Abstract
Objective
Factors leading patients with head and neck cancer (HNCA) to seek radiation or chemoradiation in an academic center versus the community are incompletely understood, as are the effects of site of treatment on treatment completion and survival.
Study Design
Historical cohort study.
Setting
Tertiary academic center, community practices.
Methods
A historical cohort study was completed of patients with mucosal HNCA identified by International Classification of Disease, Ninth Revision (ICD-9) codes receiving consultation at the authors’ institution from 2003 to 2008. Patients who received primary and adjuvant radiation at an academic center or in the community were included. The authors compared treatment completion rates and performed univariate and multivariate analyses of treatment outcomes.
Results
Of 388 patients, 210 completed treatment at an academic center and 145 at a community center (33 excluded, location unknown). Patients with HNCA undergoing radiation at an academic site had more advanced disease (P = .024) and were more likely to receive concurrent chemotherapy. Academic hospitals had a higher percentage of noncurrent smokers, higher median income, and higher percentage of oropharyngeal tumors. There was no significant difference in the rate of planned treatment completion between community and academic centers (93.7% vs 94.7%, P > .81) or rate of treatment breaks (22.4% vs 28.4%, P > .28). On Kaplan-Meier analysis, the 5-year survival rate was 53.2% (95% confidence interval [CI], 45.3%–61.1%) for academic centers and 32.8% (95% CI, 22.0%–43.6%) for community hospitals (P <.001).
Conclusion
In this cohort, although treatment completion and treatment breaks were similar between academic and community centers, survival rates were higher in patients treated in an academic setting.
Keywords: head and neck cancer, treating institution, radiation, outcomes
There is a considerable movement to improve the quality of medical care within the United States. The Institute of Medicine has emphasized the critical need for quality improvement,1 and this discussion has found a central place in cancer care. A number of outcomes have been used to evaluate the quality of head and neck cancer care, including treatment outcomes and adherence to nationally established evidence-based treatment guidelines.2 The effect of receiving treatment at a low-volume versus high-volume center has been evaluated in many non–head and neck cancers, with the majority of literature supporting improved prognosis with treatment at high-volume centers.3–8 Chen et al9,10 have shown improved survival associated with high-volume facilities for early stage and advanced stage laryngeal cancer. Similarly, Akman and colleagues11 found that surgery at specialized cancer centers resulted in improved locoregional control in laryngeal cancer. With regard to radiation therapy, it is well known that treatment prolongation secondary to treatment breaks results in lower rates of locoregional control and survival.12–14
Less well understood is whether the location of treatment influences outcomes in head and neck cancer. Undergoing treatment at an academic medical center can be challenging for patients because of geographical distance, lack of transportation, lack of funding for travel or housing, lack of social support, and preventative substance abuse issues. The evidence is conflicting as to what effect receiving radiation at local community centers versus academic centers may have on patient outcomes. Benasso and colleagues15 evaluated the effect of treating institution type on survival in patients radiated at a coordinating center versus affiliated centers within a randomized controlled trial and found improved survival for patients receiving chemoradiation at a coordinating center with similar treatment compliance at both types of centers. Kubicek et al,16 however, evaluated patients with head and neck cancer receiving radiation in the community versus at an academic center and did not find any difference in patient outcomes. This group did not address differences in treatment completion.
To gain additional insight into differences related to site of radiation administration, we evaluated (1) adherence to radiation treatment protocols by assessing completion of entire prescribed treatment and evidence of treatment interruptions and (2) the effect of type of treating institution on survival.
Methods
Setting and Subjects
We obtained University of Minnesota Institutional Review Board approval for a retrospective cohort study evaluating differences in patient characteristics, treatment, and cancer outcomes in the head and neck cancer population at the University of Minnesota. A cohort of patients with head and neck cancer who received consultation for treatment at the University of Minnesota from 2002 through 2008 was identified via a search of head and neck cancer–related International Classification of Disease, Ninth Revision (ICD-9) codes (search algorithm available from corresponding author upon request). Codes representing primary tumor sites of the oral cavity, oropharynx, hypopharynx, larynx, nasopharynx, nose, and paranasal sinuses were used to identify patients. To identify our cohort of patients receiving either radiation or chemoradiation, we reviewed medical records.
Data Collection
We collected data on demographics, general medical data, tumor variables, treatment, and outcomes via medical record review.
Demographic characteristics included sex, age at diagnosis, follow-up time (time from first to last available follow-up), marital status at diagnosis, work status at diagnosis, and insurance type. We interrogated the distance traveled by these patients by documenting distance in miles from the university using zip codes. The median income of the patient’s home zip code was recorded. Finally, the patient’s county of origin was recorded and classified using the US Department of Agriculture rural-urban continuum codes. These 9 codes allow for categorization of all counties in the United States on a continuum from metropolitan to nonmetropolitan by degree of urbanization and proximity to metro areas.
General medical data included information about health behaviors at baseline (tobacco use and alcohol use) and health comorbidities, including diabetes, heart disease, lung disease, stroke, arthritis, and mental illness/depression. Tumor variables included anatomic site, histopathology, and TNM staging data. Treatment data included site of radiation therapy (academic or community). The state of Minnesota has 2 academic, tertiary care radiation treatment sites, which are the University of Minnesota and the Mayo Clinic. Both of these were included as academic centers, as were several academic centers outside of Minnesota from which 9 patients sought care. All other treatment sites were described as community. For patients not treated at our institution, a standard information medical record request form was submitted, including patient Health Insurance Portability and Accountability Act (HIPAA) authorization, to obtain outside radiation treatment records. These records were reviewed to determine planned dose (in centigray) and completed dose, as well as planned and completed fractions. Type of radiation treatment was documented as primary, primary with chemotherapy (concurrent), adjuvant, adjuvant with chemotherapy, and palliative. Previous history of radiation was also recorded. We also captured data about margin status and extracapsular extension of lymph nodes if available when surgery was performed.
Outcomes data included treatment completion and treatment breaks (dichotomized to yes or no), based on data obtained above. Survival data were recorded via information in the medical record and Social Security Death Index. Vital status was recorded as dead or alive. Disease status at last follow-up visit was documented as no evidence of disease, alive with disease, alive (unknown disease status), dead of disease, dead of other cause, or dead of unknown cause.
Statistical Analysis
Statistical analysis was completed using SAS, version 9.2 (SAS Institute, Cary, North Carolina). P values less than .05 were considered statistically significant. Categorical data from patients treated in the community versus academic centers were compared using the χ2 or Fisher exact test. Continuous (or ordered) data items were compared using the Wilcoxon rank sum test. A survival analysis was completed to compare the survival rate over time using the Kaplan-Meier method and the log-rank test. A Cox proportional hazard model identified risk factors that were significantly related to survival using a backward selection approach. Variables related to survival with a P value of less than .1 were initially included. The final model included type of institution plus any factors that were significant with a P value <.05.
Finally, a propensity analysis was used to address differences in the nonrandomized treatment allocation, by predicting the probability for an individual patient to receive radiation in an academic versus community setting17 using multiple logistic regression. The predictors are the patient characteristics and pretreatment factors that were significantly related to the type of institution or the outcome of survival. Goodness of fit of the final logistic model was verified using the Hosmer and Lemeshow test (P = .77). Because of missing information on marital status, the probability of being married was imputed for 8 of 326 subjects (2.5%). The propensity analysis was completed via 2 methods. In method I, subjects were divided into 5 subgroups based on their propensity scores, with an equal number of subjects in each subgroup. As a result, within each propensity subgroup, the subjects were balanced for these covariates that might be associated with radiation in an academic hospital. The Cox regression model for overall survival, including hospital type as a covariate, was stratified by the propensity subgroup. In method II, as an alternative to stratifying the analysis based on the propensity score, the predicted probability was used as a continuous covariate in the Cox regression model together with hospital type.
Results
Our ICD-9 code search identified 388 patients with mucosal head and neck cancer treated with radiation therapy from 2003 to 2008. We could not determine site of treatment for 33 patients, and these patients were therefore excluded. Of the 355 patients remaining, 145 (41%) were treated at community hospitals and 210 (59%) at academic hospitals. Of the group treated at academic hospitals, 197 underwent radiation at the University of Minnesota, whereas 13 received radiation at an alternative academic center.
Patient Characteristics
Demographic variables are recorded in Table 1. Treatment groups were similar with regard to sex, comorbidity, marital status, work status, insurance, and alcohol use. The community group had more current smokers and slightly older patients. This patient population traveled considerably farther to reach the university and lived in lower income areas relative to the university population. Patients treated in an academic setting were much more likely to live in an urban location.
Table 1.
Study Population: Demographics
| Categorical Data Items | Community, No. (%) | Academic, No. (%) | P Valuea |
|---|---|---|---|
| Total | 145 | 210 | |
| Sex | 1.000 | ||
| Male | 102 (70.3) | 147 (70.0) | |
| Female | 43 (29.7) | 63 (30.0) | |
| Smoker | .017b | ||
| Never or former | 78 (53.8) | 136 (64.8) | |
| Current | 63 (43.4) | 63 (30.0) | |
| Unknown | 4 (2.8) | 11 (5.2) | |
| Comorbidities | |||
| Diabetes | .220 | ||
| Yes | 14 (9.7) | 12 (5.7) | |
| No | 130 (89.7) | 187 (89.0) | |
| Unknown | 1 (0.7) | 11 (5.2) | |
| Heart disease | .149 | ||
| Yes | 24 (16.6) | 22 (10.5) | |
| No | 120 (82.8) | 178 (84.8) | |
| Unknown | 1 (0.7) | 10 (4.8) | |
| Stroke | 1.000 | ||
| Yes | 2 (1.4) | 2 (1.0) | |
| No | 142 (97.9) | 198 (94.3) | |
| Unknown | 1 (0.7) | 10 (4.8) | |
| Lung disease | .068 | ||
| Yes | 24 (16.6) | 19 (9.0) | |
| No | 120 (82.8) | 181 (86.2) | |
| Unknown | 1 (0.7) | 10 (4.8) | |
| Arthritis | .400 | ||
| Yes | 20 (13.8) | 21 (10.0) | |
| No | 124 (85.5) | 179 (85.2) | |
| Unknown | 1 (0.7) | 10 (4.8) | |
| Psychiatric disease | .614 | ||
| Yes | 19 (13.1) | 22 (10.5) | |
| No | 125 (86.2) | 177 (84.3) | |
| Unknown | 1 (0.7) | 11 (5.2) | |
| Marital status at diagnosis | .363 | ||
| Married | 83 (57.2) | 135 (64.3) | |
| Not married | 55 (38.0) | 72 (34.3) | |
| Unknown | 7 (4.8) | 3 (1.4) | |
| Work status at diagnosis | .154 | ||
| Employed | 66 (45.5) | 119 (56.6) | |
| Not employed | 42 (29.0) | 52 (24.8) | |
| Unknown | 37 (25.5) | 39 (18.6) | |
| Insurance | .350 | ||
| No insurance | 1 (0.7) | 0 | |
| Private | 71 (49.0) | 116 (55.2) | |
| Medicare/Medicaid | 55 (37.9) | 68 (32.4) | |
| Other government insurance | 2 (1.4) | 5 (2.4) | |
| Other | 0 | 0 | |
| Unknown | 16 (11.0) | 21 (10.0) | |
| Rural/urban (code = 1) | 80/145 (55.2) | 170/210 (81.0) | <.001b |
|
| |||
| Ordered or Continuous Data Items | Community, Median (Min/Max) | Academic, Median (Min/Max) | P Valuec |
|
| |||
| Age at diagnosis, y | 58 (21/92) | 56 (19/90) | .043b |
| Unknown, No. (%) | 1 (0.7) | 0 | |
| Drinker | .549 | ||
| Never | 36 (24.8) | 45 (21.4) | |
| Former | 37 (25.5) | 53 (25.2) | |
| Current | 67 (46.2) | 100 (47.6) | |
| Unknown | 5 (3.4) | 12 (5.7) | |
| Days between first and last visit | 646 (25/2711) | 1020 (60/6895) | <.001b |
| Distance from home, miles | 34 (2/349) | 16 (1/1499) | <.001b |
| Income, $ | $45,147 (24,028/95,372) | $54,358 (20,330/100,270) | .001b |
| Unknown, No. (%) | 0 | 1 (0.5) | |
The P value was derived from the χ2 or the Fisher exact test.
Indicates statistical significance with a P <.05.
The P value was derived from the Wilcoxon rank sum test.
Cancer-specific variables are recorded in Table 2. Anatomic site of tumor origin differed between the community and academic treatment groups; the academic group tended to have a greater number of oral cavity/oropharynx primaries and fewer hypopharynx/larynx tumors. Further delineation of the oral cavity and oropharynx tumors showed that 59.6% of oral cavity and 70.3% of oropharynx primaries were treated at an academic institution, whereas 40.4% of oral cavity and 29.7% of oropharynx primaries were treated at a community center (P = .038 for academic vs community oral cavity/oropharynx). The patients treated in an academic setting had more advanced cancer as measured by N classification and overall TNM stage. T and M classification did not differ between groups. Of the patients who underwent surgery at the university, the rate of positive margins was not different between community and academic patients, but the rate of extracapsular spread from lymph nodes was greater in the academic group. Follow-up time was longer in the academic treatment group.
Table 2.
Cancer End Points
| Categorical Data Items | Community, No. (%) | Academic, No. (%) | P Valuea |
|---|---|---|---|
| Primary tumor site | <.001b | ||
| Oral cavity | 40 (27.6) | 58 (27.6) | |
| Oropharynx | 30 (20.7) | 71 (33.8) | |
| Hypopharynx/larynx | 49 (33.8) | 31 (14.8) | |
| Sinus/nose | 14 (9.7) | 27 (12.9) | |
| Other | 9 (6.2) | 23 (11.0) | |
| Unknown | 3 (2.1) | 0 | |
| Positive margins | .096 | ||
| Yes | 25 (17.2) | 23 (11.0) | |
| No | 64 (44.1) | 107 (51.0) | |
| Unknown | 56 (38.6) | 80 (38.1) | |
| Nodes with extracapsular spread | .030b | ||
| Yes | 13 (9.0) | 37 (17.6) | |
| No | 68 (46.9) | 87 (41.4) | |
| Unknown | 64 (44.1) | 86 (41.0) | |
| Disease status at last follow-up: | .009b | ||
| Alive, no evidence of disease | 58 (40.0) | 115 (54.8) | |
| Alive with disease or dead | 83 (57.2) | 92 (43.8) | |
| Unknown | 4 (2.8) | 3 (1.4) | |
| Alive at last follow-up | .065 | ||
| Yes | 69 (47.6) | 121 (57.6) | |
| No | 75 (51.7) | 87 (41.4) | |
| Unknown | 1 (0.7) | 2 (1.0) | |
|
| |||
| Ordered or Continuous Data Items | Community, Median (Min/Max) | Academic, Median (Min/Max) | P Valuec |
|
| |||
| T classification | .558 | ||
| 1 | 25 (17.2) | 32 (15.2) | |
| 2 | 31 (21.4) | 54 (25.7) | |
| 3 | 21 (14.5) | 38 (18.1) | |
| 4 | 37 (25.5) | 41 (19.5) | |
| Unknown | 31 (21.4) | 45 (21.4) | |
| N classification | .006b | ||
| 0 | 57 (39.3) | 60 (28.6) | |
| 1 | 22 (15.2) | 24 (11.4) | |
| 2 | 32 (22.1) | 80 (38.1) | |
| 3 | 5 (3.4 ) | 6 (2.9) | |
| Unknown | 29 (20.0) | 40 (19.0) | |
| M Classification (M1) | 2/115 (1.7) | 4/171 (2.3) | 1.000 |
| TNM stage | .024b | ||
| 1 | 15 (10.3) | 13 (6.2) | |
| 2 | 18 (12.4) | 20 (9.5) | |
| 3 | 23 (15.9) | 29 (13.8) | |
| 4 | 58 (40.0) | 108 (51.4) | |
| Unknown | 31 (21.4) | 40 (19.0) | |
The P value was derived from the χ2 or the Fisher exact test.
Indicates statistical significance with a P <.05.
The P value was derived from the Wilcoxon rank sum test.
Radiation-specific data are documented in Table 3. The vast majority of the cases in this study completed radiation treatment (94.3%), and there was no significant difference in the completion rate between community hospitals (93.7%) and academic hospitals (94.7%). Correspondingly, there was a similar rate of radiation treatment breaks for community and university hospitals, 22.4% and 28.4%, respectively. The academic treatment group had a greater number of patients who received chemotherapy with radiation (over radiation alone, P <.001).
Table 3.
Radiation Results
| Categorical Data Items | Community, No. (%) | Academic, No. (%) | P Valuea |
|---|---|---|---|
| Type of radiation | .447 | ||
| Primary | 64 (44.1) | 100 (47.6) | |
| Adjuvant | 79 (54.5) | 104 (49.5) | |
| Unknown | 2 (1.4) | 6 (2.9) | |
| Type of radiation | <.001b | ||
| Primary or adjuvant without chemotherapy | 69 (47.6) | 60 (28.6) | |
| Primary or adjuvant with chemotherapy | 74 (51.0) | 144 (68.6) | |
| Unknown | 2 (1.4) | 6 (2.9) | |
| Outside treatment for chemotherapy | <.001b | ||
| Yes | 70 (48.3) | 5 (2.4) | |
| No | 74 (51.0) | 204 (97.1) | |
| Unknown | 1 (0.7) | 1 (0.5) | |
| Previous radiation | .147 | ||
| Yes | 0 (0.0) | 4 (1.9) | |
| No | 145 (100) | 204 (97.1) | |
| Unknown | 0 (0.0) | 2 (1.0) | |
|
| |||
| Continuous Data Items | Community, Median (Min/Max) | Academic, Median (Min/Max) | P Valuec |
|
| |||
| Planned treatment | 35 (10/62) | 35(15/65) | .573 |
| Unknown, No. (%) | 32 (22.1) | 12 (5.7) | |
| Actual treatment | 34 (4/62) | 34 (1/65) | .584 |
| Unknown, No. (%) | 27 (18.6) | 7 (3.3) | |
| Planned dose, cGy | 6800 (3000/7740) | 6996 (1840/8000) | .967 |
| Unknown, No. (%) | 21 (14.5) | 11 (5.2) | |
| Actual dose, cGy | 6660 (720/7740) | 6643 (200/8000) | .937 |
| Unknown, No. (%) | 15 (10.3) | 5 (2.4) | |
The P value was derived from the χ2 or the Fisher exact test.
Indicates statistical significance with a P <.05.
The P value was derived from the Wilcoxon rank sum test.
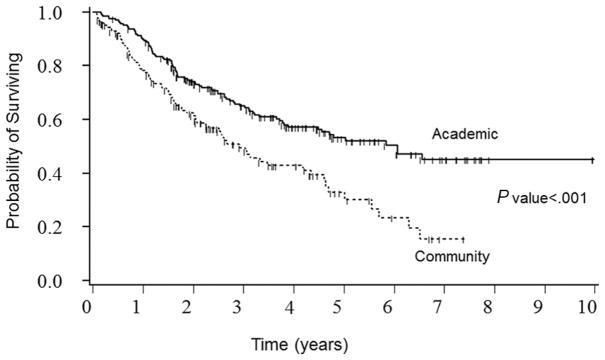
Outcomes varied by site of treatment. The patients treated at the academic centers had a better disease-free survival. The survival rate was also higher in academic centers (log-rank P <.001; Figure 1). The 5-year survival rate and 95% confidence interval (CI) for academic and community hospitals were 53.2% (CI, 45.3%–61.1%) and 32.8% (CI, 22.0%–43.6%), respectively. As seen below, on multivariate analysis, several other variables affected outcomes, including marital status, smoking status, and some medical comorbidities.
Figure 1.
Kaplan-Meier survival curves by type of treating institution.
Because treatment was not randomized, we used a Cox proportional hazards multivariate model to understand which variables had the greatest impact on survival. Results from the univariate analysis are shown in Table 4. Our initial model included the type of treating institution and other significant risk factors and included the 352 cases with information on survival status and survival time plus a classification for type of hospital for this analysis (data could not be obtained in 3 patients). As shown in the final model in Table 5, after adjustment for significant confounding variables (P < .05), including marital status, smoking status, lung disease, and arthritis, patients treated at the academic hospitals had a lower risk of dying, with a risk ratio of 0.7 (P = .049; CI, 0.5–1.0). There were no significant interactions between hospital setting and the other significant covariates in the final model. A test of the proportional hazards assumption for type of hospital showed that this property was not violated (P > .7); therefore, the decreased risk associated with academic hospitals was consistent over the follow-up time. Although the risk of nonsurvival was 30% lower in the academic setting, the 95% confidence interval cannot exclude trivial or no differences. Thus, from this portion of the analysis alone, one cannot conclude, with any reasonable degree of certainty, the true impact of academic versus community setting on outcomes.
Table 4.
Univariate Cox Models for Patient and Treatment Characteristicsa Related to Overall Survival
| Risk Factor | No. | Coefficient (SE) | Wald χ2 | Risk Ratio | 95% CI for Risk Ratio | P Value |
|---|---|---|---|---|---|---|
| Academic hospital | 352 | −0.60 (0.16) | 14.40 | 0.55 | 0.40–0.75 | <.001 |
| Female sex | 385 | 0.21 (0.16) | 1.66 | 1.23 | 0.90–1.69 | .197 |
| Age at diagnosis, per year | 384 | 0.02 (0.01) | 10.39 | 1.02 | 1.01–1.03 | .001 |
| Income, per $10,000 | 384 | −0.10 (0.05) | 4.17 | 0.91 | 0.82–1.00 | .041 |
| Married | 375 | −0.55 (0.16) | 12.14 | 0.58 | 0.43–0.79 | .001 |
| Distance from home, per 100 miles | 385 | 0.03 (0.05) | 0.423 | 1.03 | 0.94–1.13 | .516 |
| Private insurance | 340 | −0.36 (0.16) | 4.99 | 0.70 | 0.51–0.96 | .026 |
| Metro zip code | 385 | −0.19 (0.17) | 1.28 | 0.83 | 0.60–1.15 | .259 |
| Drink alcohol | 362 | |||||
| Never [reference] | 1.00 | |||||
| Former | 0.15 (0.23) | 0.46 | 1.17 | 0.75–1.82 | .499 | |
| Current | 0.13 (0.20) | 0.39 | 1.13 | 0.77–1.68 | .533 | |
| Current smoker | 365 | 0.62 (0.16) | 15.11 | 1.85 | 1.36–2.53 | <.001 |
| Lung disease | 369 | 0.96 (0.20) | 24.20 | 2.62 | 1.78–3.84 | <.001 |
| Arthritis | 369 | 0.51 (0.22) | 5.45 | 1.66 | 1.09–2.54 | .020 |
| Diabetes | 368 | 0.66 (0.25) | 7.03 | 1.94 | 1.19–3.17 | .008 |
| Heart disease | 369 | 0.49 (0.21) | 5.53 | 1.63 | 1.09–2.45 | .019 |
| Depression/mental illness | 367 | 0.45 (0.23) | 3.81 | 1.57 | 1.00–2.46 | .051 |
| Tumor site | 385 | |||||
| Oral cavity/oropharynx [reference] | 1.00 | |||||
| Hypopharynx/larynx | 0.40 (0.18) | 4.98 | 1.49 | 1.05–2.11 | .026 | |
| Sinus/nose | 0.31 (0.23) | 1.75 | 1.36 | 0.86–2.16 | .186 | |
| Other | −0.10 (0.31) | 0.10 | 0.91 | 0.50–1.66 | .755 | |
| Stage TNM | 301 | 0.04 (0.09) | 0.25 | 1.05 | 0.88–1.24 | .614 |
| Adjuvant radiation | 374 | 0.12 (0.16) | 0.58 | 1.13 | 0.83–1.53 | .445 |
| Radiation with chemotherapy | 374 | 0.15 (0.16) | 0.83 | 1.16 | 0.84–1.59 | .363 |
Abbreviations: CI, confidence interval; SE, standard error.
Other patient and treatment factors were excluded due to a very low prevalence rate, too many missing values, or colinearity with other covariates.
Table 5.
Multivariate Cox Proportional Hazard Model for Overall Survival (n = 326)
| Risk Factor | Coefficient (SE) | Wald χ2 | Risk Ratio | 95% CI for Risk Ratio | P Value | 5-Year Survival Rate (SE)a
|
|
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Current smoker | 0.52 (0.18) | 8.57 | 1.67 | 1.19–2.36 | .003 | 54.4 (4.5) | 29.1 (5.4) |
| Married | −0.34 (0.17) | 3.92 | 0.71 | 0.51–1.00 | .048 | 32.3 (5.5) | 52.0 (4.4) |
| Lung disease | 0.85 (0.21) | 15.54 | 2.33 | 1.53–3.55 | <.001 | 49.8 (3.8) | 7.4 (6.4) |
| Arthritis | 0.58 (0.22) | 6.57 | 1.78 | 1.15–2.76 | .010 | 48.2 (3.7) | 21.3 (8.5) |
| Academic hospital | −0.34 (0.17) | 3.89 | 0.71 | 0.51–1.00 | .049 | 30.7 (5.9) | 52.2 (4.3) |
Abbreviations: CI, confidence interval; SE, standard error.
The 5-year survival was derived from the univariate analysis for each factor.
Our propensity analysis developed a model to estimate the predicted probability for each individual to receive radiation at an academic center instead of a community hospital (Table 6), and these scores were used in the survival analyses. The propensity score for 326 subjects had a median of 0.60 with a range of 0.03 to 0.91. In model 1 (Table 7), the estimated risk of dying in an academic hospital was 63% of the risk in the community hospital after stratifying the analysis by the 5 ordered subgroups that were based on the likelihood to be treated in an academic hospital. In model II, the propensity score is a regression adjustment added to the survival analysis (Table 8). The estimated risk of dying following radiation treatment in an academic hospital was again 63% of the risk in the community hospital. The similar results achieved in models I and II demonstrate the robustness of our propensity score.
Table 6.
Logistic Regression with Variables Significantly Related to Type of Institution or Survival to Create the Propensity Score for Treatment at an Academic Hospital
| Risk Factor | Coefficient (SE) | Wald χ2 | Odds Ratio | 95% CI for Odds Ratio | P Value |
|---|---|---|---|---|---|
| Current smoker | −0.47 (0.26) | 3.17 | 0.63 | 0.37–1.05 | .075 |
| Metro zip code | −0.22 (0.07) | 10.57 | 0.81 | 0.71–0.92 | .001 |
| Age at diagnosis, per year | −0.02 (0.01) | 2.32 | 0.98 | 0.97–1.00 | .128 |
| Distance from home, per 100 miles | 0.13 (0.11) | 1.27 | 1.13 | 0.91–1.41 | .260 |
| Income, per $10,000 | 0.04 (0.09) | 0.20 | 1.04 | 0.87–1.25 | .655 |
| Currently married | 0.16 (0.27) | 0.37 | 1.18 | 0.69–2.00 | .545 |
| Arthritis | −0.38 (0.39) | 0.93 | 0.69 | 0.32–1.48 | .335 |
| Lung disease | −0.74 (0.40) | 3.45 | 0.48 | 0.22–1.04 | .063 |
| Radiation with chemotherapy | 1.05 (0.27) | 15.48 | 2.87 | 1.70–4.85 | <.001 |
| Tumor site | |||||
| Oral cavity/oropharynx [reference] | 1.00 | ||||
| Hypopharynx/larynx | −0.82 (0.31) | 7.05 | 0.44 | 0.24–0.81 | .008 |
| Sinus/nose | 0.06 (0.40) | 0.02 | 1.06 | 0.48–2.31 | .887 |
| Other | 0.29 (0.50) | 0.34 | 1.33 | 0.51–3.52 | .562 |
Stage was excluded from the analysis due to the degree of missing information. Abbreviations: CI, confidence interval; SE, standard error.
Table 7.
Model I: Cox Model for Overall Survival Stratified by Propensity Subgroups (n = 326)
| Risk Factor | Coefficient (SE) | Wald χ2 | Risk Ratio | 95% CI for Risk Ratio | P Value |
|---|---|---|---|---|---|
| Academic hospital | −0.47 (0.19) | 5.93 | 0.63 | 0.43–0.91 | .015 |
Abbreviations: CI, confidence interval; SE, standard error.
Table 8.
Model II: Cox Model for Overall Survival Including Propensity Score (n = 326)
| Risk Factor | Coefficient (SE) | Wald χ2 | Risk Ratio | 95% CI for Risk Ratio | P Value |
|---|---|---|---|---|---|
| Propensity score (0.1 increase) | −0.12 (0.04) | 7.35 | 0.89 | 0.82–0.97 | .007 |
| Academic hospital | −0.46 (0.19) | 5.97 | 0.63 | 0.43–0.91 | .015 |
Abbreviations: CI, confidence interval; SE, standard error.
Discussion
Radiation therapy is a mainstay of head and neck cancer treatment, both as a primary and adjuvant treatment. When this therapy is required, patients traveling from far distances must choose where to receive treatment. This decision may be complex, depending on a variety of motivating factors—financial, social, geographical, cancer specific, disease specific, or other. It is important to understand differences in outcomes related to type of treating institution as well as the differences in patient populations that receive treatment at community versus academic centers.
Our historical cohort study demonstrated that receiving radiation therapy at an academic center for mucosal head and neck cancer was associated with improved survival over treatment at a community center after accounting for other patient characteristics. The observed survival differences were large (30% relative differences). Our findings were robust to multivariable and also 2 approaches to propensity analyses, supporting the thesis that there is a significant survival advantage for patients treated in an academic setting.
Our findings support those of the Benasso et al15 study in which patients with head and neck cancer treated with chemoradiotherapy at the coordinating center of a clinical trial had improved survival over those treated at affiliated centers. A similar survival advantage was not appreciated by Kubicek and colleagues,16 who reviewed their cohort of patients with head and neck cancer treated by radiation therapy and reported a similar sample size and patient population. The authors suggest that subset analysis in their study may not have had the power to detect a difference in the groups. The work by Chen et al9,10 on laryngeal cancer is consistent with our findings. In these large database studies, treatment for early and advanced stage larynx cancer at both a high volume site and a teaching facility was associated with improved survival.
It is important to note that we found higher survival at academic centers despite the fact that patients radiated at a university had more advanced N classification and TNM stage. They more often received chemotherapy as well. Patients treated at both types of centers, however, had similar rates of completion of planned treatment and treatment breaks, and therefore our hypothesis that improved survival at the academic centers would be secondary to fewer treatment breaks was rejected. Our findings suggest that more subtle differences in treatment administration and support at academic centers need to be investigated to understand the survival differences. Perhaps technological differences exist in equipment used for radiation treatment or the strength of supportive care is unequal in academic versus community centers. For example, intensity-modulated radiation therapy (IMRT) is frequently used in both academic and community settings for treating head and neck cancer. Accurate IMRT target definition and treatment planning for head and neck cancers are time intensive and involve a learning curve for both physician and physicist. Academic centers more often have both radiation oncologists who specialize in head and neck cancer and peer review of treatment plans. Also, participation in multidisciplinary care teams enables the coordination of more complex supportive care during and following completion of radiation. Alternatively, unknown or immeasurable differences in patient characteristics between the 2 treatment locations could factor into survival differences.
Patients choosing to undergo radiation in the community instead of at an academic center had several other defining characteristics that were adjusted for in our analysis but may point to the underlying causes for survival differences. The community group had greater numbers of current smokers, which resulted in a 1.7 times increased risk of dying over nonsmokers. In addition, patients choosing treatment in the community tended to reside in less urban locations and had to travel approximately twice the distance of the academic group to reach our clinic. Patients receiving radiation in the community lived in zip codes with considerably lower median household incomes ($45,147 vs $54,358, P = .001). Despite this, insurance status was not different between groups. The income differences between groups and the associated survival differences in our study echo the findings of Groome and colleagues,18 who found diminishment in cause-specific survival and increased locoregional failure associated with lower socioeconomic status in glottic cancer. These potential disparities in care related to income, socioeconomic status, and geography should be further explored.
There are several limits of our study. These include the nonrandomized nature of treatment allocation in our retrospective cohort. However, these kinds of treatment allocation are not feasible through randomization, and this type of study methodology is the best approach to obtain preliminary insight. We also did not control for treatment volume; therefore, our findings may just reflect the previously noted finding that high-volume treatment centers tend to have better outcomes. The propensity score stratification balance was assessed using the technique proposed by Rosenbaum and Rubin19 and verified that patients in the academic and community setting have similar distributions of the covariates within each stratum. The main limitation of propensity analysis in observational studies is that they do not control for unobserved covariates or hidden bias; however, we considered all possible information available for the subjects in our analysis. As is frequently a problem with retrospective studies, missing information can limit the usefulness of some otherwise important variables. Finally, information that may shed more light on patient decision making when choosing a treatment site could not be obtained from the medical record.
Conclusion
Our historical cohort study of patients with head and neck cancer suggests that despite similar rates of treatment completion and rate of treatment breaks between groups, patients treated in academic centers had more advanced cancer but better survival. Further evaluation of the underlying etiologies for these differences should be sought to improve survival for all patients with head and neck cancer.
Acknowledgments
The authors express their most sincere thanks to the American Academy of Otolaryngology–Head and Neck Surgery for graciously supporting this work via the Rande H. Lazar Health Services Research Grant.
Footnotes
Author Contributions
Amy Anne D. Lassig, study conception and design, interpretation of data, drafting article; Anne M. Joseph, study design, revising article; Bruce R. Lindgren, statistical analysis, revising article; Patricia Fernandes, acquisition of data, drafting and revising article; Sarah Cooper, acquisition of data, revising article; Chelsea Schotzko, acquisition of data, revising article; Samir Khariwala, study design, revising article; Margaret Reynolds, acquisition of data, revising article; Bevan Yueh, study design, interpretation of data, revising article.
Disclosures
Competing interests: None.
Sponsorships: Rande H. Lazar Health Services Research Grant.
Funding source: American Academy of Otolaryngology–Head and Neck Surgery.
References
- 1.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the Twenty-First Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 2.Lewis CM, Hessel AC, Roberts DB, et al. Prereferral head and neck cancer treatment: compliance with National Comprehensive Cancer Network treatment guidelines. Arch Otolaryngol Head Neck Surg. 2010;136:1205–1211. doi: 10.1001/archoto.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhoef C, Van de Weyer R, Schaapveld M, Bastiaannet E, Plukker JT. Better survival in patients with esophageal cancer after surgical treatment in university hospitals: a plea for performance by surgical oncologists. Ann Surg Oncol. 2007;14:1678–1687. doi: 10.1245/s10434-006-9333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98:163–171. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 5.Gouma DJ, Van Geenen RC, Van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez JC, Perez EA, Moffat FL, Livingstone AS, Franceschi D, Koniaris LG. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245:952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung MC, Hamilton K, Sherman R, et al. Impact of teaching facility status and high-volume centers on outcomes for lung cancer resection: an examination of 13,469 surgical patients. Ann Surg Oncol. 2009;16:3–13. doi: 10.1245/s10434-008-0025-9. [DOI] [PubMed] [Google Scholar]
- 8.Zirasagar S, Lien YC, Lin HC, Lee HC, Liu TC, Tsai J. Procedure volume of gastric cancer resections versus 5-year survival. Eur J Surg Oncol. 2008;34:23–29. doi: 10.1016/j.ejso.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Chen AY, Pavluck A, Halpern M, Ward E. Impact of treating facilities’ volume on survival for early-stage laryngeal cancer. Head Neck. 2009;31:1137–1143. doi: 10.1002/hed.21072. [DOI] [PubMed] [Google Scholar]
- 10.Chen AY, Fedewa S, Pavluck A, Ward EM. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116:4744–4752. doi: 10.1002/cncr.25364. [DOI] [PubMed] [Google Scholar]
- 11.Akman FC, Dag N, Ataman OU, et al. The impact of treatment center on the outcome of patients with laryngeal cancer treated with surgery and radiotherapy. Eur Arch Otorhinolaryngol. 2008;265:1245–1255. doi: 10.1007/s00405-008-0664-2. [DOI] [PubMed] [Google Scholar]
- 12.Fowler JF. Biological factors influencing optimum fractionation in radiation therapy. Acta Oncologica. 2001;40:712–717. doi: 10.1080/02841860152619124. [DOI] [PubMed] [Google Scholar]
- 13.Suwinski R, Sowa A, Rutkowski T. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56:399–412. doi: 10.1016/s0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 14.Groome PA, O’Sullivan B, Mackillop WJ, et al. Compromised local control due to treatment interruptions and late treatment breaks in early glottic cancer: population-based outcomes study supporting need for intensified treatment schedules. Int J Radiat Oncol Biol Phys. 2006;64:1002–1012. doi: 10.1016/j.ijrobp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Benasso M, Lionetto R, Corvo R, Ponzanelli A, Vitale V, Rosso Impact of the treating institution on the survival of patients with head and neck cancer treated with concomitant alternating chemotherapy and radiation. Eur J Cancer. 2003;39:1895–1898. doi: 10.1016/s0959-8049(03)00487-8. [DOI] [PubMed] [Google Scholar]
- 16.Kubicek GJ, Wang F, Reddy E, Shnayder Y, Cabrera CE, Girod DA. Importance of treatment institution in head and neck cancer radiotherapy. Otolaryngol Head Neck Surg. 2009;141:172–176. doi: 10.1016/j.otohns.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 18.Groome PA, Schulze KM, Keller S, et al. Explaining socioeconomic status effects in laryngeal cancer. Clin Oncol (R Coll Radiol) 2006;18:283–292. doi: 10.1016/j.clon.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]