Abstract
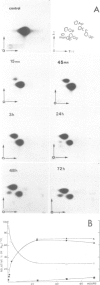
We have investigated the specificity of the enzymes Q-insertase and mannosyl-Q transferase that replace the guanosine at position 34 (wobble base) in the anticodon of several tRNAs by Q or mannosyl-Q derivatives. We have restructured in vitro the normal anticodon of yeast tRNA-Asp-GUC, yeast tRNAArgICG and yeast tRNALeuUAG. With yeast tRNA-Asp-GUC, we have replaced one or several nucleotides in the vicinity of G34 by one of the four canonical nucleotides or by pseudouridylic acid; we have also constructed a tRNAAsp with eight bases instead of seven in the anticodon loop. With yeast tRNAArgICG and yeast tRNALeuUAG, we have replaced their anticodon by the trinucleotide GUC, coding for aspartic acid. The chimerical tRNAs were microinjected into the cytoplasm of Xenopus laevis oocytes and after 72 h the amount of Q34 and mannosyl-Q34 incorporated was measured. Our results show that the U33G34U35 sequence, within an anticodon loop of seven bases in chimerical yeast tRNA-Asp-GUC, tRNAArgGUC or tRNALeuGUC, is the main determinant for Q-insertase activity at position 34; the rest of the tRNA sequence has only a slight influence. For mannosyl-Q transferase, however, a much broader structural feature of the tRNA than just the U33G34U35 sequence is important for the efficiency of Q34 transformation into mannosyl-Q34.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce A. G., Uhlenbeck O. C. Enzymatic replacement of the anticodon of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1982 Mar 2;21(5):855–861. doi: 10.1021/bi00534a007. [DOI] [PubMed] [Google Scholar]
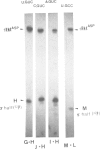
- Carbon P., Haumont E., De Henau S., Keith G., Grosjean H. Enzymatic replacement in vitro of the first anticodon base of yeast tRNAAsp: application to the study of tRNA maturation in vivo, after microinjection into frog oocytes. Nucleic Acids Res. 1982 Jun 25;10(12):3715–3732. doi: 10.1093/nar/10.12.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
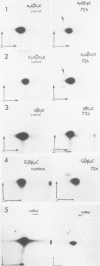
- Fournier M., Haumont E., de Henau S., Gangloff J., Grosjean H. Post-transcriptional modification of the wobble nucleotide in anticodon-substituted yeast tRNAArgII after microinjection into Xenopus laevis oocytes. Nucleic Acids Res. 1983 Feb 11;11(3):707–718. doi: 10.1093/nar/11.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatica M., Tarragó A., Allende C. C., Allende J. E. Aminoacylation of transfer RNA microinjected into Xenopus laevis oocytes. Nature. 1975 Aug 21;256(5519):675–678. doi: 10.1038/256675a0. [DOI] [PubMed] [Google Scholar]
- Howes N. K., Farkas W. R. Studies with a homogeneous enzyme from rabbit erythrocytes catalyzing the insertion of guanine into tRNA. J Biol Chem. 1978 Dec 25;253(24):9082–9087. [PubMed] [Google Scholar]
- Nishikura K., De Robertis E. M. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J Mol Biol. 1981 Jan 15;145(2):405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- Okada N., Nishimura S. Isolation and characterization of a guanine insertion enzyme, a specific tRNA transglycosylase, from Escherichia coli. J Biol Chem. 1979 Apr 25;254(8):3061–3066. [PubMed] [Google Scholar]
- Okada N., Noguchi S., Kasai H., Shindo-Okada N., Ohgi T., Goto T., Nishimura S. Novel mechanism of post-transcriptional modification of tRNA. Insertion of bases of Q precursors into tRNA by a specific tRNA transglycosylase reaction. J Biol Chem. 1979 Apr 25;254(8):3067–3073. [PubMed] [Google Scholar]
- Shindo-Okada N., Okada N., Ohgi T., Goto T., Nishimura S. Transfer ribonucleic acid guanine transglycosylase isolated from rat liver. Biochemistry. 1980 Jan 22;19(2):395–400. doi: 10.1021/bi00543a023. [DOI] [PubMed] [Google Scholar]
- Werner C., Krebs B., Keith G., Dirheimer G. Specific cleavages of pure tRNAs by plumbous ions. Biochim Biophys Acta. 1976 May 3;432(2):161–175. doi: 10.1016/0005-2787(76)90158-1. [DOI] [PubMed] [Google Scholar]