Abstract
Background
Intrauterine infection is a significant cause of early preterm birth. We have developed a fetal-neonatal model in the rhesus macaque to determine the impact of chronic intrauterine infection with Ureaplasma parvum on early neonatal reflexes and brain development.
Methods
Time-mated, pregnant rhesus macaques were randomized to be inoculated with U. parvum (serovar 1; 105cfu) or control media at ~120 dGA. Neonates were delivered by elective hysterotomy at 135–147 dGA (term=167d) stabilized and cared for in our nonhuman primate neonatal intensive care unit. Neonatal reflex behaviors were assessed from birth and fetal and postnatal brain MRIs were performed.
Results
A total of 13 preterm and 5 term macaque infants entered the study. 10 preterm infants survived to 6 months of age. U. parvum infected preterm neonates required more intensive respiratory support than control infants. MRI suggest potential perturbation of brain growth and white matter maturation with exposure to intra-amniotic infection.
Conclusion
We have demonstrated the feasibility of longitudinal fetal-neonatal studies in the preterm rhesus macaque after chronic intrauterine infection. Future studies will examine long-term neurobehavioral outcomes, cognitive development, neuropathology and in vivo brain imaging to determine the safety of antenatal antibiotic treatment for intrauterine infection.
INTRODUCTION
Intrauterine infection can lead to premature labor and preterm birth during early gestation1. Ureaplasma spp. are microbes most commonly associated with preterm birth, identified in 47% of placentae from women in preterm labor with histological chorioamnionitis 2. Early preterm birth (<28 weeks gestation) is associated with high mortality and morbidity in survivors as well as a disproportionate share of perinatal health care costs 3. Bronchopulmonary dysplasia and neurodevelopmental abnormalities are sequelae of early prematurity associated with intrauterine infectious and inflammatory processes 4.
Studies from our laboratory have established a nonhuman primate (NHP) model of infection-induced preterm birth using intra-amniotic inoculation with U. parvum 5. This maternal-fetal model results in a robust intrauterine pro-inflammatory response, a sequential up-regulation of amniotic fluid (AF) leukocytes, pro-inflammatory cytokines, prostaglandin E2 and F2α and matrix metalloproteinase-9, increased uterine activity leading to premature labor 5. Histological chorioamnionitis and a systemic fetal inflammatory response, fetal lung injury and pneumonitis were also observed. Clinically, isolation of Ureaplasma spp. in amniotic fluid from preterm births is associated with adverse psychomotor development and increased risk of cerebral palsy diagnosis at 2 years age 6. Additionally, intra-amniotic U. parvum inoculation has been associated with central microgliosis and disrupted neuronal development in the neocortex of fetal mouse brains 7. However, the long-term, neurodevelopmental effects of perinatal Ureaplasma infections have not been adequately studied 8.
This paper describes our new model for the assessment of postnatal outcomes following chronic intra-amniotic infection with Ureaplasma parvum. This model enables the longitudinal study of physiological and neurodevelopmental consequences of infection-associated preterm birth and provides a platform for the assessment of insults across the perinatal period, along with evaluation of new antenatal and postnatal therapeutic strategies.
METHODS
Maternal-Fetal Animal Model of Chronic Intra-Amniotic Infection
Study protocols were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee. Strict guidelines for humane care were followed, with adherence to the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Animals were allocated to the study by assignment from the ONPRC breeding colony. Time-mated pregnant rhesus monkeys (Macaca mulatta) were adapted to a vest and mobile catheter protection device 9 and intrauterine surgery was performed at a mean of 106 days gestational age (dGA, range: day 101–111; n=12) to implant catheters in the amniotic cavity, and the maternal femoral vein and artery. Pre- and post-operative Cefazolin sodium (Apotex Çorp., Weston, FL USA) was administered intravenously to prevent infection, and tocolytic medications (e.g., Terbutaline sulfate and/or Atosiban) were used to control post-surgical uterine irritability as previously published 5,10. Animals were divided into three groups as shown in Fig. 1, with experiments performed concurrently.
Fig 1. Flowchart of Study Groups.
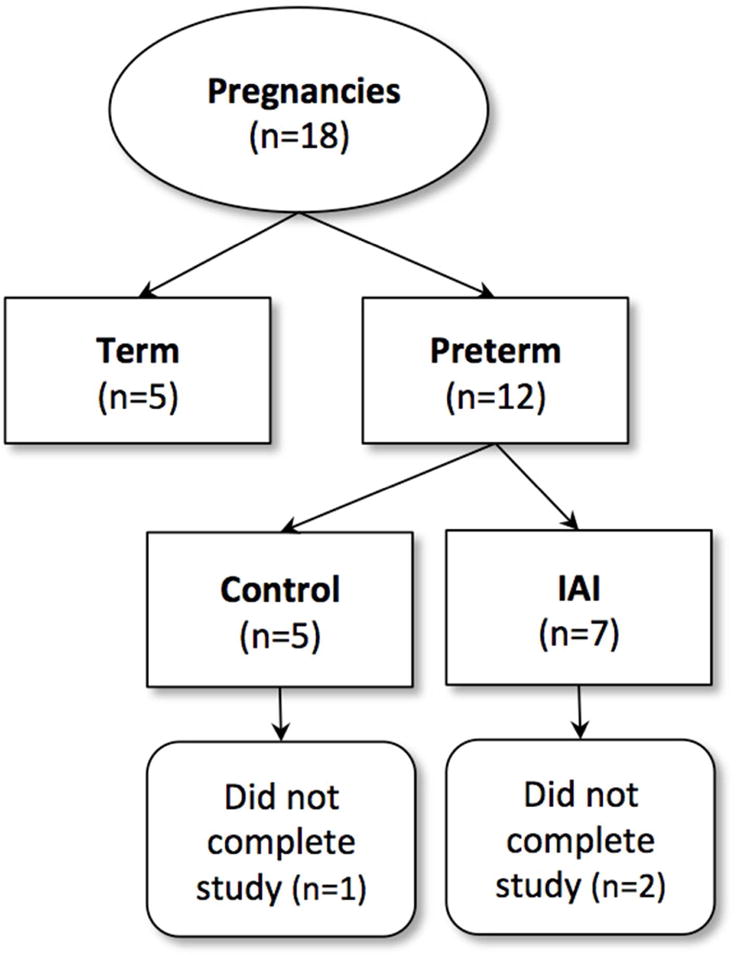
Preterm infants from catheterized pregnancies were randomized to control and intra-amniotic infection (IAI). An additional group of term control animals were obtained from non-catheterized pregnancies from the ONPRC colony. Deaths that occurred during the neonatal period prior to the completion of the study.
Microorganism
As previously published 5,10 a low passaged clinical isolate of U. parvum (serovar 1) (Diagnostic Mycoplasma Laboratory, University of Alabama at Birmingham, AL) was utilized. To induce chronic U. parvum IAI, intra-amniotic inoculations with 1.4×105 CFU/mL were performed at 124±1.9 days gestational. Sterile media was administered to gestational-age matched controls.
Maternal-Fetal Physiological Monitoring and Sampling
Uterine activity
Intra-amniotic pressure was recorded, digitized and analyzed using PowerLab system and LabChartPro (ADInstruments, Colorado Springs, CO) 10. The integrated area under the curve for intra-amniotic pressure was used as the measure of uterine activity and reported as the mean of the cumulative hourly contraction area (HCA, mmHg.sec/hr). The average peak HCA per day prior to inoculation (with U. parvum or media control) represents baseline contractility and was compared to the peak HCA per day from inoculation until delivery (post-inoculation).
Amniotic Fluid (AF) Cytokines
AF was sampled at 0, 12, 24, 48 and 72hrs post-inoculation and then twice weekly. AF concentrations of the pro-inflammatory mediators interleukin-1β (IL-1β); IL-6; tumor necrosis factor-alpha (TNF-α) and prostaglandins PGE2 and PGF2α were determined using commercially available human or rhesus monkey–specific enzyme-linked immunosorbent assay kits (Life Technologies, Grand Island, NY USA) previously validated for use in the rhesus monkey 5,10,11. The minimum detectable levels of IL-1β, IL-6 and TNF-α assays were <1–2pg/mL. The inter-assay coefficient of variance was below 15% for all assays.
Culture and PCR Detection of U.parvum
Quantitative cultures/PCR for U. parvum were performed on samples of AF, placenta, fetal membranes and neonatal nasal swabs 10,12,13.
Preterm Delivery and Neonatal Intensive Care
Elective hysterotomies were performed for delivery of preterm infants between 135–147 dGA (n=12). All preterm neonates were immediately transferred to our Special Care Nursery for resuscitation and stabilization. Control and IAI preterm infants received prophylactic postnatal azithromycin (20mg/kg/d, IV for 5 days, Fresenius Kabi USA, LLC, Grand Island, NY) to reflect standard clinical practice for suspected neonatal Ureaplasma infection and to prevent secondary pneumonitis 14,15.
Respiratory Stabilization and Support
A standardized neonatal resuscitation algorithm (modified Neonatal Resuscitation Program 16) was utilized to ensure consistency across experimental groups. Neonatal resuscitation began with room air (21% inspired oxygen concentration (FiO2)) and positive pressure ventilation (PPV) using a Neo-Tee® infant T-piece Resuscitator (Mercury Medical, Clearwater, FL). Targeted peak inspiratory pressure (PIP, 20–30cm/H2O) and positive end-expiratory pressure (PEEP, 5cm/H2O) were maintained. Neonates with clinical evidence of respiratory failure and/or signs of increasing respiratory distress (e.g. tachypnea >60 breaths per minute with intercostal retractions, and with required FiO2> 40%) were intubated with a 1.0–2.0 ID endotracheal tube and administered bovine-derived surfactant (100 mg phospholipids/kg of birth weight, 4mL/kg intratracheal; Survanta Beractant, AbbVie Inc., North Chicago, IL) and synchronized intermittent mandatory ventilation (SMIV, 40 breaths/min, peak inspiratory pressure 15cm/H2O and positive end expiratory pressure 5cm/H2O Servo300 Maquet, Solino, Sweden). Infants were weaned from ventilator support when breathing in excess of the ventilator and oxygen saturation was stable while receiving Fi02 of 0.21. Newborn Health Assessments (modified APGAR scores 17) were performed immediately at 5 & 20 min after birth, with a score of 10–12 indicating good physical condition.
Intravenous Access and Parenteral Nutrition
IV access was achieved by placement of a peripheral IV catheter in the distal saphenous or cephalic vein (Introcan Safety IV Catheter, 22G × 1″, Teflon Straight, Braun Medical Inc., Bethlehem, PA). Within the first hour of age, a peripherally inserted central catheter (PICC) was also placed in the saphenous vein (First PICC™ S/L 26GA, 1.9F, Argon Medical Devices, Athens, TX) for administration of total parenteral nutrition (TPN). Radiographs confirmed correct placement and terminus of the PICC within the inferior vena cava at the level of the liver. Standard preterm neonatal TPN multivitamins and trace elements (ExactaMix, Baxter Healthcare Corporation, Englewood, CO) were administered at 120mL/kg/day, increasing daily by 20mL/kg/day. Enteral feeding with donated human breast milk (0.5–1.0mL) was introduced every 2hrs, over a 3–5 day period per os or via nasal/oral gastric gavage (3.5 PVC Feeding Tube w/Radiopaque Line, Covidein, Mansfield, MA) until a suckling reflex was evident. Bovine milk infant formula (Similac® for Supplementation, Abbott Nutrition, Lake Forest, IL) was then introduced ad lib, and total intake adjusted based on parenteral and enteral volumes. Adequate enteral feeding volumes and presence of regular bowel movements were criteria for removal of the PICC line and cessation of parenteral feeding.
Infant Behavioral Assessment Scale (IBAS)
From the day of birth and then every 2–3 days as clinical condition allowed, all infants were assessed using neuromotor, sensory-motor and basic survival reflex tests (as previously published 17–19) by trained personnel with inter-operator reliability of >90%. Reflexes tested included rooting (elicited by lightly brushing the cheek), snouting (lightly brushing the nose), suck (nipple placed in mouth), startle (auditory startle to sudden loud noise), grasping (with each hand or foot), clasping (ability for infant to support bodyweight by holding on to “mother”), proprioception (placing of hands and feet when moving the infant down towards a counter surface), visual orientation and following (near and far distances) and auditory orientation (to lip-smacking with the infant facing away from the tester). The post-conception age at which animals achieved a criterion response for each reflex (using a scoring scale outlined by Sackett et al 19) was recorded.
Fetal and Neonatal Brain Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) were performed on a Siemens 3T Tim Trio system (Erlangen, Germany). In utero examinations of the fetal brain were carried out at 139dGA following published procedures 20. Anatomical and diffusion MRI data were acquired and analyzed for neonatal brains at term equivalent age, term plus one month and term plus six months of age (see Supplemental Methods).
Statistical Analyses
Data in the text are presented as mean ± SD. MRI results are presented for control (n=3), IAI (n=4) and term control animals (n=3). Uterine activity data, colony counts and pro-inflammatory mediators are shown for control (n=3) and IAI (n=5) animals. Neonatal reflexes data are shown for control (n=4), IAI (n=5) and term control (n=3). A single control pregnancy was not catheterized due to previous surgical history of hysterotomy. This animal is included in postnatal assessments only. One-way ANOVA, paired T-test or Two-way ANOVA with repeated measures and post-hoc Sidak’s multiple comparison test were used for analyses of neonatal reflexes and AF pro-inflammatory mediators. P<0.05 was considered statistically significant. Analyses were performed using Prism v6.0 for MacOSX (GraphPad Software Inc., La Jolla, CA).
RESULTS
Maternal-Fetal Model of Chronic Intra-Amniotic Infection
After inoculation with U. parvum, there was an exponential growth of microorganisms in the AF that peaked between 8.4×104 and 1.2×106 CFU/mL of AF within 4d and stabilized thereafter (Fig. 2). A single animal had peak AF colony growth of 1.1×107 CFU/mL at 15 days. Interestingly, despite a lower inoculum than previously used 10, colony growth rates and peak levels were comparable to our previous studies. In AF, infant nasal/throat swabs, placenta and fetal membranes were 100% positive for U. parvum by culture/PCR at the time of delivery. All control animals were negative by culture/PCR. For the neonates that completed the study, the inoculation to delivery interval was 18±2d (range: 15–21d). The two IAI neonates that were euthanized before 6 months of postnatal age showed early signs of preterm labor and were delivered after 9 and 13 days infection, attributed to secondary AF infection (Staphylococcus epidermidis) and significant growth restriction, respectively.
Figure 2. Amniotic fluid Ureaplasma parvum (colony-forming units [cfu/mL] growth curve [log scale]).
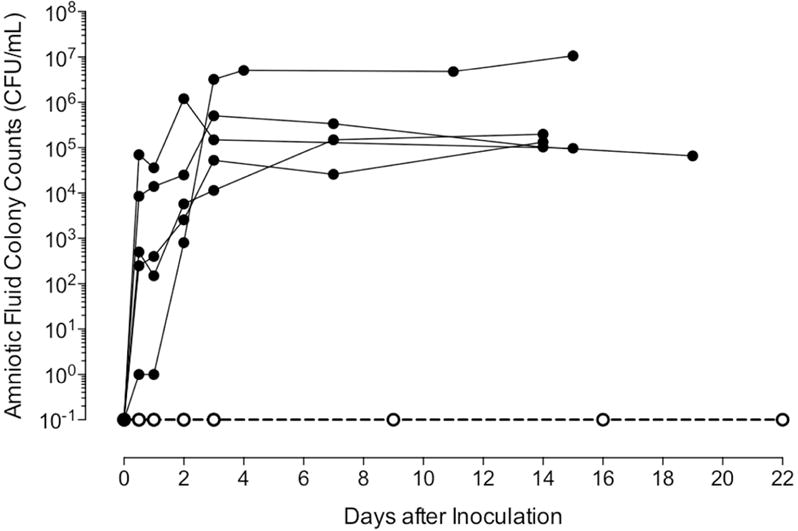
Data are presented as mean ± SD for animals following intra-amniotic inoculation (IAI; filled circles) and in gestationally age-matched controls (open circles) from inoculation to delivery. All control animals were negative for U. parvum for the duration of the experiment.
Before intra-amniotic inoculation, the uterus was quiescent. The maximal HCA per day was calculated for the pre-inoculation baseline and post-inoculation periods and are shown in Table 1. The peak daily uterine contractility was significantly elevated in the post-inoculation period for the U. parvum infected group when compared to baseline. Uterine activity of control animals remained stable with no increase in the period following control inoculation with sterile media. Uterine activity was not determined in uncatheterized Term pregnancies.
Table 1.
Amniotic Fluid Proinflammatory Mediators and Uterine Activity after Intra-Amniotic Ureaplasma parvum Inoculation
| Variable | Control | Intra-Amniotic Infection | ||
|---|---|---|---|---|
| Baseline | Post-Inoculation | Baseline | Post-Inoculation | |
| PGE2 (pg/mL) | 32 ± 8 | 55 ±14 | 80 ± 23 | 2580 ± 1045* |
| PGF2a (pg/mL) | 35 ± 8 | 84 ±22 | 40 ± 6 | 733 ± 162* |
| TNF-alpha (pg/mL) | not detectable | not detectable | not detectable | 1035 ± 320* |
| IL-1B (pg/mL) | not detectable | not detectable | not detectable | 239 ± 71* |
| IL-6 (ng/mL) | 0.5 ± 0.1 | 1.0 ±0.7 | 0.7 ± 0.1 | 57.8 ± 9.3* |
| UA (mmHg.sec/hr) | 1174 ± 300 | 1256 ±285 | 909 ± 232 | 1545 ± 260** |
Data are Mean ± SEM. Concentrations of amniotic fluid pro-inflammatory mediators represent the average concentration prior to intra-amniotic inoculation (baseline) and the maximal concentration reached post-inoculation. Uterine Activity (UA) is expressed as the average of the maximal hourly cumulative area (HCA) under the contraction curve per day prior to inoculation (baseline) and post-inoculation. Intra-amniotic infection (IA) animals received inoculation with U. parvum (serovar1) 10^5 CFU/mL and gestationally age-matched controls received vehicle inoculation only. PG, prostaglandin; TNF, tumor necrosis factor; IL, interleukin.
Indicates AF pro-inflammatory mediator post-inoculation values were significantly increased from control baseline, control peak and IAI baseline values;
Post-inoculation UA was significantly increased compared baseline in the IAI group (p<0.05 paired 2-way ANOVA, Šídák’s multiple comparison).
Amniotic Fluid Pro-Inflammatory Mediators
Coinciding with the increased uterine activity following intra-amniotic inoculation with U. parvum, AF concentrations of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and prostaglandins (PGE2 and PGF2α) were significantly elevated post-inoculation when compared to baseline (both control and IAI) and to media control post-inoculation values (Table 1; P<0.05). There were no differences between baseline and post-inoculation pro-inflammatory mediators for control animals.
Delivery and Neonatal Outcomes
The average gestational age at C-section delivery was 144±2.2d for control (n=5) and 141±4.6d in IAI (n=7) animals (Term=~167dGA21). Birth weight was slightly higher in the control vs. IAI groups (394±91g and 355±52g, respectively), however this difference was not significant (P=0.46). Of the preterm infants, one control and two IAI animals were euthanized prior to 6 months of age and completion of the study. The control animal that did not complete the study was euthanized at 9d postnatal age after developing clinical signs of necrotizing enterocolitis (NEC) that did not respond to antibiotic treatment (7 days of 5mg/kg/day S.Q. Gentamicin (Fresenius Kabi, Lake Zurich, IL) and 100mg/kg B.I.D. I.V. Ampicillin (Sandoz, Holzkirchen, Germany)) and complete gastrointestinal rest. A second animal from the IAI group that developed NEC responded to treatment and completed the study. Two neonatal deaths occurred in the IAI group at 12hrs and 21hrs post-birth were due to respiratory failure, complicated by secondary infection and growth restriction. Both animals had histologic evidence of atelectasis and pneumonitis present at necropsy.
All infants required resuscitation at delivery and were supported with supplemental oxygen (FiO2 up to 0.30–0.40) and/or mechanical ventilation as indicated by clinical signs. During the first 30 min of resuscitation and stabilization, U. parvum IAI infants required higher concentrations of inspired oxygen compared to preterm control animals to achieve SpO2 levels between 60–80% (FiO2, 0.49 vs. 0.37 respectively, P<0.05). Neonatal respiratory distress syndrome (RDS), characterized by significant tachypnea, sternal retractions, and labored breathing was present in 3 of 7 (43%) IAI infants and 1 of 4 (25%) control preterm infants. Length of ventilation was 2.2hrs for the control infant and an average of 10hrs for all IAI infants or 5.6hrs for only those IAI infants that completed the study. Infants exposed to chronic U. parvum IAI required a significantly longer duration of TPN compared to control preterm infants before achieving full enteral feedings (10.2±1 d IAI vs. 4.3±2 d control, P<0.05).
Fetal Brain Development
Figure 3 shows a T2-weighted brain image acquired in utero of a control fetus at 139d gestational age (dGA). Brain lesions secondary to inflammatory processes can be identified with T2-weighted MRI 22. However, normal variation in white matter signal intensity associated with brain development can confound lesion identification. High signal intensity is observable in late developing white matter structures such as within the frontal and temporal lobes (Fig. 3a, arrowheads). Conspicuous lesions attributable to brain inflammation have not been observed by MRI in the IAI fetuses examined to date. Histological examinations are currently ongoing. As shown in Figure 3d–f, the 3D T2-weighted images facilitate construction of cerebral cortical surface models, which enable characterization of fetal brain development 23,24.
Figure 3. In utero T2-weighted control fetal brain images and cortical surface at 139dGA.
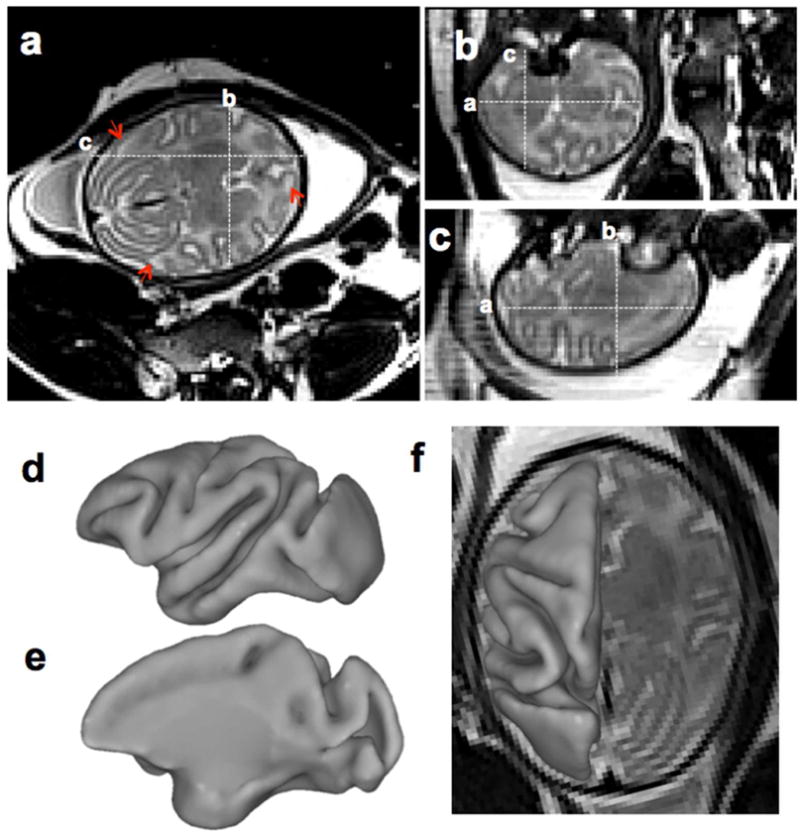
T2-weighted image was acquired using HASTE along the axial direction. From axial (a), coronal (b), and sagittal (c) view, tissue contrast between cortical grey matter, fetal white matter and deep nuclei is evident (a–c). Heterogeneous signal intensity within the developing cerebral cortex is present at this developmental stage (A, arrowheads). The cerebral cortical surface was generated from segmentations of the T2-weighted image (d–f). At this developmental stage, all primary and secondary sulci and gyri present in adult cortex are identifiable.
Neonatal Brain Growth and Development
Figure 4 shows template T1-weighted (top row), fractional anisotropy (FA, middle row), and T2-weighted (bottom row) image axial views at the three postnatal ages chosen for MRI examination. The six white matter (WM) sub-regions are overlaid onto one hemisphere of the T1-weighted images. In addition to overall changes in brain size with development, MRI signal intensity changed within WM for each of the three modalities over the first six months of life (fig. 5d).
Figure 4.
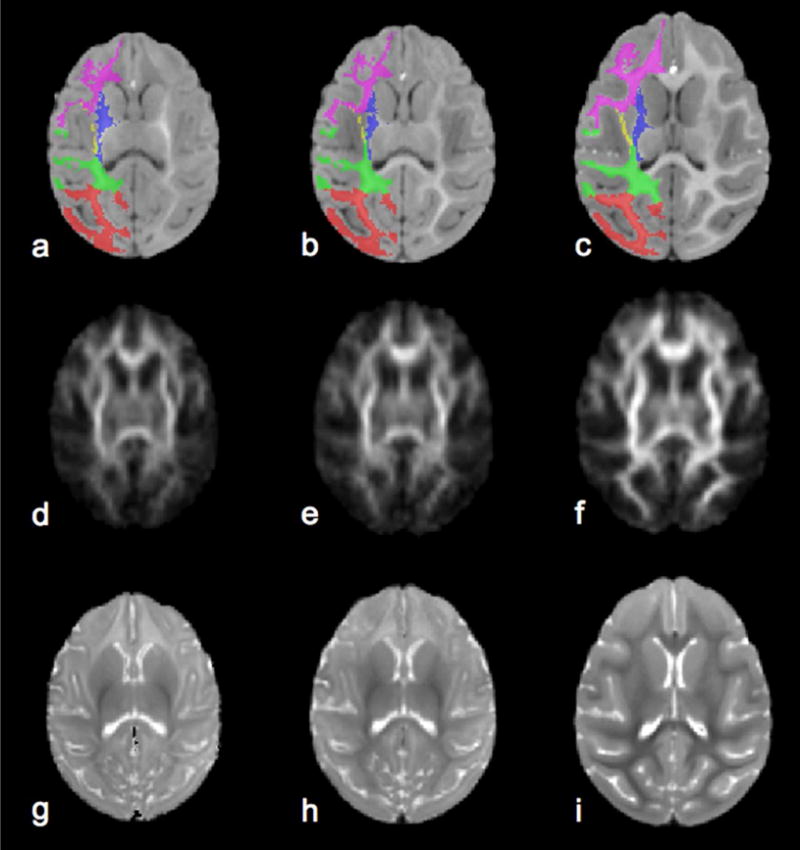
Age-specific reference T1-weighted (a–c), Fractional Anisotropy (d–f), and T2-weighted (g–i) brain templates were constructed from term control subject scans at term (a, d, g), term plus one month (b, e, f) and term plus six months age (c, f, i). For T1-weighted images six white matter parcellations are overlaid on the left brain hemisphere, where occipital white matter in red, parietal white matter in green, internal capsule in blue, external capsule in yellow, temporal white matter in cyan and frontal white matter in magenta. The T2-weighted images corresponding to a TE of 100 ms were constructed from parameter maps of proton density and T2 determined from the dual echo measurement, as described in the supplementary methods.
Figure 5. Comparison of average age-related total and regional brain volumes for control and IAI groups.
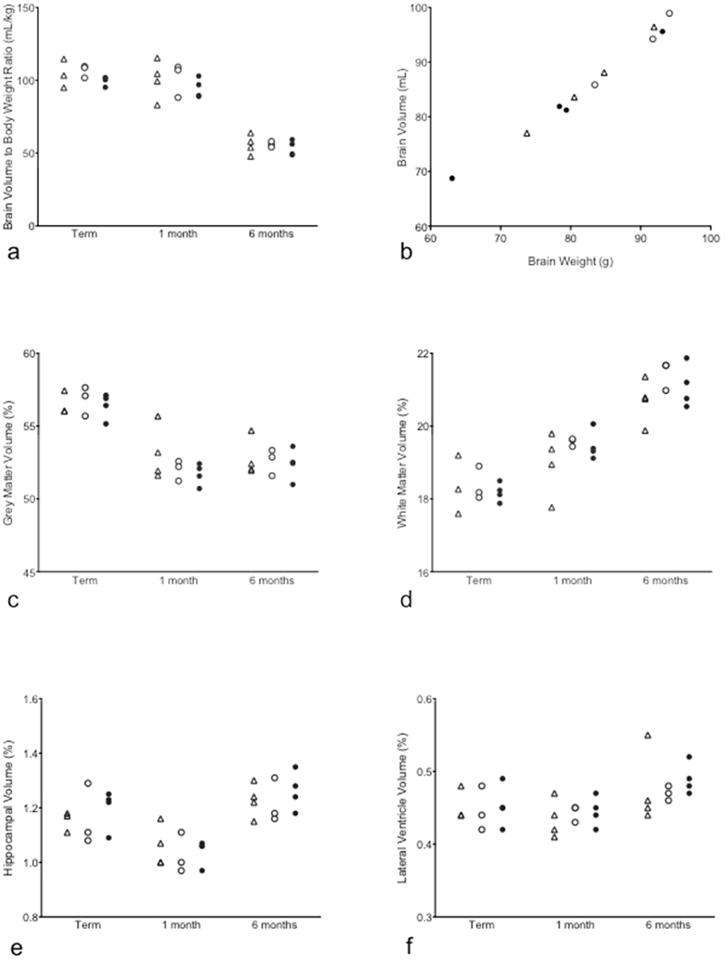
(a) The brain volumes at term, term plus 1 month and term plus 6 months age are shown for Term Control (triangle), Control (open circle) and Intra-Amniotic Infection (IAI; filled circle). (b) Brain volumes calculated from MRI is linearly related with brain weight at the same age measured at necropsy. Regional brain volumes as a proportion of total brain volume are shown for grey matter (c), white matter (d), hippocampus (e) and lateral ventricle (f).
Brain growth data for term control, control and IAI groups are shown in Fig. 5. Overall brain volume increased with age (data not shown) but decreased with age when normalized to body weight. The brain volume.to body weight ratios (corrected to term, term plus 1 month and term plus 6 months ages) were similar across all groups (Fig. 5a), with no significant differences between infants born at term or preterm. As expected, brain volume at 6 months age was proportional to fetal brain weight measured at necropsy at the same age (Fig. 5b). Brain volume fractions of grey matter, WM, hippocampus and lateral ventricles are shown in Fig. 5c–f, and were highly similar between groups. The grey matter volume fraction decreased in all groups from term to 1 month old and remained stable from 1 month to 6 months of age. From term age to 6 months old, the volume occupied by WM increased in all groups. No significant differences were observed between IAI and control groups in volume fraction of hippocampus and lateral ventricles.
Neuroimaging indices of white matter maturation are shown for the internal capsule (Fig. 6a,c) and frontal WM (Fig. 6b, d). As expected, the water T2 decreases with age (Fig. 6a, b), and FA increased with age (Fig. 6c, d) over the first six months of life. Within both regions displayed, the average value for the water T2 was slightly longer in IAI animals compared to controls at term-equivalent age, and the average value for FA was slightly lower in IAI animals compared to controls at one month of age. These findings suggest perturbed WM maturation in IAI animals relative to controls, however neither of these differences reached statistical significance.
Figure 6. Comparison of age-related average regional T2 and FA values between control and IAI groups.
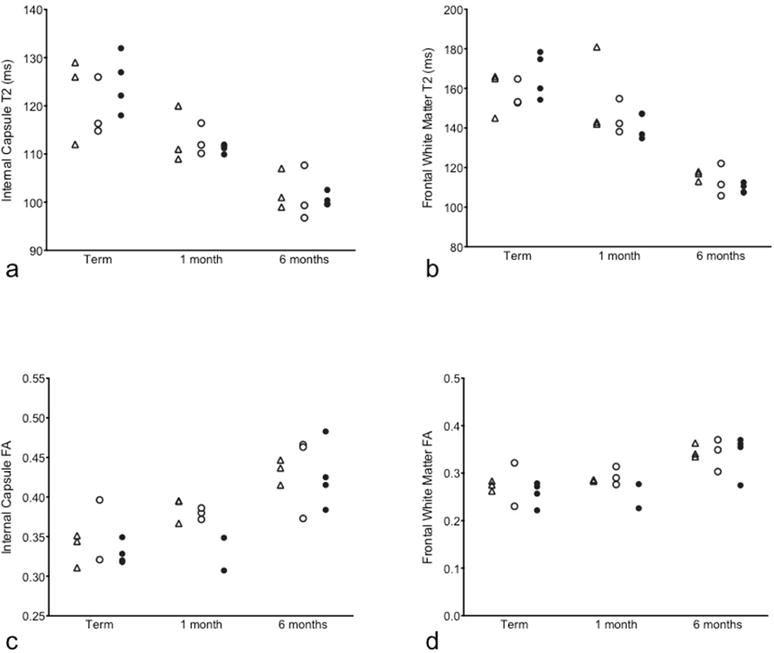
T2 and FA values in the internal capsule (a, c) and the frontal white matter (b, d) regions are shown at term, term plus one month and term plus six months age for Term Control (triangle), Control (open circle) and Intra-amniotic Infection (IAI; filled circle) animals.
Infant Behavioral Assessments
IBAS testing was performed on preterm and term neonates with most “survival” and motor reflexes present within the first two postnatal weeks of life. This is consistent with data from Sackett et al 19 in nursery-reared rhesus infants born between 151–161dGA from a study of assisted reproduction technology. When comparing post-conception age between preterm and term infants in our study (Fig. 7), term control animals reached criterion at significantly later post-conception age (but similar postnatal age) for startle, rooting, suckling, righting, grasp, clasp and auditory orient reflexes when compared to both preterm groups (P<0.05). Criterion for grasp reflex developed significantly earlier post-conception age in IAI animals compared to control (P<0.001), potentially due to earlier gestational age at delivery in this group as postnatal ages at criterion were similar. Unlike survival and motor reflexes that assess maturity of spinal and subcortical nervous system functions, the placing reflex and sensorimotor visual reflexes that require more complex neural mechanisms developed at a similar post-conception age for both term and preterm cohorts despite differences in gestational age at birth.
Figure 7. Neonatal Reflex Behaviors.
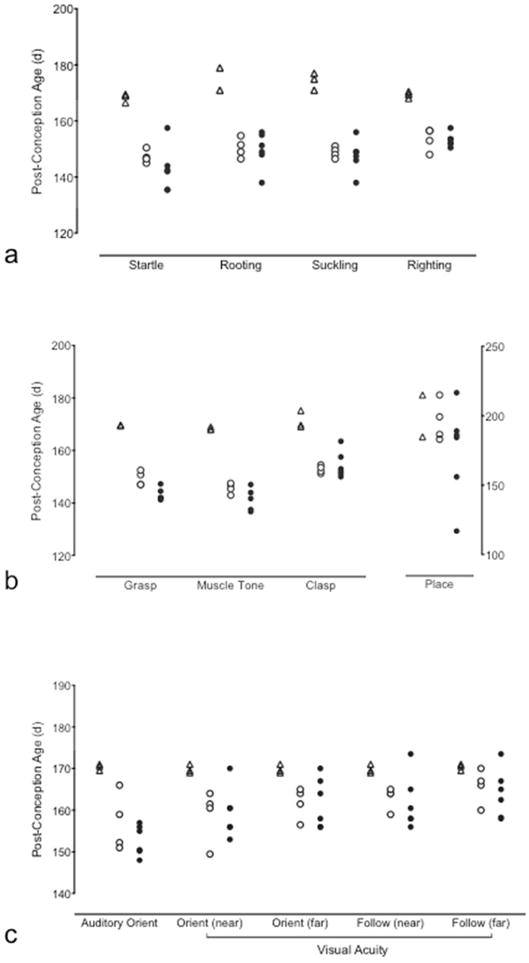
Post-conception age at which criterion was reached for (a) survival, (b) motor [values for placing are plotted against the right y-axis] and (c) sensorimotor neonatal reflexes for Term Control (triangles), Control (open circles) and Intra-Amniotic Infection (IAI; filled circles) animals. Term Control animals reached criterion at significantly later post-conception age (but similar postnatal age) for startle, rooting, suckling, righting, grasp, clasp and auditory orient reflexes when compared to preterm control and IAI animals. Criterion for grasp reflex developed significantly earlier in IAI animals compared to control. P<0.05, ANOVA.
DISCUSSION
This study outlines the successful expansion of our maternal-fetal model of intra-amniotic U. parvum infection to allow postnatal assessments of NHP infant outcomes following preterm birth. Neonatal NHP studies, including those of respiratory therapies and brain development in the preterm baboon and behavioral development in pigtail macaques have previously demonstrated the value of NHP models for the study of important perinatal outcomes that are difficult to assess clinically 19,25–29. By combining our established catheterized pregnant monkey model 10 with our newly developed NHP Special Care Nursery we are able to examine longitudinal outcomes in preterm rhesus monkeys across the fetal and postnatal periods. The long-term goal of these studies is to assess more complex aspects of postnatal cognitive development following infection-associated preterm birth and the safety of potential antenatal antibiotic treatments.
This study demonstrates our ability to perform serial in vivo fetal and postnatal MRI studies of the developing rhesus brain. Fetal MRI is a valuable diagnostic tool for predicting future neurobehavioral outcomes in preterm infants with reduced white and grey matter volumes indicative of fetal brain injury and insults such as periventricular leukomalacia 30,31. While our studies have not identified significant differences by MRI, minor changes in brain volume and development warrant further investigation. By combining clinically relevant MR imaging modalities and advances in technology and analysis techniques with an experimental NHP model of intrauterine infection we are able to leverage the power of a controlled experimental setting with data that is highly translatable to clinical practice.
We also provide early neonatal reflex data for infant monkeys following infection-associated preterm birth. In the current study, development of reflexes requiring simpler neural processing, such as the “survival” reflexes, were associated with postnatal age in term and preterm infants. These reflexes were present soon after birth despite differences in gestational age. However, more complex reflexes requiring integration of sensory and motor functions across a number of brain regions, such as visual following and placing 19, developed at similar post-conception ages in both term and preterm control or IAI infants. This suggests that premature birth and/or infection in these monkeys did not delay development of these later neonatal reflexes. Small differences in gestational age and bodyweight at birth between animals in the preterm control and IAI groups are a potential source of variation between the groups. However, no associations between birth weight and the acquisition of neonatal reflexes were identified (data not shown). Additionally, the necessary use of postnatal antibiotics to treat Ureaplasma infected infants (and therefore also preterm controls) and clinical use for treatment of NEC may be considered a potential confounder, but represent normal clinical outcomes and practice. While we have not identified marked differences in early reflex outcomes, ongoing examination of recognition and procedural memory, which require more advanced prefrontal cortex, striatal and cerebellar maturity may identify functional differences in preterm infants not observed in the early neonatal period.
The current study characterizes a chronic intra-amniotic infection resulting in long-term exposure of the fetus to infection and inflammation. We have previously inoculated rhesus monkeys with 107CFU/mL or 105CFU/mL U. parvum at 128 or 138dGA, respectively 5,10. Despite differences in inoculum size, AF colony counts reached similar peak levels around 106CFU/mL across studies 5,10. Ammonia produced by Ureaplasma spp. has been shown to reach inhibitory concentrations in in vitro culture media 5,32 and likely inhibits growth in AF. However, we also observed a muted response in AF pro-inflammatory mediator concentrations in the chronic model compared to short-term infection. Gestational age may mediate this effect, with the inoculation-to-delivery interval and production of pro-inflammatory mediators decreasing as age at inoculation increased 5,10. This is consistent with increased sensitivity of the uterus to inflammatory (pro-labor) mediators and heightened uterine contractility as gestation progresses. Chronic exposure to inflammation, beginning at an earlier gestational age likely mimics clinical cases of intrauterine infection, with microorganisms detected in AF as early as 16 weeks gestation in women, many weeks before the onset of labor 33. The length of in utero exposure to U. parvum and inflammation may also mediate the severity of fetal brain injury and level of postnatal functional deficits associated with intrauterine inflammation 34–37. The mechanisms of perinatal inflammatory brain injury remain poorly understood. Co-morbidities including prematurity, hypoxic-ischemic insults, intrauterine growth restriction, neonatal resuscitation efforts and polymicrobial infection further complicate our understanding of these processes, particularly with respect to the “second-hit hypothesis”, in which perinatal inflammation may increase vulnerability to injury from other fetal or postnatal sources.
These studies have focused on the need to characterize the neurodevelopmental consequences of intrauterine infection. However, the authors recognize the importance of perinatal infection in the etiology of preterm lung injury and bronchopulmonary dysplasia 38. A technical limitation of this study is that volume-targeted ventilation was not used. Our use of respiratory support was determined by the clinical needs of each infant based on Neonatal Resuscitation Guidelines 16. Steps were taken to reduce the FiO2 and duration of intubation and invasive respiratory support. Continuous mask CPAP was not required beyond the resuscitation period but CPAP was used intermittently (5min intervals) in the first 48hrs of life in response to apneic events or precipitous drop in SpO2. Whilst lung inflammation and pathology have not been reported in this study, future work will include assessments of lung injury and function in these animals, including consideration of the greater level of respiratory support required by IAI infants and the consequences this may have on both lung and brain development 26,39,40.
We have previously shown in a fetal rhesus study that intra-amniotic U. parvum infection leads to a robust lung inflammatory response and that maternal azithromycin treatment can ameliorate this injury and also eradicate U. parvum from the AF, fetal tissues, resulting in a prolongation of gestation 10. However, the importance of providing clear evidence of functional outcomes for such treatments has been highlighted by the Oracle Trials and the potential effect antenatal antibiotic exposure may have on neurodevelopmental outcomes 41,42. Correlating functional and histological findings is a priority of future studies utilizing this new NHP model in order to examine the long-term safety of antimicrobial interventions for premature birth.
The development of this fetal-neonatal model demonstrates our ability to study the intra-uterine environment, during infection and pharmaceutical or physiological interventions, providing direct physiological measurements across gestation. By combining these measurements with assessments of postnatal functional outcomes correlated with both in vivo imaging techniques and in vitro assays, we have the ability to assess a broad range of insults across the perinatal period. The emergence of new threats such as the Zika virus highlight the need for basic research utilizing relevant animal models with the ability to advance our understanding and treatment of human obstetrical and perinatal questions.
Supplementary Material
Acknowledgments
We acknowledge the contributions of Kimberly Ray, Edward Henson, Norma Drinkwater, Nikki Sternberger, Sheila Roberts, Megan O’Brien and the Clinical Medicine, Surgical Services and Behavioral Services Units, Division of Comparative Medicine, ONPRC, Dr. L. Xiao, Ms Donna Crabb and Ms Amy Ratliff (University of Alabama at Birmingham) and Dr. Tom Burbacher (Director of the Infant Primate Research Laboratory, WaNPRC, Seattle). Portions of this work have been presented at the 61st annual meeting of the Society for Reproductive Investigation, Florence, Italy, March 2014; European Workshop on Neonatal Transition, Prato, Italy, August 2014 and the 41st annual meeting of the Fetal and Neonatal Physiological Society, St Vincent, Italy, September 2014.
Financial Support: This work was supported by the following grants: Collins Medical Trust (pilot program funding), National Institute of Child Health and Human Development HD055053, and the National Institutes of Health, number 8P51 OD 011092-53.
Footnotes
Disclosure Statement: The authors declare no financial interests or conflicts.
Category of Study: Translational
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 4.Dammann O, Leviton A, Gappa M, Dammann CE. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. BJOG. 2005;112(Suppl 1):4–9. doi: 10.1111/j.1471-0528.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 5.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 6.Berger A, Witt A, Haiden N, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–8. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 7.Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thebaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65:430–6. doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 8.Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed. 2014;99:F87–92. doi: 10.1136/archdischild-2012-303351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 10.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207:475 e1–e14. doi: 10.1016/j.ajog.2012.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–63. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 12.Waites KB, Duffy LB, Schwartz S, Talkington DF. Mycoplasma and Ureaplasma. In: Isenberg H, editor. Clinical microbiology handbook. Washington, D.C.: American Society for Microbiology Press; 2004. [Google Scholar]
- 13.Xiao L, Glass JI, Paralanov V, et al. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J Clin Microbiol. 2010;48:2715–23. doi: 10.1128/JCM.01877-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscardi RM, Othman AA, Hassan HE, et al. Azithromycin to prevent bronchopulmonary dysplasia in ureaplasma-infected preterm infants: pharmacokinetics, safety, microbial response, and clinical outcomes with a 20-milligram-per-kilogram single intravenous dose. Antimicrob Agents Chemother. 2013;57:2127–33. doi: 10.1128/AAC.02183-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan HE, Othman AA, Eddington ND, et al. Pharmacokinetics, safety, and biologic effects of azithromycin in extremely preterm infants at risk for ureaplasma colonization and bronchopulmonary dysplasia. J Clin Pharmacol. 2011;51:1264–75. doi: 10.1177/0091270010382021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Textbook of Neonatal Resuscitation. 6th. USA: American Academy of Pediatrics and American Heart Association; 2011. [Google Scholar]
- 17.Ruppenthal GC, Sackett GP. Research protocol and technician’s manual. 2nd. Infant Primate Research Laboratory, University of Washington; 1992. http://wanprc.org/iprl: [Google Scholar]
- 18.Schneider ML, Coe CL, Lubach GR. Endocrine activation mimics the adverse effects of prenatal stress on the neuromotor development of the infant primate. Dev Psychobiol. 1992;25:427–39. doi: 10.1002/dev.420250604. [DOI] [PubMed] [Google Scholar]
- 19.Sackett G, Ruppenthal G, Hewitson L, Simerly C, Schatten G. Neonatal behavior and infant cognitive development in rhesus macaques produced by assisted reproductive technologies. Dev Psychobiol. 2006;48:243–65. doi: 10.1002/dev.20132. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Pettersson DR, Studholme C, Kroenke CD. Characterization of Laminar Zones in the Mid-Gestation Primate Brain with Magnetic Resonance Imaging and Histological Methods. Front Neuroanat. 2015;9:147. doi: 10.3389/fnana.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novy MJ, Walsh SW. Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol. 1983;145:920–31. doi: 10.1016/0002-9378(83)90841-4. [DOI] [PubMed] [Google Scholar]
- 22.Dean JM, van de Looij Y, Sizonenko SV, et al. Delayed cortical impairment following lipopolysaccharide exposure in preterm fetal sheep. Ann Neurol. 2011;70:846–56. doi: 10.1002/ana.22480. [DOI] [PubMed] [Google Scholar]
- 23.Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131:2028–41. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefevre J, Germanaud D, Dubois J, et al. Are Developmental Trajectories of Cortical Folding Comparable Between Cross-sectional Datasets of Fetuses and Preterm Newborns? Cereb Cortex. 2016;26:3023–35. doi: 10.1093/cercor/bhv123. [DOI] [PubMed] [Google Scholar]
- 25.Yoder BA, Coalson JJ. Animal models of bronchopulmonary dysplasia. The preterm baboon models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L970–7. doi: 10.1152/ajplung.00171.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verney C, Rees S, Biran V, Thompson M, Inder T, Gressens P. Neuronal damage in the preterm baboon: impact of the mode of ventilatory support. J Neuropathol Exp Neurol. 2010;69:473–82. doi: 10.1097/NEN.0b013e3181dac07b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 2007;27:12506–15. doi: 10.1523/JNEUROSCI.3063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer J, Grant K, Burbacher T, et al. Relationship between prenatal cortisol exposure and behavioral development in macaque monkeys. Psychoneuroendocrinology. 2015;61:30–1. [Google Scholar]
- 29.Golub MS, Hogrefe CE, Vandevoort CA. Binge drinking prior to pregnancy detection in a nonhuman primate: behavioral evaluation of offspring. Alcohol Clin Exp Res. 2014;38:551–6. doi: 10.1111/acer.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–48. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 31.Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Cassell GH, Blanchard A, Duffy L. Mycoplasma Clinical and Pathogenic Microbiology. St Louis: Mosby-Year Book; 1994. [Google Scholar]
- 33.Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 34.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–26. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuban KC, O’Shea TM, Allred EN, et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr Neurol. 2015;52:42–8. doi: 10.1016/j.pediatrneurol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dada T, Rosenzweig JM, Al Shammary M, et al. Mouse model of intrauterine inflammation: sex-specific differences in long-term neurologic and immune sequelae. Brain Behav Immun. 2014;38:142–50. doi: 10.1016/j.bbi.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75:376–80. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viscardi RM, Kallapur SG. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin Perinatol. 2015;42:719–38. doi: 10.1016/j.clp.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rees SM, Loeliger MM, Munro KM, et al. Cerebellar development in a baboon model of preterm delivery: impact of specific ventilatory regimes. J Neuropathol Exp Neurol. 2009;68:605–15. doi: 10.1097/NEN.0b013e3181a39b3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeliger M, Inder T, Cain S, et al. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics. 2006;118:1640–53. doi: 10.1542/peds.2006-0653. [DOI] [PubMed] [Google Scholar]
- 41.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372:1319–27. doi: 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- 42.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet. 2008;372:1310–8. doi: 10.1016/S0140-6736(08)61202-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


