiFU) as a stimulus to remotely activate inner ear vestibular otolith organs. The otolith organs are the primary sensory apparati responsible for detecting orientation of the head relative to gravity and linear acceleration in three-dimensional space. These organs also respond to loud sounds and vibration of the temporal bone. The oyster toadfish, Opsanus tau, was used to facilitate unobstructed acoustic access to the otolith organs in vivo. Single-unit responses to amplitude-modulated LiFU were recorded in afferent neurons identified as innervating the utricle or the saccule. Neural responses were equivalent to direct mechanical stimulation, and arose from the nonlinear acoustic radiation force acting on the otolithic mass. The magnitude of the acoustic radiation force acting on the otolith was measured ex vivo. Results demonstrate that LiFU stimuli can be tuned to mimic directional forces occurring naturally during physiological movements of the head, loud air conducted sound, or bone conducted vibration.
I. INTRODUCTION
The inner ear vestibular otolith organs are inertial sensors responsible for detecting gravity and linear acceleration in three-dimensional space. In mammals, the otolithic utriculus and sacculus provide neural inputs essential to the vestibulo-ocular, -collic, and -sympathetic reflexes (Wilson et al., 1995; Goldberg et al., 2012; Cohen et al., 2013; Yates et al., 2014), and inputs essential for neural representation of self movement/position in space (Carter and Ray, 2008; Fortenberry et al., 2012; Jacob et al., 2014; Rancz et al., 2015). Otolith organs also respond to bone conducted vibration (BCV) and air conducted sound (ACS) (Curthoys and Vulovic, 2011), and serve as the primary auditory organs in extant species of fish (Popper and Fay, 1993; Harada et al., 2001; Weeg et al., 2002). The name otolith has Greek origins in ōto meaning ear and lithos meaning stone. In mammals, the lithos is comprised of dense calcium carbonate-rich otoconia, which are bound together and coupled to sensory hair bundles through a gelatinous otoconial membrane (Lins et al., 2000; Thalmann et al., 2001; Andrade et al., 2012). Acceleration of the head generates a gravito-inertial force on the composite otoconial mass, deflecting hair bundles relative to the cell body in the direction opposite the acceleration, and ultimately leading to mechano-electrical-transduction (MET) by hair cells and transmission of the sensory information to the brain (Grant and Best, 1987; Rabbitt et al., 2003; Davis et al., 2007; Goldberg et al., 2012). Each individual otolith afferent neuron responds preferentially to a specific direction and frequency, encoding the signal through modulation of action potential firing rate and timing (Fernandez et al., 1972; Jamali et al., 2009; Curthoys and Vulovic, 2011). Disorders afflicting the vestibular otolith organs can have a major debilitating impact on daily activities and the quality of life (Furman et al., 2010; Gioacchini et al., 2014; Kaga, 2014; Bisdorff et al., 2015; Plishka, 2015). It is therefore important for both clinical testing and basic science to have a controlled stimulus that can selectively activate individual otolith organs in prescribed spatial directions and temporal waveforms. The present report introduces low-intensity focused ultrasound (LiFU) for this purpose.
Available methods used to stimulate vestibular otolith organs can be divided grossly into high-frequency and low-frequency regimes. In the high-frequency regime, pulsed packets (∼1–10 ms) of auditory frequency air conducted sound or bone conducted vibration are used to evoke compensatory otolith-driven motor outputs. Vestibular evoked myogenic potentials (VEMPs) are measured clinically in the cervical sternocleidomastoid muscles (cVEMP) and/or extraocular muscles (oVEMP) in response the high-frequency stimulus (Curthoys et al., 2014; Curthoys and Grant, 2015). VEMPs reflect function of the otolith organs as well as the neural circuits responsible for the muscle activation (Naranjo et al., 2016). The current understanding is that cervical cVEMPs arise primarily from activation of saccular afferent fibers through ipsilateral motor inputs, while ocular oVEMPs arise primarily from activation of utricular fibers (Kushiro et al., 1999; Kushiro et al., 2000; Newlands et al., 2003; Curthoys et al., 2011; Rosengren and Colebatch, 2011; Oh et al., 2016). Short latency vestibular stimulus-evoked potentials also occur in response to ACS or BCV, and contribute to the overall auditory brain stem response (ABR) for high ACS stimulus strengths (Todd et al., 2014a; Todd et al., 2014b). Both bone conducted vibration and air conducted sound vibrate hair bundles, leading to auditory frequency modulation of MET currents and triggering of action potentials in a subset of high-frequency sensitive utricular and saccular afferent neurons (McCue and Guinan, 1994, 1995; Curthoys et al., 2012; Zhu et al., 2014). Curthoys et al. describe high-frequency sensitive otolith afferent neurons as driving the “transient” system (Curthoys et al., 2017), and are denoted as “transient-type” in the present report (Curthoys et al., 2017). Transient-type afferent neurons have irregularly spaced inter-spike intervals in mammals. Sensitive transient-type neurons are capable of firing action potentials locked to a precise phase of the stimulus at frequencies exceeding 2 kHz (Fay et al., 1994; Curthoys et al., 2012). Transient-type afferent neurons can also be stimulated in animals using repeated high-intensity accelerations (Elidan et al., 1987; Jones and Jones, 1999; Honaker et al., 2015). When stimulated by tonic maintained (DC) stimuli, transient-type neurons adapt firing rates over time and therefore are not suited to encode tonic low-frequency signals such as changes in head orientation relative to gravity (Baird and Lewis, 1986; Goldberg et al., 1990; Si et al., 1997; Newlands et al., 2003). Otolith afferent neurons sensitive to tonic, low-frequency stimuli have regularly spaced inter-spike intervals in mammals, and are described by Curthoys et al. as driving the “sustained” or static system (Curthoys et al., 2017), and are termed “sustained-type” in the present report (Curthoys et al., 2017). Auditory-frequency ACS, BCV, or acceleration stimuli preferentially activate transient-type afferent neurons with little to no activation of sustained-type neurons (Halmagyi et al., 2005; Curthoys et al., 2012; Curthoys et al., 2014) and therefore do not activate the critical compensatory circuits they drive (Goldberg et al., 1990; Minor and Goldberg, 1991; Yates, 1998; Minor et al., 1999; Goldberg, 2000; Carter and Ray, 2008).
Methods currently used to activate sustained-type otolith afferent neurons include changing orientation of the head relative to gravity (tilt table), applying translational acceleration (linear sled), or altering gravito-inertial acceleration (centrifugation or space flight) (Curthoys et al., 1998; Cohen et al., 1999; Clément et al., 2001; Ramat et al., 2001). These methods require moving the animal or patient, which presents technical/cost challenges, activates organs bilaterally, and limits animal experiments involving cellular electrophysiology or optical imaging. The only methods currently available to controllably stimulate individual vestibular organs at low frequency without physical movement require micro-surgical access to the labyrinth. Electrical, mechanical, and infrared stimulation techniques have been developed, but these methods are invasive and are not well suited for controlled directional stimulation of otolith organs (Cohen et al., 1964; O'Leary, 1970; Schor and Miller, 1982; Dickman et al., 1988; Rabbitt et al., 1995; Rajguru et al., 2011; Rabbitt et al., 2016). Further complicating these studies is the fact that the population of otolith afferents does not divide into two distinct sustained-type and transient-type groups, but instead forms a continuum of sensitivities ranging from the low-gain regularly discharging sustained-type afferent neurons that are sensitive only to low-frequency stimulation, to the irregularly discharging transient-type afferent neurons that precisely lock action potential timing to high-frequency sound and vibration. This diversity further compels development of a stimulus capable of delivering precise forces to the otolith organs with controlled temporal and directional properties.
Here, we introduce low intensity focused ultrasound (LiFU), as a vestibular otolith stimulus capable of preferentially modulating sustained-type or transient-type afferent neurons. Results demonstrate that focused ultrasound generates a radiation force on the otolithic mass in the direction of the incident ultrasound wave, and that the temporal waveform can be tuned to mimic physiological gravito-inertial acceleration and/or vibrational stimuli.
II. METHODS
All animal procedures were approved by the University of Utah Institutional Animal Care and Use Committee and comply with policies and regulations of the Journal of the Acoustical Society of America. Oyster toadfish, Opsanus tau, of either sex, ∼500 g, were used as an established animal model of the vestibular system to facilitate ultrasound stimulation of the utricle and saccule in vivo (Boyle and Highstein, 1990; Rabbitt et al., 1995; Highstein et al., 1996). Fish were provided by Marine Biological Laboratory (Woods Hole, MA) with eleven fish contributing useful data. Previously described methods (Boyle and Highstein, 1990; Boyle et al., 1991; Rabbitt et al., 1995; Highstein et al., 1996; Holstein et al., 2004) were used for preparation, and for extracellular and intracellular single-unit neural recording. Fish were immersed in oxygenated seawater containing MS222 (3-aminobenzoic acid ethyl ester, Sigma, 25 g/L) and partially immobilized by an injection of pancuronium bromide (Sigma, 0.05 mg/kg) in the tail muscle. Each fish was secured in a plastic tank with their dorsal surface covered by moist tissues. A (2 cm dia.) dorsal craniotomy was performed to give direct acoustic access to the utricle, saccule, semicircular canals and their respective nerve branches. A 10 cm polystyrene culture dish (VWR, 10861-594) with a circular hole cut in the bottom was sealed (Locktite, 1710908) onto the skin around the craniotomy. The culture dish was filled with fluorocarbon (3 M, FC-880) for acoustic coupling to the LiFU transducer face and transmission of ultrasound waves to perilymph (Marsh et al., 2002).
A 5 MHz spherically focused ultrasound transducer (Olympus, C309-SU P) was driven by a power amplifier (EIN, 240 L RF) and amplitude-modulated (Textronix, AFG320) to deliver sinusoidal continuous-wave LiFU (cwLiFU) [Fig. 1(A)], or triggered in pulses to deliver short packets of constant-amplitude LiFU (pLiFU) [Fig. 1(B)]. Continuous 5 MHz ultrasound waves were amplitude modulated at 0.1–100 Hz, and packets were delivered at 5–100 pps (pulse width 1–10 ms). The driving voltage was adjusted to generate radiation forces equivalent to ∼0–0.4 g (g = acceleration of gravity) on the otolithic mass (measured using excised liths ex vivo). The transducer was positioned using a manual micromanipulator 2.5 cm above the target organ to focus LiFU dorsal to ventral (-z direction) as illustrated in Fig. 1(C). The focus diameter at the otolith organ target was 0.6 mm and the depth of focus was 8.4 mm.
FIG. 1.
LiFU envelope waveforms for pulsed [(B) pLiFU, pulses-s−1 pps, pulse width (PW)] and amplitude-modulated continuous-wave ultrasound [(A) cwLiFU, period T]. Insets show individual cycles of 5 MHz ultrasound with amplitude determined by the envelope. The acoustic radiation force () is proportional to the acoustic intensity, and the rate-of-change in force is the time derivative (). (C) Schematic of the toadfish vestibular labyrinth showing the horizontal canal (HC), anterior canal (AC), posterior canal (PC), utriculus (U), sacculus (S), and brain (BR). [Adapted from Popper and Fay (Popper and Fay, 1993) and Ghanem et al. (Ghanem et al., 1998).] Ultrasound (US) was applied from dorsal to ventral (-z) using a spherically focused probe 16 mm in diameter immersed in fluid and located ∼25 mm above the target organ.
In addition to LiFU stimulation, a polished glass pipette fixed to a piezoelectric actuator was used to apply controlled mechanical stimulation to the otolith (Rabbitt et al., 1995). The tip of the pipette was positioned on the membranous sac of the saccule and preloaded to drive displacement of the otolith tangent to the sensory epithelium using piezoelectric actuation. Afferent nerve responses to mechanical stimulation were used to definitively identify the organ innervated by the afferent and to characterize response characteristics to direct mechanical displacement including afferent gain and frequency sensitivity.
In a subset of experiments, mechanical stimulation and LiFU were applied simultaneously to characterize interaction between the two stimuli. Vestibular organs were excised after the experiment, and fixed in 2% paraformaldehyde for subsequent experiments measuring acoustic radiation force. For force measurements, tissue was suspended by a force calibrated elastic wire and exposed to cwLiFU. The net acoustic radiation force acting on the tissue (or lith) was determined by measuring displacement of the elastic wire (Qimaging, EXi) and using the calibrated spring constant of the wire to extract force.
In separate experiments, the temperature rise caused by LiFU was measured using a calibrated micro-thermistor located at the focus of the LiFU beam in extracellular fluid media. The microthermistor was a fast response bifilar wire with tolerance of +/− 0.5 °C (QTMB, Quality Thermistor, Inc., Boise, ID).
Phase-locking of action potentials to the stimulus was quantified using the winding ratio and the vector strength. The winding ratio (j:k) quantifies the number of action potentials “k” evoked for “j” stimulus cycles (Lakshmanan and Murali, 1996). For example, the most sensitive afferents respond with a single action potential for each LiFU pulse giving winding ratio 1:1, or k = 1, j = 1. Vector strength was calculated to quantify the precision of phase locking using , where is the phase angle between the spike and the stimulus pulse onset and is the number of spikes in the interval. The vector strength approaches 0 if the timing of action potentials is random relative to the stimulus, and approaches 1 if the timing of the action potential occurs with exactly the same phase (latency) for each stimulus presentation. The firing rate (Spk-s-1) was computed for each neuron using the inverse of the inter-spike-interval between adjacent action potentials. The mean firing rate and standard deviation were computed for each afferent during spontaneous activity to quantify regularity. Results from individual afferents were combined to define the average coefficient of variation for a group of neurons using , where the ratio was set to zero for units silent at rest.
III. RESULTS
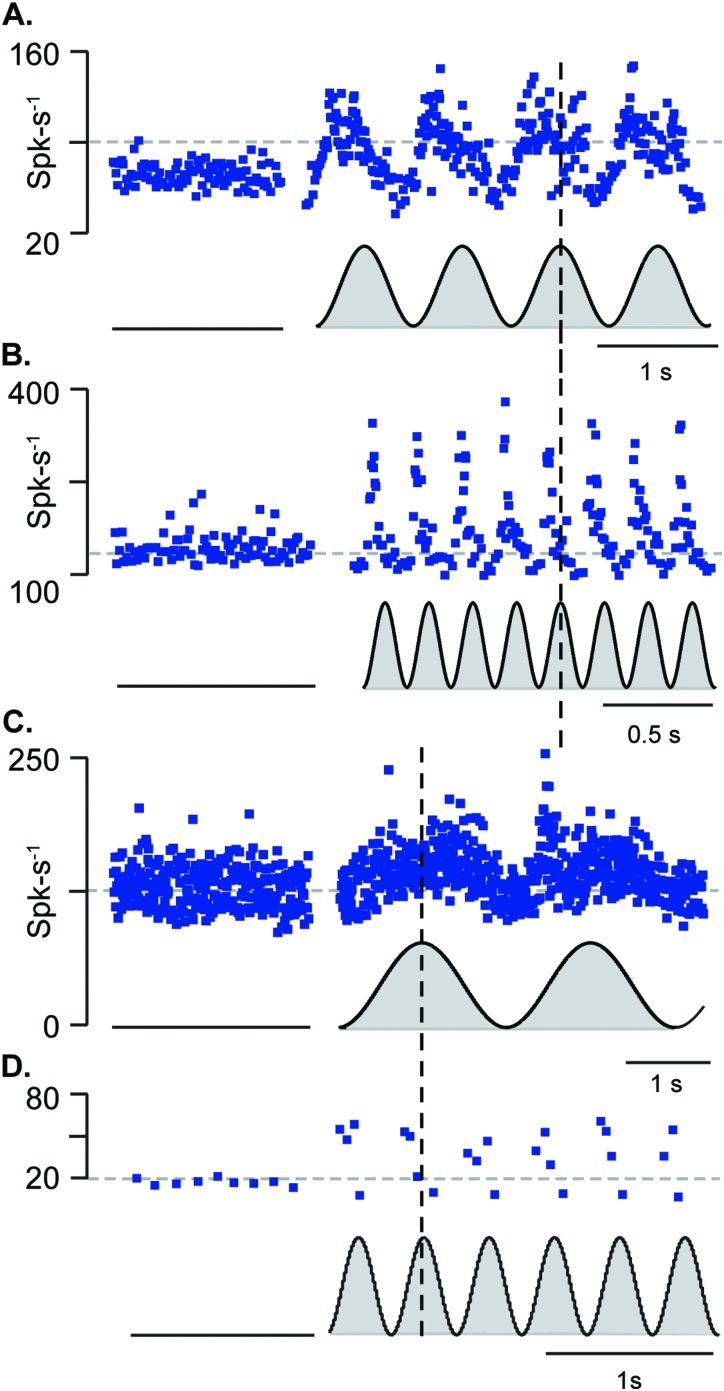
Low frequency sinusoidally modulated LiFU [cwLiFU, Fig. 1(A)] directed at the utriculus or sacculus evoked action potential firing rate changes in much the same way as sinusoidal linear acceleration reported previously (Boyle et al., 2001; Goldberg et al., 2012). Figure 2 shows utricular afferent responses when the ultrasound amplitude was slowly modulated to generate envelopes ranging from zero to peak (black, gray filled to zero) for stimuli at 1, 5, 0.3, and 3 Hz [Figs. 2(A)–2(D), respectively]. Neurons in Figs. 2(A), 2(B) responded with peak firing rate ∼90° phase advanced relative to the peak LiFU stimulus. In contrast, units shown in Figs. 2(C), 2(D) responded in phase and increased action potential firing rate roughly in proportion to the LiFU stimulus envelope. The acoustic radiation force generated by ultrasound is known to be proportional to the incident wave acoustic intensity, or incident pressure squared (Doinikov, 2003; Trahey et al., 2004; Sarvazyan et al., 2010) [also see Fig. 8(C)]. Unlike responses to low-frequency sinusoidal acceleration generated with mechanical stimulation, responses to cwLiFU (Fig. 2) were rectified and responded preferentially during one phase of the stimulus [a direct comparison of mechanical and cwLiFU is shown in Figs. 7(A), 7(B), and 7(D)]. Responses are consistent with activation by the acoustic radiation force, which only generates force in the positive direction of the incident ultrasound wave. Afferent neurons shown in Fig. 2 continuously responded to the envelope of the 5 MHz ultrasound (sustained-type responses), but did not strongly phase lock to the envelope of the ultrasound stimulus (n = 30). The maximum temperature rise caused by LiFU was <1 °C in the present experiments, ruling out temperature as responsible for afferent responses (Park et al., 2010; Rabbitt et al., 2016) and supporting direct mechanical activation by the ultrasound.
FIG. 2.
Sustained-type low frequency sensitive afferent neurons modulated firing rate in response to amplitude-modulated continuous-wave LiFU (∼0.4 equivalent “G” peak). (A) Example high-gain sustained-type utricular afferent discharge rate (ISI−1, blue dots, s−1) responding in phase with the LiFU acoustic radiation force and (B) a second unit responding with a ∼90° phase advance relative to the stimulus. (C) Low-gain sustained-type utricular afferent responding in phase with force and (D) saccular afferent responding in phase with the stimulus.
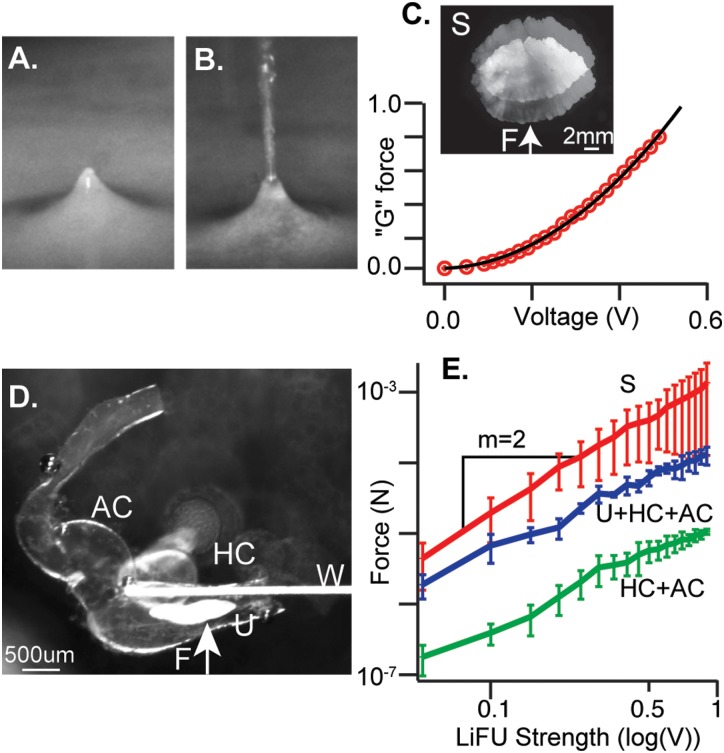
FIG. 8.
(Color online) LiFU delivers controlled force to the otolithic mass. (A) 5 MHz LiFU transducer immersed in water and focused on the air-water interface generates a radiation force on the surface. (B) Higher power generates ∼1 mm diameter streaming fountain. (C) Saccular otoliths were excised, suspended using a calibrated elastic wire, and exposed to continuous wave LiFU. Force was measured as a function of stimulus voltage. Force increased as the square of the voltage and was converted to “G” using the otolith mass and acceleration of gravity (). (D) cwLiFU was applied to a portion of the excised labyrinth containing the horizontal canal ampulla (HC), anterior canal ampulla (AC), and utricular macula (U). (E) Force was measured with a calibrated elastic wire (W) on isolated saccular otolith (S), U+HC+AC portion of the membranous labyrinth, and on the HC+AC portion of the labyrinth after removal of the utricular otolith. Force was greatly reduced after removal of the utricular otolith, demonstrating that LiFU generates force primarily by reflection off the otolithic mass, and not by absorption.
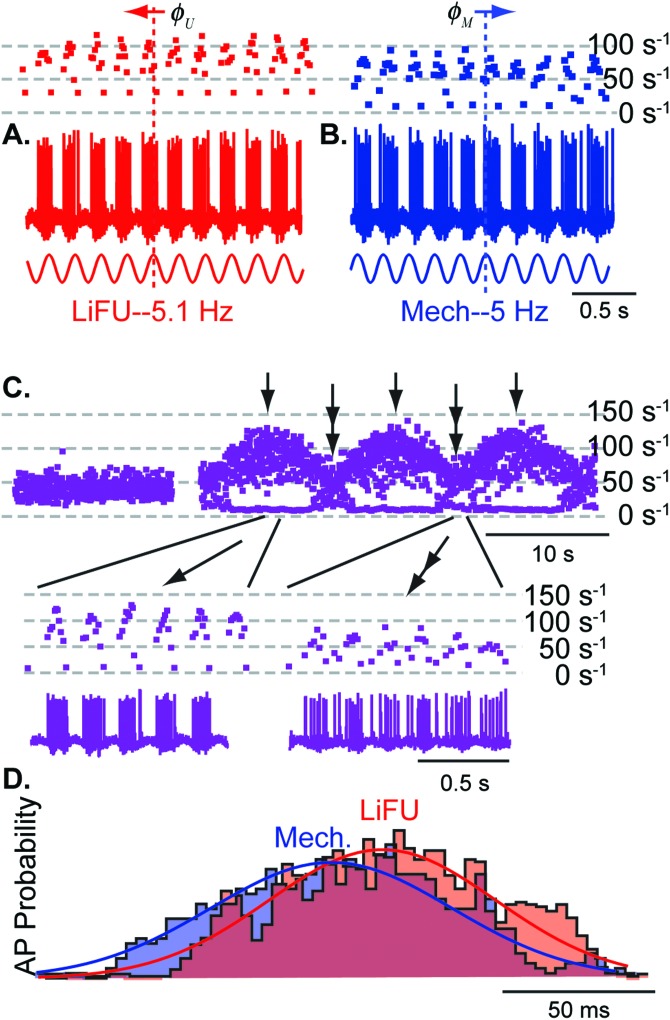
FIG. 7.
Continuous-wave LiFU modulates action potential discharge rate and mimics low-frequency mechanical stimulation. Representative sustained-type saccular afferent neuron showing continuous modulation of action potential discharge rate in response to (A) continuous wave LiFU at 5.1 Hz (ISI−1, red dots), (B) sinusoidal mechanical displacement at 5 Hz (ISI−1, blue dots), and (C) combined stimuli with responses beating at the difference frequency of 0.1 Hz (ISI−1, purple dots). The LiFU stimulus was stronger than the mechanical stimulus [(A) vs (B)], so a residual remained during destructive interference. (D) Stimulus phase histograms showing probability of evoking action potentials in response to 5.1 Hz continuous 5 MHz LiFU stimulus (red) and 5 Hz sinusoidal mechanical stimulus (blue).
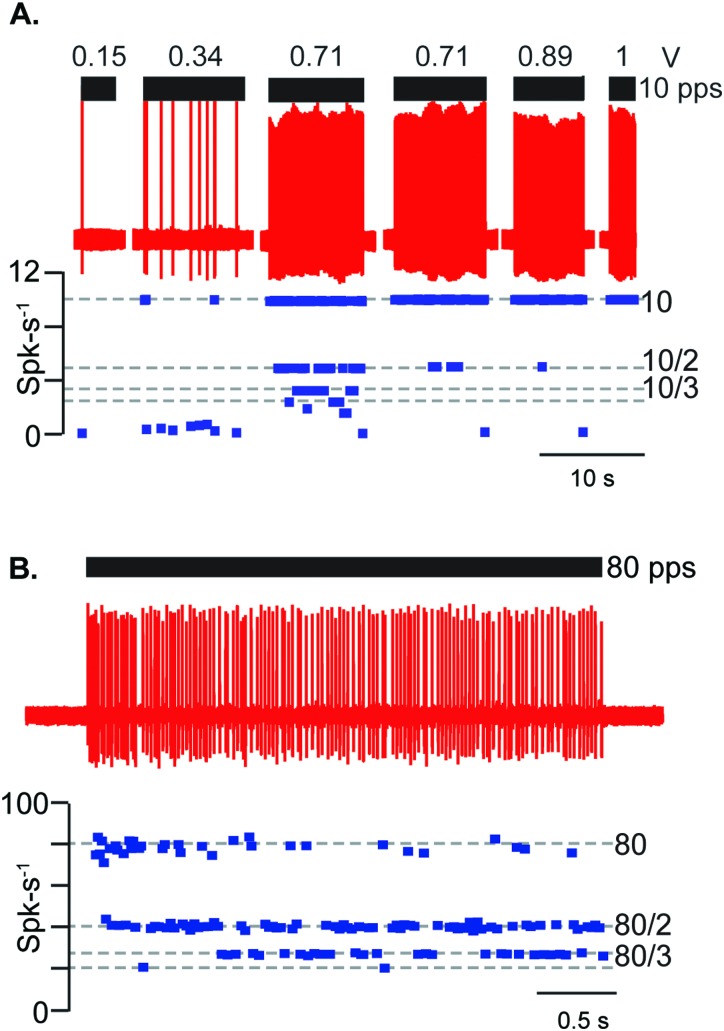
Transient-type otolith afferents responded to the rate-of-change of the 5 MHz LiFU envelope. To maximize the rate-of-change we used short packets of pulsed ultrasound, where the time derivative of the pLiFU envelope was a positive Dirac delta at the onset of each packet plus a negative Dirac delta at the termination of each packet [pLiFU, Fig. 1(B)]. Transient-type afferent neurons fired an action potential phase locked at a specific latency relative to each pLiFU pulse. Figure 3(A) shows a saccular afferent neuron responding to a pLiFU pulse train delivered at 10 pps as the voltage driving the transducer was increased from 0.15 to 1 V (see Fig. 8 for the radiation force corresponding to each voltage). Raw action potentials are shown (red) along with the instantaneous firing rate (spk-s−1). This saccular afferent phase-locked at winding ratios from k = 4 (one action potential every 4th pulse, dotted line at 2.5 Hz) for a 0.71 V driving voltage to k = 1 (one action potential for each pulse, dotted line at 10 Hz) for a 1 V driving voltage. The saccular afferent shown in Fig. 3(B) responded to 80 pps LiFU initially at a winding ratio k = 1, then adapted to firing at winding ratios of k = 2–3. In all afferents, action potentials were evoked by the LiFU amplitude envelope (the packet), and not by any individual ultrasound cycles within the packet (there were 2000–2500 cycles of 5 MHz ultrasound per packet). Of 100 afferents tested with pLiFU, 23 units were silent at rest. 77 units had an average background firing rate of 59.7 +/− 45.4 spk-s−1 and regularity of 21.7 +/− 28.8 spk-s−1. Across all units, the average coefficient of variation was 0.26 +/− 0.21 (see Sec. II). Although variance was high, on average afferent neurons with higher responded with higher vector strength. 68 afferent neurons had a of greater than 0.25 and responded with average vector strength of 0.82 +/− 0.24, while 32 had a less than 0.25 and responded with average vector strength of 0.56 +/− 0.36.
FIG. 3.
Transient-type auditory frequency sensitive saccular afferent neurons phase lock action potentials with packets of pulsed LiFU. (A) 1 ms packets pLiFU repeated at 10 pps evoked phase-locked action potential discharge with ISIs (blue dots) at winding ratios of k = 1–6 (dashed lines). Winding ratios approached 1 (one action potential per pulse) as the stimulus strength (reported as preamplifier voltage here 0.15–1 V) was increased. (B) Saccular afferent exhibiting adaptation to 1 ms packets of pLiFU at 80 pps with winding ratios initially at k = 1 and 2, running down during the stimulus to winding ratios of 2 and 3. Both of these saccular afferent neurons (A), (B) were silent at rest.
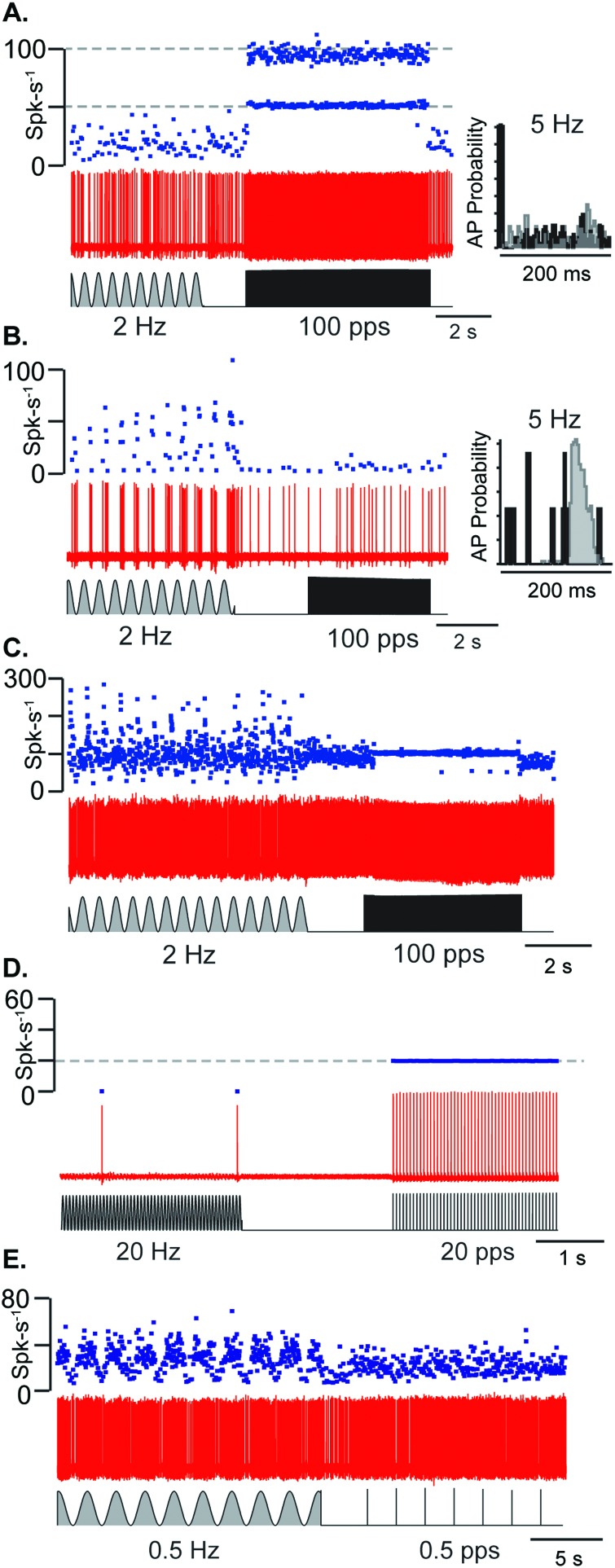
Transient-type otolith afferent neurons responded preferentially to auditory frequency pLiFU whereas sustained-type afferent neurons responded preferentially to low frequency cwLiFU. Figure 4 shows example transient-type and sustained-type neurons where the transient-type responded preferentially to pLiFU [Figs. 4(A) and 4(D)], the sustained-type responded preferentially to cwLiFU [Figs. 4(B) and 4(E)]. The distribution was a continuous and some neurons responded robustly to pulsed and continuous wave stimuli [Fig. 4(C)]. Transient-type afferents in Figs. 4(A) and 4(D) responded to pLiFU with vector strength of 0.95 to 100 pps and 0.99 to 20 pps, respectively. Sustained-type afferents in Figs. 4(B) and 4(E) respond to pLiFU at 100 pps with a vector strength of 0.23 and 0.5 pps with a vector strength of 0.01, respectively. The afferent sensitive to both pLiFU and cwLiFU [Fig. 4(C)] responded with vector strength of 0.94 for the 100 pps pLiFU. A stimulus triggered histogram of action potential times for the transient-type afferent in Fig. 4(A) (right, inset) shows spike timing was random during 2 Hz cwLiFU but phase-locked with high vector strength during pulsed pLiFU. In contrast, the stimulus triggered histogram for the sustained-type afferent in Fig. 4(B) shows spike timing was random during the pLiFU but modulated rate continuously with cwLiFU. Of 45 units tested with both pLiFU and cwLiFU, 20 units responded to only pLiFU (had no firing rate modulation to cwLiFU, units had a of 0.49 +/− 0.36), 7 units to only cwLiFU (vector strength to pLiFU was less than 0.3, units had a of 0.16 +/− 0.18), and 28 responded to both (units had a of 0.18 +/− 0.27).
FIG. 4.
Transient- and sustained-type afferent neurons have preferential responses to pLiFU and cwLiFU. (A), (D) Transient-type afferents respond preferentially and phase-lock to pLiFU at 100 and 20 pps, respectively. Stimulus triggered histogram of action potential times in (A) shows preference for pLiFU with most action potentials being fired within 10 ms of pulse onset and random firing to cwLiFU. (D) Unit was silent at rest. (B), (E) Sustained-type afferents modulating firing rate with high gain to cwLiFU at 2 and 0.5 Hz, respectively, and exhibiting no phase-locking to pLiFU. Stimulus triggered histogram of action potential times in (B) shows firing rate modulation with cwLiFU and random firing to pLiFU. (C) Afferent responds with high gain to both pLiFU at 100 pps and cwLiFU at 2 Hz.
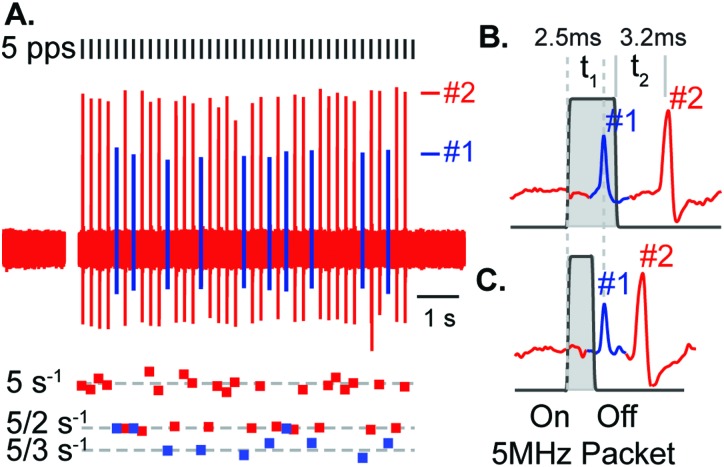
Transient-type afferents responded to the rate-of-change of acoustic radiation force, triggered by either the positive Dirac at the onset of the pLiFU packet or by the negative Dirac at the termination of the pLiFU packet [see Fig. 1(B), dF/dt]. Figure 5 shows two saccular afferents recorded simultaneously in response to a 5 pps pLiFU pulse train. For a stimulus packet width of 3 ms [Fig. 5(B)], unit #1 responded with a latency of 2.5 ms relative to the onset of the pLiFU packet, and unit #2 responded with a latency of 3.2 ms relative to the termination of the packet. The same latencies occurred using a pulse with of 1.5 ms, which could only occur if unit #1 responded to the onset of the pLiFU packet and unit #2 responded to the termination. This suggests both afferent neurons were excited by the acoustic radiation force, with unit #1 responding during ventrally directed force on the otolith (putatively because unit #1 innervated hair cells with ventral polarity) and unit #2 responding to dorsally directed force (putatively because unit #2 innervated hair cells with dorsal polarity).
FIG. 5.
Simultaneous recording of two saccular afferents in response to pLiFU at 5 pps. (A) Individual units were identified by spike sorting (#1 blue, #2 red) and fired action potentials at winding ratios k = 1–2 (#2) and k = 2–3 (#1). (B), (C) Changing LiFU packet PW revealed that action potentials in unit #1 were triggered by the onset of the LiFU packet (#1, 2.5 ms latency re: On), and that action potentials in unit #2 were triggered by termination of the LiFU packet (#2, 3.2 ms latency re: Off). Afferents were triggered by the rate-of-change in force or, in terms of gravito-inertial stimuli, the rate-of-change in acceleration (termed “jerk”). These jerk sensitive afferent neurons are analogous to the auditory-frequency sensitive irregularly discharging afferents in mammals (transient-type neurons).
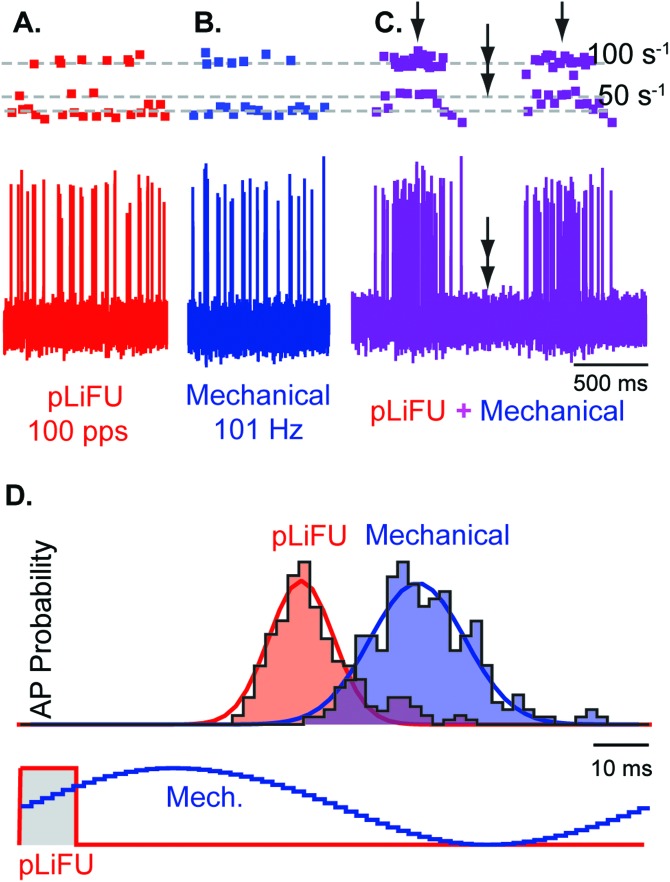
To examine if LiFU activated neurons by mechanical displacement of the otolith, we recorded responses while combining LiFU with direct piezoelectric driven mechanical stimulation. Figure 6 shows responses of a transient-type saccular afferent responding to pLiFU delivered at 100 pps [Fig. 6(A), red], phase locking action potentials [Fig. 6(A), red dots] to the pLiFU stimulus at winding ratios of k = 1, 2, and 3 (dashed lines). The same afferent neuron responded to direct 101 Hz sinusoidal mechanical vibration [Fig. 6(B)] of the saccular otolith with phase locked action potentials [Fig. 6(B), blue dots] at winding ratios of k = 1 and 3. When 100 pps LiFU and 101 Hz mechanical stimuli were applied at the same time, the two stimuli beat through each other at the difference frequency of 1 Hz, generating periods of constructive interference [Fig. 6(C), single arrows], and periods of destructive interference completely silencing the unit [Fig. 6(C), double arrows]. Superposition of LiFU and direct mechanical stimulation strongly suggest that responses were due to displacement of the otoconial mass, and rule out other biophysical mechanisms such as ultrasonic heating. The vector strength and timing of action potentials is illustrated in Fig. 6(D) in the form of phase histograms for repeated 10 ms pLiFU pulses at 100 pps [Fig. 6(D), red curves], and for repeated sinusoidal mechanical stimulation [Fig. 6(D), blue curves] at 101 Hz. The latency of action potential firing for this afferent relative to the peak mechanical displacement of the otolith was 4.2 ms with vector strength of 0.87. In response to pLiFU, the same afferent phase locked with a latency of 3.1 ms relative to the onset of the pLiFU transducer pulse with vector strength of 0.92. The increased vector strength for pLiFU relative to direct mechanical stimulation most likely reflects differences in the stimulus waveforms (cf. Fig. 7).
FIG. 6.
Pulsed LiFU evokes phase locked action potentials and mimics stimulation by direct mechanical vibration. Representative transient-type sensitive saccular afferent with phase locked responses at winding ratios k = 1–4 for (A) 1 ms LiFU packets at 100 pps, (B) sinusoidal piezoelectric mechanical stimulation at 101 Hz, and (C) combined stimuli with responses beating at the difference frequency of 1 Hz. Amplitudes of the two stimuli were adjusted to evoke similar responses when presented alone. Single arrows denote times of constructive interference when the stimuli add and double arrows show times of destructive interference when the stimuli cancel. (D) Stimulus phase histograms showing phase locking of action potentials in response 100 pps pulsed 5 MHz LiFU (red) and 101 Hz sinusoidal mechanical stimulation of the otolith (blue).
When comparing responses it is important to recognize that LiFU generates a direct force on the otoconial mass while the mechanical stimulus forced displacement of the saccular otolith by direct contact with the membranous sac overlying the edge of the macula. We adjusted the strength of the two stimuli to evoke nearly equivalent afferent response magnitudes, but we did not adjust the relative phase of the stimuli. A difference in the phase of otolith displacement would be expected for these diverse stimuli because of otolith mass, and differences in the viscous drag and mechanical coupling of the mechanical stimulus probe relative to the acoustic radiation force (Grant and Best, 1987; Rabbitt et al., 2003; Dunlap et al., 2012).
The equivalency between LiFU and mechanical stimulation illustrated in Fig. 6 for pulsed stimuli also held true for low frequency amplitude-modulated continuous-wave stimuli. Figure 7 shows responses of a sustained-type saccular afferent neuron to sinusoidal 5.1 Hz cwLiFU [Fig. 7(A)], and to 5 Hz sinusoidal mechanical stimulation [Fig. 7(B)]. This afferent did not strongly phase lock action potentials to the either stimulus but did modulate action potential discharge rate. The cwLiFU stimulus intensity used in this recording was stronger than the mechanical stimulus and evoked higher firing rate peaks [Fig. 7(A) vs 7(B)]. This afferent neuron responded with a phase advance () relative to cwLiFU and a phase lag () relative to direct mechanical stimulation, with a difference between the two of ∼180°. The otolith mass in the fish sacculus is very large (Popper and Fay, 1993), indicating the phase difference was primarily due to inertia of the otolith itself (Grant and Best, 1987; Rabbitt et al., 2003). When the two stimuli were presented together, the afferent neuron modulated firing rate at the beat frequency of 0.1 Hz [Fig. 7(C)]. Responses were maximized during periods of constructive interference between the stimuli [Fig. 7(C), single arrows] and minimized during periods of destructive interference [Fig. 7(C), double arrows] as expected from responses to individual stimuli [Figs. 7(A), 7(B)]. Phase histograms [Fig. 7(D)] revealed nearly identical action potential discharge patterns in response to cwLiFU (red) and mechanical stimulation (blue, shifted by 180° to facilitate comparison).
Focused ultrasound generates force in tissue proportional to the acoustic intensity in the direction of the incident wave (Doinikov, 2003; Trahey et al., 2004; Sarvazyan et al., 2010). The acoustic radiation force was visualized in the present study by immersing the transducer in media and focusing the beam on the air-media interface [Fig. 8(A)]. The LiFU beam deflected the media-air interface upward until the radiation force was balanced by gravity and surface tension. Increasing power generated sufficient localized force to break the surface tension, and ultimately generated a streaming fountain [Fig. 8(B)]. To quantify the magnitude of the acoustic radiation force acting on the utricular and saccular otoliths, we suspended excised tissue in media using a calibrated flexible tungsten wire and measured LiFU force directly using deflection of the wire. Figure 8(C) shows an excised saccular otolith suspended by the flexible wire, and imaged using a double exposure before and during exposure to cwLiFU. Deflection was measured using image registration (Thévenaz et al., 1998) and converted to force. The acoustic radiation force () grew with the square of the driving voltage [Fig. 8(C), data symbols, parabolic curve fit], as expected from acoustic theory, and was converted to equivalent acceleration in “” force by dividing by the mass of the saccular otolith times the acceleration of gravity (). Saccular otoliths in the toadfish are larger than the 1 mm LiFU focal spot of the transducer resulting in maximum acoustic radiation force. The absolute force on the saccular otolith is shown as a function of voltage on a log-log scale in Fig. 8(E) (red line, slope = 2). To measure force on the utricular otolith we suspended an excised section of the labyrinth in media [Fig. 8(D), w], and measured the acoustic radiation force before and after removal of the otolith. The tissue section included the utriculus (U), horizontal canal (HC), and the anterior canal (AC) ampullae [Fig. 8(D)]. The force acting on the full tissue sample is shown in Fig. 8(E) (blue line, U+HC+PC), and was less than the force on the saccular otolith because the focal spot was smaller than the saccular otolith but larger than the utricular otolith. Removing the utricular otolith from the tissue reduced the acoustic radiation force by an order of magnitude [Fig. 8(E), green line, HC+AC], demonstrating that ∼90% of the radiation force was due to reflection off the dense otolith, while the residual ∼10% was due to absorption of ultrasound by endolymph, perilymph, and soft tissue (Doinikov, 2003; Sarvazyan et al., 2010).
To test sensitivity of the semicircular canals to LiFU, the ultrasound beam was focused on the HC ampulla and responses of individual afferent neurons were recorded in the nerve branch exclusively contacting the horizontal canal crista (Boyle and Highstein, 1990). Mechanical stimulation of the canal duct was used to confirm normal sensitivity of the afferent prior to application of ultrasound stimuli (Rabbitt et al., 1995). In 19 canal afferent neurons tested using the same LiFU stimuli applied to otolith organs, 2 responded with modulated action potential ISIs but did not phase lock individual action potentials to LiFU pulses, while 10 phase locked with a vector strength of 0.69 +/− 0.26. These relatively modest responses are consistent with the reduced acoustic radiation force acting on the canals vs the otolith organs (Fig. 8).
IV. DISCUSSION
LiFU has been applied previously to stimulate various neural tissue using 0.25–5 MHz transducers (Gavrilov et al., 1976; Tyler et al., 2008; Tufail et al., 2010; Bystritsky et al., 2011). Although the mechanism(s) of action in the brain remain a topic of research, it appears LiFU stimulation does not damage tissue providing the temperature rise is controlled and intensity ≲3 W/m2 (Min et al., 2011; Yoo et al., 2011; Kim et al., 2014; Rezayat and Toostani, 2016). The present study used a similar approach to stimulate vestibular otolith organs while recording first order afferent responses. Results demonstrate LiFU responses of afferent neurons in the utriculus and sacculus arising from the acoustic radiation force acting on the otolithic mass, which leads to transduction by mimicking gravito-inertial acceleration. Equivalency of mechanical vs LiFU stimuli for both transient-type and sustained-type afferents (Figs. 6 and 7) is very revealing because these two types of afferents innervate hair cells in different regions of the sensory epithelium, have a distinct electrophysiological characteristics, and contact hair cells with markedly different hair bundles and micromechanical properties. Alternatively, if LiFU acted through direct action on hair bundles or individual hair cells, one might expect more loosely coupled hair bundles near the striola to be preferentially or differentially activated by LiFU relative to mechanical stimulation (Curthoys and Grant, 2015). This was not the case, evidenced by the fact that mechanical-LiFU equivalency to extend across the entire population of afferents. The simplest explanation is that LiFU forcing is equivalent to direct mechanical force applied to the entire otolithic mass by the piezoelectric actuator. It is also important to note that the wavelength of 5 MHz ultrasound is at least 2 orders of magnitude longer than hair bundle width, and that the depth of focus was at least 3 orders of magnitude longer than the vestibular organs tested. In addition, direct measurement of the acoustic radiation force acting on excised saccular and utricular otoliths generated forces equivalent to that generated by 0–0.8 g of linear acceleration. The radiation force generated by the impedance mismatch between the otolithic mass and surrounding fluids and tissues is sufficient to explain all neural responses.
We used the oyster toadfish as the experimental model to facilitate application of LiFU to individual otolith organs and to simultaneously record afferent neurons in vivo. When considering application of LiFU it is important to recognize two differences between otolith organs in the fish compared with mammalians. First, the otolithic mass in fish consists of a single fused otolith in each organ, while the otolithic mass in mammals consists of a matrix of small otoconia adhered to each other through an extracellular matrix (Popper and Fay, 1973; Platt, 1977; Lins et al., 2000; Andrade et al., 2012). This difference would be expected to reduce the net acoustic impedance of otoconial mass in mammals relative to the otolith in fish. Based on the density and calcium carbonate content of mammalian otoconia, however, it is likely that the acoustic impedance of the mammalian otoconial complex, like fish otoliths, is very high relative to endolymph. This implies a large acoustic impedance miss-match would be present in mammals as well, which would be expected to lead to a large acoustic radiation force similar to that observed directly on excised otolith stones from fish. Second, the fish saccular otolith is very large relative to the saccular otoconial mass in mammals. Decreasing size reduces the total radiation force due to the reduction in the frontal surface area reflecting the ultrasound wave (e.g., Fig. 8), but size reduction would not be expected to reduce sensitivity of the organ to ultrasound because of the larger surface to volume ratio. The acoustic radiation force is approximately equal to the radiation pressure times the area (; radiation pressure, frontal area), while the force due to acceleration is the mass times the acceleration (; volume, density, acceleration). The equivalent acceleration generated by LiFU is, , or , where is the area to volume ratio. As the size of the organ decreases the area to volume ratio increases , thus increasing the equivalent acceleration generated by the acoustic radiation force. This suggests mammals are likely to be even more sensitive to LiFU than recorded here in toadfish.
Focused ultrasound has several potential advantages as an otolith organ stimulus relative to traditional methods. First, the temporal waveform can be readily controlled to deliver forces directly to the otolithic mass over a very broad frequency bandwidth ranging from tonic DC forces to auditory frequencies above the upper limit of vestibular sensation. Second, the direction of the force can be controlled by the direction of the incident ultrasound wave. Third, ultrasound can be focused to preferentially activate individual otolith organs unilaterally. These three features facilitate activation specific afferent neurons based on organ, direction, and spectral composition of the stimulus. Fourth, focused ultrasound stimulation does not require moving the animal, and does not require the use of loud sounds or vibration that activate the cochlea concurrently.
Although present results demonstrate several strengths of LiFU as an otolith organ stimulus, there are notable limitations. Importantly, focused ultrasound works by mimicking inertial force normally present during physiological stimulation with the acoustic radiation force. Therefore, the stimulus requires a responding otolith organ with functional MET channels, hair cells, and afferent neurons. Also, safety to the ear has not been established in that we did not test the stimulus in mammals or address sensitivity of the cochlea. However, the minimal temperature rise recorded and the low power used suggest that no tissue damage will occur. Based on responses of semicircular canal afferent neurons, pLiFU directed at the organ of Corti would be expected to evoke afferent responses and the sensation of sound. Sensitivity, and how well the stimulus can be focused to a specific region of the cochlea are unknown. Targeting specific inner ear organs through the temporal bone is another unknown. Targeting with a waveguide contacting perilymph in excised tissue or via a large surgical opening is likely straightforward, but it is not known if adequate targeting of vestibular otolith organs or the organ of Corti can be achieved through the temporal bone in animal models or in humans. Based on the present results, if methods could be developed to target LiFU to vestibular organs in humans the stimulus could be useful for vestibular testing. The ability to tune the frequency content and stimulus direction could provide advantages relative to air and bone conducted sound, or linear acceleration stimuli. Although there are very significant technical challenges, it might also be possible in the future to develop prostheses using focused ultrasound to treat otolith hypofunction or orthostatic intolerance by closed-loop selective activation of the appropriate sustained-type or transient-type otolith afferents.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health Grant No. R01 DC006685.
References
- 1. Andrade, L. R. , Lins, U. , Farina, M. , Kachar, B. , and Thalmann, R. (2012). “ Immunogold TEM of otoconin 90 and otolin—Relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo,” Hear. Res. 292, 14–25. 10.1016/j.heares.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baird, R. A. , and Lewis, E. R. (1986). “ Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and lagena,” Brain Res. 369, 48–64. 10.1016/0006-8993(86)90512-3 [DOI] [PubMed] [Google Scholar]
- 3. Bisdorff, A. R. , Staab, J. P. , and Newman-Toker, D. E. (2015). “ Overview of the International Classification of Vestibular Disorders,” Neurol. Clin. 33, 541–550. 10.1016/j.ncl.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 4. Boyle, R. , Carey, J. P. , and Highstein, S. M. (1991). “ Morphological correlates of response dynamics and efferent stimulation in horizontal semicircular canal afferents of the toadfish, Opsanus tau,” J. Neurophysiol. 66, 1504–1521. [DOI] [PubMed] [Google Scholar]
- 5. Boyle, R. , and Highstein, S. M. (1990). “ Resting discharge and response dynamics of horizontal semicircular canal afferents of the toadfish, Opsanus tau,” J. Neurosci. 10, 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle, R. , Mensinger, A. F. , Yoshida, K. , Usui, S. , Intravaia, A. , Tricas, T. , and Highstein, S. M. (2001). “ Neural readaptation to Earth's gravity following return from space,” J. Neurophysiol. 86, 2118–2122. [DOI] [PubMed] [Google Scholar]
- 7. Bystritsky, A. , Korb, A. S. , Douglas, P. K. , Cohen, M. S. , Melega, W. P. , Mulgaonkar, A. P. , DeSalles, A. , Min, B. K. , and Yoo, S. S. (2011). “ A review of low-intensity focused ultrasound pulsation,” Brain Stimul. 4, 125–136. 10.1016/j.brs.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 8. Carter, J. R. , and Ray, C. A. (2008). “ Sympathetic responses to vestibular activation in humans,” Am. J. Physiol. Reg. Integr. Comp. Physiol. 294, R681–R688. 10.1152/ajpregu.00896.2007 [DOI] [PubMed] [Google Scholar]
- 9. Clément, G. , Moore, S. T. , Raphan, T. , and Cohen, B. (2001). “ Perception of tilt (somatogravic illusion) in response to sustained linear acceleration during space flight,” Exp. Brain Res. 138, 410–418. 10.1007/s002210100706 [DOI] [PubMed] [Google Scholar]
- 10. Cohen, B. , Martinelli, G. P. , Raphan, T. , Schaffner, A. , Xiang, Y. , Holstein, G. R. , and Yakushin, S. B. (2013). “ The vasovagal response of the rat: Its relation to the vestibulosympathetic reflex and to Mayer waves,” FASEB J. 27, 2564–2572. 10.1096/fj.12-226381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen, B. , Suzuki, J. I. , and Bender, M. B. (1964). “ Eye movements from semicircular canal nerve stimulation in the cat,” Ann. Otol. Rhinol. Laryngol. 73, 153–169. 10.1177/000348946407300116 [DOI] [PubMed] [Google Scholar]
- 12. Cohen, B. , Wearne, S. , Dai, M. , and Raphan, T. (1999). “ Spatial orientation of the angular vestibulo-ocular reflex,” J. Vestib. Res. 9, 163–172. [PubMed] [Google Scholar]
- 13. Curthoys, I. S. , and Grant, J. W. (2015). “ How does high-frequency sound or vibration activate vestibular receptors?,” Exp. Brain Res. 233, 691–699. 10.1007/s00221-014-4192-6 [DOI] [PubMed] [Google Scholar]
- 14. Curthoys, I. S. , Haslwanter, T. , Black, R. A. , Burgess, A. M. , Halmagyi, G. M. , Topple, A. N. , and Todd, M. J. (1998). “ Off-center yaw rotation: Effect of naso-occipital linear acceleration on the nystagmus response of normal human subjects and patients after unilateral vestibular loss,” Exp. Brain Res. 123, 425–438. 10.1007/s002210050587 [DOI] [PubMed] [Google Scholar]
- 15. Curthoys, I. S. , Iwasaki, S. , Chihara, Y. , Ushio, M. , McGarvie, L. A. , and Burgess, A. M. (2011). “ The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin,” Clin. Neurophysiol. 122, 611–616. 10.1016/j.clinph.2010.07.018 [DOI] [PubMed] [Google Scholar]
- 16. Curthoys, I. S. , MacDougall, H. G. , Vidal, P. P. , and de Waele, C. (2017). “ Sustained and transient vestibular systems: A physiological basis for interpreting vestibular function,” Front. Neurol. 8, 117. 10.3389/fneur.2017.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curthoys, I. S. , and Vulovic, V. (2011). “ Vestibular primary afferent responses to sound and vibration in the guinea pig,” Exp. Brain Res. 210, 347–352. 10.1007/s00221-010-2499-5 [DOI] [PubMed] [Google Scholar]
- 18. Curthoys, I. S. , Vulovic, V. , Burgess, A. M. , Manzari, L. , Sokolic, L. , Pogson, J. , Robins, M. , Mezey, L. E. , Goonetilleke, S. , Cornell, E. D. , and MacDougall, H. G. (2014). “ Neural basis of new clinical vestibular tests: Otolithic neural responses to sound and vibration,” Clin. Exp. Pharmacol. Physiol. 41, 371–380. 10.1111/1440-1681.12222 [DOI] [PubMed] [Google Scholar]
- 19. Curthoys, I. S. , Vulovic, V. , Sokolic, L. , Pogson, J. , and Burgess, A. M. (2012). “ Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound,” Brain Res. Bull. 89, 16–21. 10.1016/j.brainresbull.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 20. Davis, J. L. , Xue, J. , Peterson, E. H. , and Grant, J. W. (2007). “ Layer thickness and curvature effects on otoconial membrane deformation in the utricle of the red-ear slider turtle: Static and modal analysis,” J. Vestib. Res. 17, 145–162. [PMC free article] [PubMed] [Google Scholar]
- 21. Dickman, J. D. , Reder, P. A. , and Correia, M. J. (1988). “ A method for controlled mechanical stimulation of single semicircular canals,” J. Neurosci. Meth. 25, 111–119. 10.1016/0165-0270(88)90147-1 [DOI] [PubMed] [Google Scholar]
- 22. Doinikov, A. (2003). “ Acoustic radiation forces: Classical theory and recent advances,” Rec. Res. Devel. Acoust. 1, 39–67. [Google Scholar]
- 23. Dunlap, M. D. , Spoon, C. E. , and Grant, J. W. (2012). “ Experimental measurement of utricle dynamic response,” J. Vestib. Res. 22, 57–68 10.3233/VES-2011-0431. [DOI] [PubMed] [Google Scholar]
- 24. Elidan, J. , Langhofer, L. , and Honrubia, V. (1987). “ The neural generators of the vestibular evoked response,” Brain Res. 423, 385–390. 10.1016/0006-8993(87)90868-7 [DOI] [PubMed] [Google Scholar]
- 25. Fay, R. R. , Edds-Walton, P. L. , and Highstein, S. M. (1994). “ Directional sensitivity of saccular afferents of the toadfish to linear acceleration at audio frequencies,” Biol. Bull. 187, 258–259. 10.1086/BBLv187n2p258 [DOI] [PubMed] [Google Scholar]
- 26. Fernandez, C. , Goldberg, J. M. , and Abend, W. K. (1972). “ Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey,” J. Neurophys. 35, 978–987. [DOI] [PubMed] [Google Scholar]
- 27. Fortenberry, B. , Gorchetchnikov, A. , and Grossberg, S. (2012). “ Learned integration of visual, vestibular, and motor cues in multiple brain regions computes head direction during visually guided navigation,” Hippocampus 22, 2219–2237. 10.1002/hipo.22040 [DOI] [PubMed] [Google Scholar]
- 28. Furman, J. , Cass, S. , and Whitney, S. (2010). Vestibular Disorders: A Case Study Approach to Diagnosis and Treatment ( Oxford University Press, New York: ), pp. 1–464. [Google Scholar]
- 29. Gavrilov, L. R. , Gersuni, G. V. , Ilyinsky, O. B. , Sirotyuk, M. G. , Tsirulnikov, E. M. , and Shchekanov, E. E. (1976). “ The effect of focused ultrasound on the skin and deep nerve structures of man and animal,” Prog. Brain Res. 43, 279–292. 10.1016/S0079-6123(08)64360-5 [DOI] [PubMed] [Google Scholar]
- 30. Gioacchini, F. M. , Alicandri-Ciufelli, M. , Kaleci, S. , Magliulo, G. , and Re, M. (2014). “ Prevalence and diagnosis of vestibular disorders in children: A review,” Int. J. Pediatr. Otorhinolaryngol. 78, 718–724. 10.1016/j.ijporl.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 31. Goldberg, J. , Wilson, V. , Cullen, K. , Angelaki, D. , Broussard, D. , Buttner-Ennever, J. , Fukushima, K. , and Minow, L. (2012). The Vestibular System: A Sixth Sense ( Oxford University Press, New York: ), pp. 1–560. [Google Scholar]
- 32. Goldberg, J. M. (2000). “ Afferent diversity and the organization of central vestibular pathways,” Exp. Brain Res. 130, 277–297. 10.1007/s002210050033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldberg, J. M. , Desmadryl, G. , Baird, R. A. , and Fernandez, C. (1990). “ The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents,” J. Neurophysiol. 63, 781–790. [DOI] [PubMed] [Google Scholar]
- 34. Grant, W. , and Best, W. (1987). “ Otolith-organ mechanics: Lumped parameter model and dynamic response,” Aviat. Space Environ. Med. 58, 970–976. [PubMed] [Google Scholar]
- 35. Halmagyi, G. M. , Curthoys, I. S. , Colebatch, J. G. , and Aw, S. T. (2005). “ Vestibular responses to sound,” Ann. N.Y. Acad. Sci. 1039, 54–67. 10.1196/annals.1325.006 [DOI] [PubMed] [Google Scholar]
- 36. Harada, Y. , Kasuga, S. , and Tamura, S. (2001). “ Comparison and evolution of the lagena in various animal species,” Acta Otolaryngol. 121, 355–363. [DOI] [PubMed] [Google Scholar]
- 37. Highstein, S. M. , Rabbitt, R. D. , and Boyle, R. (1996). “ Determinants of semicircular canal afferent response dynamics in the toadfish, Opsanus tau,” J. Neurophysiol. 75, 575–596. [DOI] [PubMed] [Google Scholar]
- 38. Holstein, G. R. , Rabbitt, R. D. , Martinelli, G. P. , Friedrich, V. L., Jr. , Boyle, R. D. , and Highstein, S. M. (2004). “ Convergence of excitatory and inhibitory hair cell transmitters shapes vestibular afferent responses,” Proc. Natl. Acad. Sci. U.S.A. 101, 15766–15771. 10.1073/pnas.0402824101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Honaker, J. A. , Lee, C. , Criter, R. E. , and Jones, T. A. (2015). “ Test-retest reliability of the vestibular sensory-evoked potential (VsEP) in C57BL/6J mice,” J. Am. Acad. Audiol. 26, 59–67. 10.3766/jaaa.26.1.7 [DOI] [PubMed] [Google Scholar]
- 40. Jacob, P. Y. , Poucet, B. , Liberge, M. , Save, E. , and Sargolini, F. (2014). “ Vestibular control of entorhinal cortex activity in spatial navigation,” Front. Integr. Neurosci. 8, 38. 10.3389/fnint.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jamali, M. , Sadeghi, S. G. , and Cullen, K. E. (2009). “ Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations,” J. Neurophysiol. 101, 141–149 10.1152/jn.91066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones, T. A. , and Jones, S. M. (1999). “ Short latency compound action potentials from mammalian gravity receptor organs,” Hear. Res. 136, 75–85. 10.1016/S0378-5955(99)00110-0 [DOI] [PubMed] [Google Scholar]
- 43. Kaga, K. (2014). “ Vertigo and balance disorders in children,” in Modern Otology and Neurotology ( Springer, Tokyo: ). [Google Scholar]
- 44. Kim, H. , Lee, S. D. , Chiu, A. , Yoo, S. S. , and Park, S. (2014). “ Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation,” Neurorep. 25, 475–479 10.1097/WNR.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kushiro, K. , Zakir, M. , Ogawa, Y. , Sato, H. , and Uchino, Y. (1999). “ Saccular and utricular inputs to sternocleidomastoid motoneurons of decerebrate cats,” Exp. Brain Res. 126, 410–416. 10.1007/s002210050747 [DOI] [PubMed] [Google Scholar]
- 46. Kushiro, K. , Zakir, M. , Sato, H. , Ono, S. , Ogawa, Y. , Meng, H. , Zhang, X. , and Uchino, Y. (2000). “ Saccular and utricular inputs to single vestibular neurons in cats,” Exp. Brain Res. 131, 406–415. 10.1007/s002219900312 [DOI] [PubMed] [Google Scholar]
- 47. Lakshmanan, M. , and Murali, K. (1996). Chaos in Nonlinear Oscillators: Contolling and Synchronization ( World Scientific, Singapore: ), pp. 1–340. [Google Scholar]
- 48. Lins, U. , Farina, M. , Kurc, M. , Riordan, G. , Thalmann, R. , Thalmann, I. , and Kachar, B. (2000). “ The otoconia of the guinea pig utricle: Internal structure, surface exposure, and interactions with the filament matrix,” J. Struct. Biol. 131, 67–78. 10.1006/jsbi.2000.4260 [DOI] [PubMed] [Google Scholar]
- 49. Marsh, J. N. , Hall, C. S. , Wickline, S. A. , and Lanza, G. M. (2002). “ Temperature dependence of acoustic impedance for specific fluorocarbon liquids,” J. Acoust. Soc. Am. 112, 2858–2862. 10.1121/1.1517251 [DOI] [PubMed] [Google Scholar]
- 50. McCue, M. P. , and Guinan, J. J., Jr. (1994). “ Acoustically responsive fibers in the vestibular nerve of the cat,” J. Neurosci. 14, 6058–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McCue, M. P. , and Guinan, J. J., Jr. (1995). “ Spontaneous activity and frequency selectivity of acoustically responsive vestibular afferents in the cat,” J. Neurophysiol. 74, 1563–1572. [DOI] [PubMed] [Google Scholar]
- 52. Min, B. K. , Bystritsky, A. , Jung, K. I. , Fischer, K. , Zhang, Y. , Maeng, L. S. , Park, S. I. , Chung, Y. A. , Jolesz, F. A. , and Yoo, S. S. (2011). “ Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity,” BMC Neurosci. 12, 23. 10.1186/1471-2202-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Minor, L. B. , and Goldberg, J. M. (1991). “ Vestibular-nerve inputs to the vestibulo-ocular reflex: A functional-ablation study in the squirrel monkey,” J. Neurosci. 11, 1636–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minor, L. B. , Lasker, D. M. , Backous, D. D. , and Hullar, T. E. (1999). “ Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses,” J. Neurophysiol. 82, 1254–1270. [DOI] [PubMed] [Google Scholar]
- 55. Naranjo, E. N. , Cleworth, T. W. , Allum, J. H. , Inglis, J. T. , Lea, J. , Westerberg, B. D. , and Carpenter, M. G. (2016). “ Vestibulo-spinal and vestibulo-ocular reflexes are modulated when standing with increased postural threat,” J. Neurophysiol. 115, 833–842. 10.1152/jn.00626.2015 [DOI] [PubMed] [Google Scholar]
- 56. Newlands, S. D. , Vrabec, J. T. , Purcell, I. M. , Stewart, C. M. , Zimmerman, B. E. , and Perachio, A. A. (2003). “ Central projections of the saccular and utricular nerves in macaques,” J. Comp. Neurol. 466, 31–47. 10.1002/cne.10876 [DOI] [PubMed] [Google Scholar]
- 57. Oh, S. Y. , Kim, H. J. , and Kim, J. S. (2016). “ Vestibular-evoked myogenic potentials in central vestibular disorders,” J. Neurol. 263, 210–220. 10.1007/s00415-015-7860-y [DOI] [PubMed] [Google Scholar]
- 58. O'Leary, D. P. (1970). “ An electrokinetic model of transduction in the semicircular canal,” Biophys. J. 10, 859–875. 10.1016/S0006-3495(70)86340-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park, H. J. , Lasker, D. M. , and Minor, L. B. (2010). “ Static and dynamic discharge properties of vestibular-nerve afferents in the mouse are affected by core body temperature,” Exp. Brain Res. 200, 269–275. 10.1007/s00221-009-2015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Platt, C. (1977). “ Hair cell distribution and orientation in goldfish otolith organs,” J. Comp. Neurol. 172, 283–287. 10.1002/cne.901720207 [DOI] [PubMed] [Google Scholar]
- 61. Plishka, C. M. (2015). A Clinician's Guide to Balance and Dizziness: Evaluation and Treatment ( Slack Incorporated, Thorofare, NJ: ), pp. 1–400. [Google Scholar]
- 62. Popper, A. N. , and Fay, R. R. (1973). “ Sound detection and processing by teleost fishes: A critical review,” J. Acoust. Soc. Am. 53, 1515–1529. 10.1121/1.1913496 [DOI] [PubMed] [Google Scholar]
- 63. Popper, A. N. , and Fay, R. R. (1993). “ Sound detection and processing by fish: Critical review and major research questions,” Brain Behav. Evol. 41, 14–38. 10.1159/000113821 [DOI] [PubMed] [Google Scholar]
- 64. Rabbitt, R. , Damiano, E. , and Grant, J. (2003). “ Biomechanics of the vestibular semicircular canals and otolith organs,” in The Vestibular System, edited by Highstein S. M., Popper A., and Fay R. ( Springer Verlag, New York: ), pp. 153–201. [Google Scholar]
- 65. Rabbitt, R. D. , Boyle, R. , and Highstein, S. M. (1995). “ Mechanical indentation of the vestibular labyrinth and its relationship to head rotation in the toadfish, Opsanus tau,” J. Neurophysiol. 73, 2237–2260. [DOI] [PubMed] [Google Scholar]
- 66. Rabbitt, R. D. , Brichta, A. M. , Tabatabaee, H. , Boutros, P. J. , Ahn, J. , Della Santina, C. C. , Poppi, L. A. , and Lim, R. (2016). “ Heat pulse excitability of vestibular hair cells and afferent neurons,” J. Neurophysiol. 116, 825–843. 10.1152/jn.00110.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rajguru, S. M. , Richter, C. P. , Matic, A. I. , Holstein, G. R. , Highstein, S. M. , Dittami, G. M. , and Rabbitt, R. D. (2011). “ Infrared photostimulation of the crista ampullaris,” J. Physiol. 589, 1283–1294. 10.1113/jphysiol.2010.198333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramat, S. , Zee, D. S. , and Minor, L. B. (2001). “ Translational vestibulo-ocular reflex evoked by a ‘head heave’ stimulus,” Ann. N.Y. Acad. Sci. 942, 95–113. [PubMed] [Google Scholar]
- 69. Rancz, E. A. , Moya, J. , Drawitsch, F. , Brichta, A. M. , Canals, S. , and Margrie, T. W. (2015). “ Widespread vestibular activation of the rodent cortex,” J. Neurosci. 35, 5926–5934. 10.1523/JNEUROSCI.1869-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rezayat, E. , and Toostani, I. G. (2016). “ A review on brain stimulation using low intensity focused ultrasound,” Basic Clin. Neurosci. J. 7, 187–194 10.15412/J.BCN.03070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosengren, S. M. , and Colebatch, J. G. (2011). “ Ocular vestibular evoked myogenic potentials are abnormal in internuclear ophthalmoplegia,” Clin. Neurophysiol. 122, 1264–1267. 10.1016/j.clinph.2010.10.040 [DOI] [PubMed] [Google Scholar]
- 72. Sarvazyan, A. P. , Rudenko, O. V. , and Nyborg, W. L. (2010). “ Biomedical applications of radiation force of ultrasound: Historical roots and physical basis,” Ultrasound Med. Biol. 36, 1379–1394. 10.1016/j.ultrasmedbio.2010.05.015 [DOI] [PubMed] [Google Scholar]
- 73. Schor, R. H. , and Miller, A. D. (1982). “ Relationship of cat vestibular neurons to otolith-spinal reflexes,” Exp. Brain Res. 47, 137–144. [DOI] [PubMed] [Google Scholar]
- 74. Si, X. , Angelaki, D. E. , and Dickman, J. D. (1997). “ Response properties of pigeon otolith afferents to linear acceleration,” Exp. Brain Res. 117, 242–250. 10.1007/s002210050219 [DOI] [PubMed] [Google Scholar]
- 75. Thalmann, R. , Ignatova, E. , Kachar, B. , Ornitz, D. M. , and Thalmann, I. (2001). “ Development and maintenance of otoconia: Biochemical considerations,” Ann. N.Y. Acad. Sci. 942, 162–178. [DOI] [PubMed] [Google Scholar]
- 76. Thévenaz, P. , Ruttimann, U. E. , and Unser, M. (1998). “ A pyramid approach to subpixel registration based on intensity,” IEEE Trans. Image Process. 7, 27–41. 10.1109/83.650848 [DOI] [PubMed] [Google Scholar]
- 77. Todd, N. P. , McLean, A. , Paillard, A. , Kluk, K. , and Colebatch, J. G. (2014a). “ Vestibular evoked potentials (VsEPs) of cortical origin produced by impulsive acceleration applied at the nasion,” Exp. Brain Res. 232, 3771–3784. 10.1007/s00221-014-4067-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Todd, N. P. , Paillard, A. C. , Kluk, K. , Whittle, E. , and Colebatch, J. G. (2014b). “ Source analysis of short and long latency vestibular-evoked potentials (VsEPs) produced by left vs. right ear air-conducted 500 Hz tone pips,” Hear. Res. 312, 91–102. 10.1016/j.heares.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Trahey, G. E. , Palmeri, M. L. , Bentley, R. C. , and Nightingale, K. R. (2004). “ Acoustic radiation force impulse imaging of the mechanical properties of arteries: In vivo and ex vivo results,” Ultrasound Med. Biol. 30, 1163–1171. 10.1016/j.ultrasmedbio.2004.07.022 [DOI] [PubMed] [Google Scholar]
- 80. Tufail, Y. , Matyushov, A. , Baldwin, N. , Tauchmann, M. L. , Georges, J. , Yoshihiro, A. , Tillery, S. I. , and Tyler, W. J. (2010). “ Transcranial pulsed ultrasound stimulates intact brain circuits,” Neuron 66, 681–694. 10.1016/j.neuron.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 81. Tyler, W. J. , Tufail, Y. , Finsterwald, M. , Tauchmann, M. L. , Olson, E. J. , and Majestic, C. (2008). “ Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound,” PloS One 3, e3511. 10.1371/journal.pone.0003511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weeg, M. S. , Fay, R. R. , and Bass, A. H. (2002). “ Directionality and frequency tuning of primary saccular afferents of a vocal fish, the plainfin midshipman (Porichthys notatus),” J. Comp. Physiol. A 188, 631–641 10.1007/s00359-002-0338-2. [DOI] [PubMed] [Google Scholar]
- 83. Wilson, V. J. , Boyle, R. , Fukushima, K. , Rose, P. K. , Shinoda, Y. , Sugiuchi, Y. , and Uchino, Y. (1995). “ The vestibulocollic reflex,” J. Vestib. Res. 5, 147–170. 10.1016/0957-4271(94)00035-Z [DOI] [PubMed] [Google Scholar]
- 84. Yates, B. J. (1998). “ Autonomic reaction to vestibular damage,” Otolaryngol. Head Neck Surg. 119, 106–112. 10.1016/S0194-5998(98)70179-2 [DOI] [PubMed] [Google Scholar]
- 85. Yates, B. J. , Bolton, P. S. , and Macefield, V. G. (2014). “ Vestibulo-sympathetic responses,” Comp. Physiol. 4, 851–887 10.1002/cphy.c130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yoo, S. S. , Bystritsky, A. , Lee, J. H. , Zhang, Y. , Fischer, K. , Min, B. K. , McDannold, N. J. , Pascual-Leone, A. , and Jolesz, F. A. (2011). “ Focused ultrasound modulates region-specific brain activity,” NeuroImage 56, 1267–1275. 10.1016/j.neuroimage.2011.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu, H. , Tang, X. , Wei, W. , Maklad, A. , Mustain, W. , Rabbitt, R. , Highstein, S. , Allison, J. , and Zhou, W. (2014). “ Input-output functions of vestibular afferent responses to air-conducted clicks in rats,” J. Assoc. Res. Otolaryngol. 15, 73–86. 10.1007/s10162-013-0428-6 [DOI] [PMC free article] [PubMed] [Google Scholar]